Abstract
Solid organ transplant recipients are vulnerable to severe infection during induction therapy. We report a case of a 67-year-old male who died unexpectedly 10 days after receiving a kidney transplant on February 10, 2020. There was no clear cause of death, but COVID-19 was considered retrospectively, as the death occurred shortly after the first confirmed case of COVID-19 in Canada. We confirmed the presence of SARS-CoV-2 components in the renal allograft and native lung tissue using immunohistochemistry for SARS-CoV-2 spike protein and RNA scope in situ hybridization for SARS-CoV-2 RNA. Results were reaffirmed with the Food and Drug Administration Emergency Use Authorization approved Bio-Rad SARS-CoV-2 digital droplet PCR for the kidney specimen. Our case highlights the importance of patient autopsies in an unfolding global pandemic and demonstrates the utility of molecular assays to diagnose SARS-CoV-2 post-mortem. SARS-CoV-2 infection during induction therapy may portend a fatal clinical outcome. We also suggest COVID-19 may be transmittable via renal transplant.
KEYWORDS: basic (laboratory) research/science, clinical research/practice, donors and donation: donor-derived infections, infection and infectious agents-viral, kidney transplantation/nephrology, kidney transplantation: living donor, pathology/histopathology, patient safety
Abbreviations: AKI, acute kidney injury; BiPAP, bi-level positive airway pressure; BPM, beats per minute; COVID-19, coronavirus disease 2019; CXR, chest radiograph; ddPCR, digital droplet polymerase chain reaction; EM, electron microscopy; FDA-EUA, federal drug administration emergency use authorization; ICU, intensive care unit; IHC, immunohistochemistry; KTx, kidney transplant; mm Hg, millimeter mercury; N, nucleocapsid genes; NPS, nasopharyngeal swab; RP, ribonuclease P; RT-PCR, reverse transcriptase polymerase chain reaction; S, spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMP/SMX, sulfamethoxazole/trimethoprim; SOT, solid organ transplant
1. BACKGROUND
The ongoing coronavirus disease 2019 (COVID-19) pandemic has led many transplant centers to suspend living donor transplants and reduce deceased donor transplants to limit the risk to patients and healthcare workers.1 As a result, few cases of SARS-CoV-2 infection in kidney transplants (KTx) during induction therapy have been reported, but there is clearly a risk of severe COVID-19 infection during solid organ transplantation (SOT).2 Westhoff et al. detected SARS-CoV-2 RNA in a kidney allograft by reverse transcriptase PCR (RT-PCR) and RNA scope in situ hybridization in a pancreas-KTx recipient.3 COVID-19 primarily targets respiratory tissue, but there is emerging evidence for multiorgan involvement including the kidney.4 , 5 Numerous studies suggest direct infection of kidney epithelial cells as detected by RT-PCR, in situ hybridization, immunohistochemistry (IHC), and electron microscopy (EM).4 The Bio-Rad SARS-CoV-2 digital droplet PCR (ddPCR) kit is a Food and Drug Administration Emergency Use Authorization (FDA-EUA) approved clinical assay to detect SARS-CoV-2 with superior sensitivity despite low viral load previously used to detect COVID-19 RNA in RT-PCR negative samples.5, 6, 7, 8 Here, we report the case of a patient who died unexpectedly 10 days following a living donor KTx. We use IHC, RNA scope, and ddPCR to establish the cause of death related to COVID-19 by confirming viral RNA and spike (S) protein presence in donor kidney tissue.
2. CASE REPORT
A 67-year-old male with end stage kidney disease due to insulin-dependent diabetes mellitus underwent an uncomplicated unrelated living donor renal transplant on January 31, 2020 with immediate post-graft function. Previous medical history included stable coronary artery disease with a transthoracic echocardiogram showing diastolic dysfunction and preserved biventricular ejection fraction in December 2019 and a low-risk thallium stress test in November 2019. Viral screening showed hepatitis B exposure (core antibody positive, surface antigen negative, surface antibody 47.9 IU/L), nonreactive for hepatitis C antibody, hepatitis D negative, and HIV negative. Medications included insulin, amlodipine, atorvastatin, hydroxyzine, irbesartan, terazosin, and metformin. There was no travel or COVID-19 exposure history.
His immunosuppressive medications consisted of basilixumab, tacrolimus, and prednisone. On February 9, 2020, 9 days after his transplant and uneventful hospitalization, the patient was discharged with no concerns other than a mild elevation of monocytes (1.1 × 109/L) ( Table 1). His discharge medications included tacrolimus, mycophenolic acid, a prednisone taper, lamivudine, and trimethoprim/sulfamethoxazole (TMP/SMX).
TABLE 1.
Laboratory values prior to discharge and at readmission
| Laboratory parameter | Feb. 8, 2020 | Feb. 9, 2020 | Reference values |
|---|---|---|---|
| Hemoglobin | 105 g/L | 154 g/L | 137–180 g/L |
| White blood cells | 7.2 × 109/L | 38.9 × 109/L | 4.5–11 × 109/L |
| Neutrophils | 4.9 × 109/L | 33.2 × 109/L | 2.0–8.0 × 109/L |
| Lymphocytes | 1.1 × 109/L | 1.6 × 109/L | 0.7–3.5 × 109/L |
| Monocytes | 1.1 × 109/L | 3.5 × 109/L | 0–1.0 × 109/L |
| Eosinophils | 0.1 × 109/L | 0.0 × 109/L | 0–0.7 × 109/L |
| Basophils | 0.0 × 109/L | 0.1 × 109/L | 0–0.2 × 109/L |
| Immature Granulocytes | 0.1 × 109/L | 0.5 × 109/L | 0 × 109/L |
| Platelets | 164 × 109/L | 50 × 109/L | 150–400 × 109/L |
| Sodium | 131 mmol/L | 137 mmol/L | 133–145 mmol/L |
| Potassium | 4.3 mmol/L | 5.2 mmol/L | 3.5–5.0 mmol/L |
| Chloride | 102 mmol/L | 107 mmol/L | 98–111 mmol/L |
| Bicarbonate | 22 mmol/L | 20 mmol/L | 21–31 mmol/L |
| Creatinine | 69 μmol/L | 50 μmol/L | 50–120 μmol/L |
| Urea | 4.4 mmol/L | 3.5 mmol/L | 3.0–9.0 mmol/L |
| eGFR | 93 mL/min/1.73 m2 | 107 mL/min/1.73 m2 | ≥60 mL/min/1.73 m2 |
| Calcium | 2.20 mmol/L | 2.11 mmol/L | 2.10–2.60 mmol/L |
| Magnesium | 0.62 mmol/L | 0.52 mmol/L | 0.65–1.05 mmol/L |
| Troponin T High Sensitivity | — | 50 ng/L | 0–13 ng/L |
| Albumin | 31 | 27 g/L | 33–48 g/L |
| pH | — | 7.35 | 7.36–7.44 |
| PaO2 | — | 65 mm Hg | 70–88 mm Hg |
| PaCO2 | — | 42 mm Hg | 30–40 mm Hg |
| HCO3 | — | 23 mmol/L | 22–26 mmol/L |
| Lactate | — | 5.6 mmol/L | <2 mmol/L |
| Glucose | 6.6 mmol/L | 23.5 mmol/L | 3.3–11.0 mmol/L |
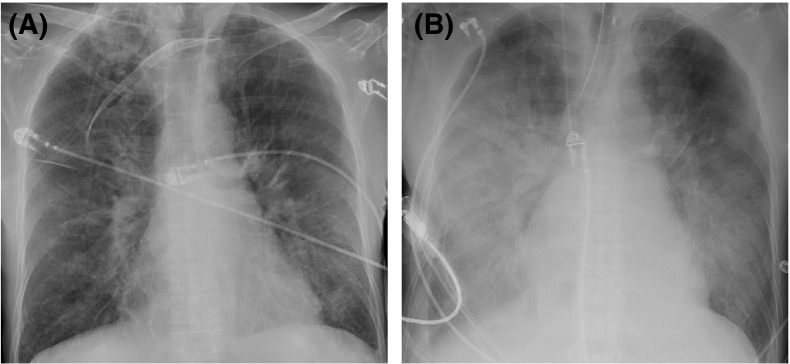
On the evening of February 9, 2020, less than 4 h after being discharged from the hospital, the patient experienced sudden onset dyspnea. Paramedics found him to be hypotensive with a wide-complex bradyarrhythmia. He became unresponsive with a peripheral capillary oxygen saturation 100% on 15 L/min via face mask. At hospital arrival, his heart rhythm was converted to a narrow-complex tachycardia with a heart rate of 130 beats per minute (BPM). 12-lead ECG showed atrial fibrillation with rapid ventricular response without ischemia. Multiple blood draws were required due to the presence of fibrin strand and platelet clumping. Laboratory blood tests showed elevated peripheral white blood cell count was 38.9 × 109/L with 85% neutrophils and a monocytosis (3.5 × 109/L), normal kidney function (serum creatinine 0.57 mg/dL), mild hyperkalemia (potassium 5.2 mmol/L), hyperglycemia (blood glucose 23.5 mmol/L [423 mg/dL]), and elevated arterial blood lactate (5.6 mmol/L) (Table 1). Initial chest radiograph (CXR) in the emergency department showed scattered areas of airspace disease present bilaterally with radiographic pulmonary edema ( Figure 1A). Empiric antibiotic therapy was initiated using piperacillin-tazobactam, TMP/SMX, and vancomycin. Vasoconstrictors, stress-dose hydrocortisone, and bi-level positive airway pressure (BiPAP) were started. Transplant nephrology was consulted and he was admitted to the intensive care unit (ICU).
FIGURE 1.
Patient’s portable anterior-posterior chest radiographs. (A) Initial radiograph in the emergency department showing scattered areas of airspace disease present bilaterally with radiographic pulmonary edema indicated by vascular redistribution and Kerley B lines. (B) In the intensive care unit 18 hours later showing progression of airspace opacification in perihilar regions of lung bilateral with bilateral pleural effusions. A left internal jugular line is present in good position.
Upon ICU admission, the patient was severely hypoxemic without respiratory distress (PaO2/FiO2 ratio 139) with clinical peripheral vasodilation. A transthoracic echocardiogram revealed normal left ventricular function, no evidence of elevated left atrial pressure, moderate right ventricular dilatation with severe tricuspid valve regurgitation, and an elevated right ventricular systolic pressure of 89 mm Hg. Diagnoses of acute respiratory distress syndrome and distributive shock were established. Further laboratory evaluation demonstrated a worsening lactic acidosis and doubling of the serum creatinine from 0.57 to 1.13 mg/dL without ultrasonographic evidence of anatomical or vascular flow abnormalities of the allograft, and a therapeutic tacrolimus level (8.0 μg/L). The patient continued to deteriorate and developed worsening hypotension nonresponsive to volume resuscitation and escalating doses of vasoconstrictor therapy. A repeat CXR, 18 hours after the initial CXR, showed progression of airspace opacification in perihilar regions of lung bilaterally with persistent bilateral pleural effusions (Figure 1B). During the patient’s short course in the ICU, he developed a recurrent bradyarrhythmia (HR = 40 BPM, third degree atrioventricular block, and a junctional escape rhythm), evolving into pulseless electrical activity requiring chest compressions, intravenous epinephrine, endotracheal intubation, and transvenous pacemaker insertion. Thereafter, the patient’s shock state rapidly progressed and became refractory to multiple high-dose vasoconstrictors, culminating in subsequent cardiac arrest from which he could not be successfully resuscitated. The patient was pronounced dead on February 10, 2020, 1 day after he was discharged well from hospital and 10-day post-KTx.
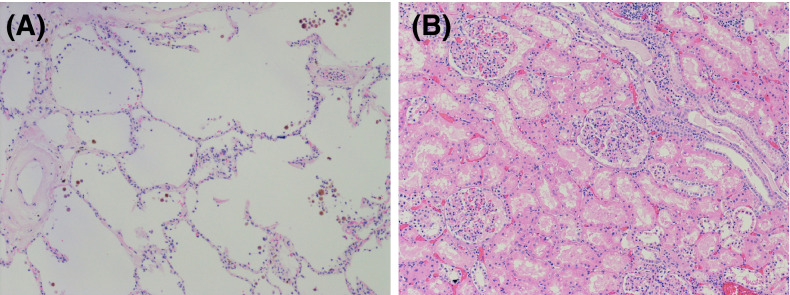
A complete autopsy was performed to elucidate the cause of death. Antemortem blood cultures were negative. Macroscopic and histologic examination of the heart revealed mild hypertensive changes with no inflammation or necrosis. There were bilateral pleural effusions (right 1.6 L and left 1.3 L). The lungs were mildly edematous, and a small subsegmental peripheral thromboembolism was identified in the left lower lobe. Histologically, the lungs showed minimal diffuse alveolar damage not felt to account for the clinical disease severity ( Figure 2A). The renal allograft was macroscopically and histologically unremarkable with both ureteric anastomoses intact, no thrombosis, and no evidence of acute rejection (Figure 2B). Thus, the cause of death in this otherwise stable patient remained unknown.
FIGURE 2.
Microscopic histopathologic images of left lung and renal allograft tissue with hematoxylin and eosin staining. (A) Microscopic sections of left lung specimen showing minimal diffuse alveolar damage with occasional capillary microthrombi. Scale, 10× magnification. (B) Histologically unremarkable microscopic sections of kidney allograft showing lack of thrombosis and no evidence of acute rejection. Scale, 10× magnification.
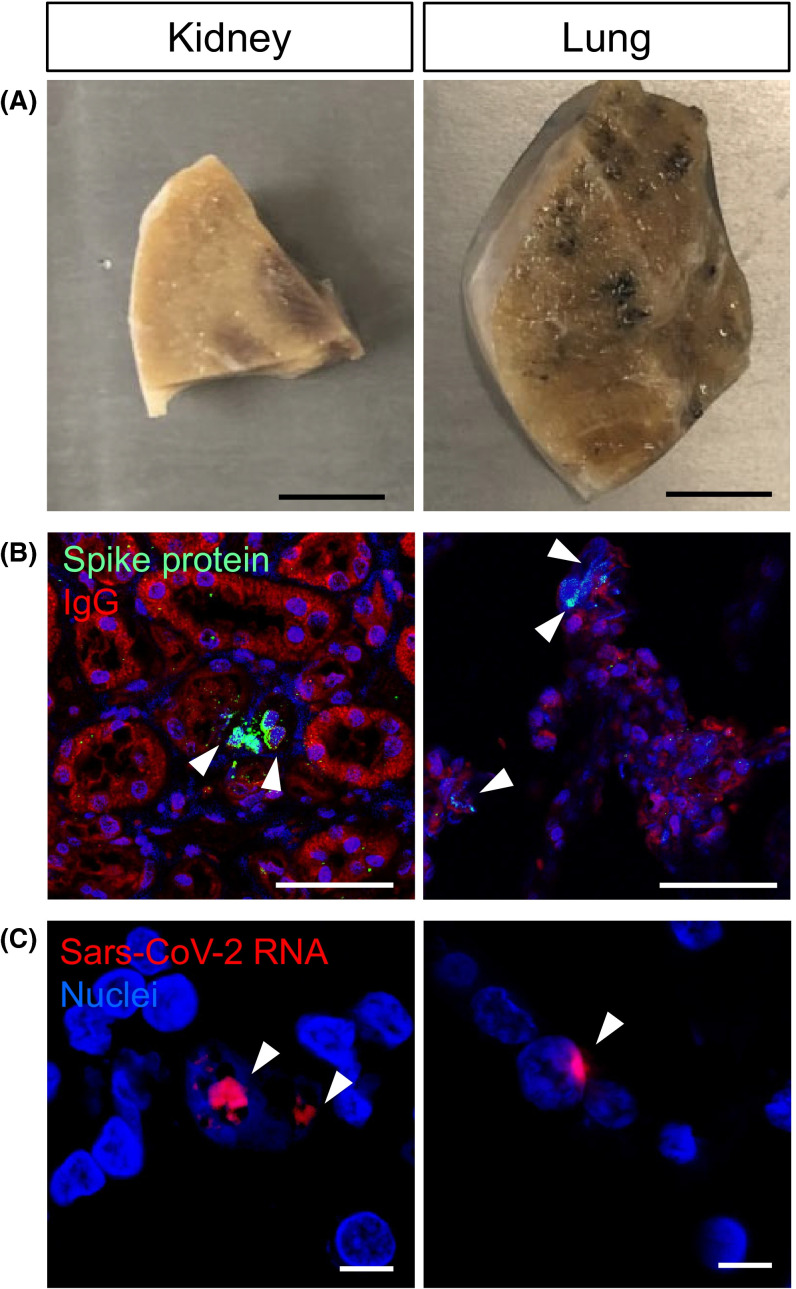
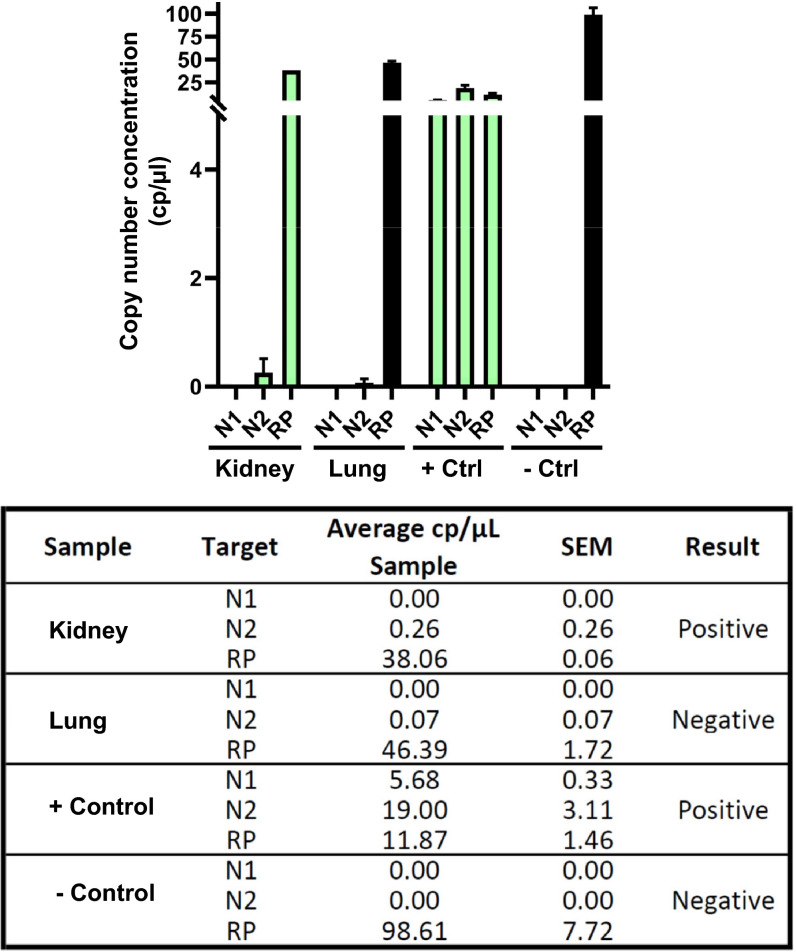
Without an alternative explanation for the patient’s death, we retrospectively questioned COVID-19 infection. Since a nasopharyngeal swab sample (NPS) or serum analysis was not available, we deployed nonstandard approaches. First, we acquired autopsy specimens of the allograft and lung tissue for analysis ( Figure 3A). Remarkably, antibodies directed against SARS-CoV-2 S protein were positive in the allograft and native lung tissue of the patient (Figure 3B). RNA scope in situ hybridization was used to detect SARS-CoV-2 RNA in the allograft (Figure 3C) as previously described.3 , 7 With both IHC and RNAscope, we noted very few viral particles, with more in the donor kidney compared to native lung tissue. To confirm this finding, we used RT-PCR but were unable to detect SARS-CoV-2 RNA (data not shown). Next, we turned to an FDA-EUA clinically validated BioRad ddPCR assay approved for human diagnosis, previously used to detect SARS-CoV-2 RNA in RT-PCR negative samples.6 , 8 Using ddPCR, we confirmed SARS-CoV-2 nucleocapsid N2 gene in the renal allograft ( Figure 4). A smaller signal was observed in lung tissue, but lower than the clinically validated threshold.
FIGURE 3.
Detection of SARS-CoV-2 in kidney and lung tissue by immunohistochemistry and RNA scope. (A) Sections from kidney allograft and lung specimens from the patient. Scale bar, 1 cm. (B) Immunohistochemistry detection by indirect immunofluorescence using antibody directed against SARS-CoV-2 spike protein. Arrowhead indicating spike protein (green) positive cells. Scale bar, 50 µm. (C) In-situ hybridization using RNA scope to detect SARS-CoV-2 viral RNA (red) indicated by arrowheads in both kidney and lung tissue. Scale bar, 10 µm.
FIGURE 4.
Digital droplet PCR using the Bio-Rad SARS-CoV-2 Droplet Digital PCR Kit. Green bars indicate samples which were deemed positive for SARS-CoV-2. SARS-CoV-2 nucleocapsid genes (N1, N2) and human RNAse P (RP). Samples and controls were run in duplicate.
3. DISCUSSION
Using three methods of viral protein and/or RNA detection, we present a COVID-19 positive patient who died on February 10, 2020, preceding the first confirmed case in Alberta, Canada. Moreover, this is the first Canadian COVID-19 fatality, which were previously established as a travel-related case on March 5, 2020, and a nursing home death on March 9, 2020, respectively.9 Our patient demonstrates the possibility of a severe adverse outcome for COVID-19 infection during induction therapy and the potential for SARS-CoV-2 renal allograft invasion mediated SOT transmission. Many case registries and meta-analyses conclude that COVID-19 portends a worse outcome in SOT recipients.10 , 11 When hospitalized, KTx recipients appear to have higher mortality and rates of end-organ complications than the general population.12 Due to the suspension of elective surgeries during the first wave of the pandemic, there are very few published reports of COVID-19 infection in the immediate post KTx induction period. However, one fatal case described a similar 24-hour escalation from clinically stable to cardiac arrest and reports of other recent KTx recipients followed a foreboding clinical course with rapid clinical deterioration.2 , 12 An analysis in Spain found 28% of patients that acquired COVID-19 infection following a KTx died.13
In hindsight, clinical presentation of septic shock with multiorgan dysfunctions, radiographic progressive bilateral patchy airspace disease, and nonspecific pathologic findings of minimal diffuse alveolar damage, capillary microthrombi, and pulmonary edema were consistent with natural history of severe COVID-19 infection in KTx recipients and published autopsy cases.14 Taken together, the detection of both COVID-19 RNA and protein, lack of an alternative cause of death, and severe clinical presentation implicated SARS-CoV-2 infection as the cause of death.
The analysis of our patient tissue consistently showed low levels of detectable SARS-CoV-2, which may be attributable to the challenges of IHC and RNA amplification using formalin-fixed samples or low viral load. COVID-19 detection was constrained to remaining cadaveric tissue post-autopsy by lack of available wide-spread Canadian NPS PCR testing in February 2020. Reliable post-mortem detection of genetic material within formalin-fixed paraffin-embedded (FFPE) tissues is technically challenging due to degradation during sample preparation which may have decreased our overall yield and explain our low signal.7 Initial NPS PCR viral load in respiratory epithelium has been correlated with disease severity,15 but a low viral detection and severe septic shock are not necessarily incongruent or unexpected with concurrent anti-rejection therapy. Severe illness presentation is suspected to occur when systemic viral load overcomes host T cell ability to suppress infection16; thus, severe cases with low viral burden could occur in T cell suppressed individuals. Severe cases with low viral loads have been reported, and time to viral clearance may be a better marker of disease severity in comorbid, older, and immunosuppressed individuals.15
How the patient acquired COVID-19 remains unclear. We speculated that either the patient contracted COVID-19 as an Alberta inpatient prior to recognized community spread or that COVID-19 was acquired via renal transplantation. KTx mediated transmission is theorized based on emerging, controversial evidence that SARS-CoV-2 may directly target kidneys. COVID-19 associated acute kidney injury (AKI) is traditionally attributed to critical illness, but clinical observations and autopsy reports suggest direct renal targeting as well. COVID-19-associated AKI is disproportionately common compared to other coronaviruses and hospitalized cases manifesting AKI appear more fatal.17 RNA and viral particles have been detected within cadaveric kidneys by EM and IHC.5 Critics assert that multivesicular bodies can mimic viral particles and mere detection of RNA within an organ does not necessarily indicate that the virus is intact, replication competent, and clinically significant.18 A more convincing case report of NPS RT-PCR confirmed COVID-19 in a pancreas–kidney transplant recipient identified SARS-CoV-2 RNA with RT-PCR and RNA scope in situ hybridization within the kidney allograft,3 combining a sensitive testing modality with clinically correlated infection. Using ddPCR to identify S proteins and RNA within the donor kidney supports their findings and our unexpected finding of increased SARS-CoV-2 detected in donor kidney relative to native lung suggests KTx-mediated transmission. If we accept that COVID-19 can invade the kidney, self-propagate within it, and potentially invade the host, it logically follows that COVID-19 is transmittable via KTx if the donor allograft is colonized. More studies with highly sensitive molecular techniques are needed to determine the precise mechanism of renal invasion and therefore potential transmission via SOT.
Regardless of how the patient acquired SARS-CoV-2, our case report presents an ominous outcome of COVID-19 infection during anti-rejection induction. As the pandemic unfolds, recent KTx recipients remain vulnerable to fatal infection due to comorbid diseases, prolonged hospitalization in overburdened critical care facilities, and high-dose immunosuppression. Management remains unclear and some providers are now opting against full induction therapy, which appears to result in increased rejection without mortality benefit.19 As such, many guidelines are advocating for a temporary suspension in elective living and non-urgent deceased donor transplant in areas with high COVID-19 incidence.20 Suspending transplant surgery has public health consequences, including increased waitlist mortality. Until SARS-CoV-2 transplant transmissibility is established, COVID-19 serum testing of donor and recipient prior to SOT is vital and transplant specialists must critically assess the risks and benefits of continuing elective and non-urgent SOT with requisite induction therapy. Finally, this case carries significant epidemiologic consequences and highlights the vital role of autopsy in an unfolding pandemic in providing valuable diagnostic information.
ACKNOWLEDGMENTS
Our deepest condolences go out to the patient’s family. We would like to thank Dr. Simardeep Gill, Dr. Serdar Yilmaz, and Ms. Sherry Buckle for their help. JC is supported by the Department of Medicine, Division of Nephrology at the University of Calgary (startup funds) and by a new investigator award from the Kidney Research Scientist Core Education and National Training Program/Canadian Institutes of Health Research.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
Funding information Institute of Nutrition, Metabolism and Diabetes
Footnotes
Emily Lauren Simms and Hyunjae Chung contributed equally to this work.
REFERENCES
- 1.Zhang H, Dai H, Xie X. Solid organ transplantation during the COVID-19 pandemic. Front Immunol. 2020;11:1392. doi: 10.3389/fimmu.2020.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolonko A, Dudzicz S, Wiecek A, Król R. COVID-19 infection in solid organ transplant recipients: a single-center experience with patients immediately after transplantation. Transpl Infect Dis. 2020;00 doi: 10.1111/tid.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westhoff TH, Seibert FS, Bauer F, et al. Allograft infiltration and meningoencephalitis by SARS-CoV-2 in a pancreas-kidney transplant recipient. Am J Transplant. 2020;20:3216–3220. doi: 10.1111/ajt.16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batlle D, Soler MJ, Sparks MA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31:1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falzone L, Musso N, Gattuso G, et al. Sensitivity assessment of droplet digital PCR for SARS-CoV-2 detection. Int J Mol Med. 2020;46:957–964. doi: 10.3892/ijmm.2020.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Babka A, Kearney BJ, et al. Molecular detection of SARS-CoV-2 in formalin-fixed, paraffin-embedded specimens. JCI Insight. 2020;5 doi: 10.1172/jci.insight.139042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alteri C, Cento V, Antonello M, et al. Detection and quantification of SARS-CoV-2 by droplet digital PCR in real-time PCR negative nasopharyngeal swabs from suspected COVID-19 patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canadian Government. COVID-19 epidemiological and economic research data. [Government Website] Updated December 3, 2020. Available at https://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/epidemiological-economic-research-data.html. Accessed December 3, 2020
- 10.Marinaki S, Tsiakas S, Korogiannou M, Grigorakos K, Papalois V, Boletis I. A systematic review of COVID-19 infection in kidney transplant recipients: a universal effort to preserve patients’ lives and allografts. J Clin Med. 2020;9:2986. doi: 10.3390/jcm9092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahalingasivam V, Craik A, Tomlinson LA, et al. COVID-19 and kidney transplantation: a systematic review. Kidney Int Report. 2020; [published ahead of print October 21. [DOI] [PMC free article] [PubMed]
- 12.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. New Eng J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 10.1111/ajt.16369 [published online ahead of print 2020]. [DOI] [PMC free article] [PubMed]
- 14.Borczuk AC, Salvatore SP, Seshan SV, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Pathol. 2020;33:2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calomeni E, Satoskar A, Ayoub I, Brodsky S, Rovin BH, Nadasdy T. Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int. 2020;98:233–234. doi: 10.1016/j.kint.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bae S, McAdams-DeMarco M, Massie A, et al. Early changes in kidney transplant immunosuppression regimens during the COVID-19 pandemic. Transplant. 2021;105(1):170–176. doi: 10.1097/TP.0000000000003502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaunat O, Legeai C, Anglicheau D, et al. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT) Kidney Int. 2020;98:1568–1577. doi: 10.1016/j.kint.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.