Abstract
Background
Patients with underlying cardiovascular disease and coronavirus disease 2019 (COVID‐19) infection are at increased risk of morbidity and mortality.
Objectives
This study was designed to characterize the presenting profile and outcomes of patients hospitalized with acute coronary syndrome (ACS) and COVID‐19 infection.
Methods
This observational cohort study was conducted using multisource data from all acute NHS hospitals in England. All consecutive patients hospitalized with diagnosis of ACS with or without COVID‐19 infection between 1 March and 31 May 2020 were included. The primary outcome was in‐hospital and 30‐day mortality.
Results
A total of 12 958 patients were hospitalized with ACS during the study period, of which 517 (4.0%) were COVID‐19‐positive and were more likely to present with non‐ST‐elevation acute myocardial infarction. The COVID‐19 ACS group were generally older, Black Asian and Minority ethnicity, more comorbid and had unfavourable presenting clinical characteristics such as elevated cardiac troponin, pulmonary oedema, cardiogenic shock and poor left ventricular systolic function compared with the non‐COVID‐19 ACS group. They were less likely to receive an invasive coronary angiography (67.7% vs 81.0%), percutaneous coronary intervention (PCI) (30.2% vs 53.9%) and dual antiplatelet medication (76.3% vs 88.0%). After adjusting for all the baseline differences, patients with COVID‐19 ACS had higher in‐hospital (adjusted odds ratio (aOR): 3.27; 95% confidence interval (CI): 2.41–4.42) and 30‐day mortality (aOR: 6.53; 95% CI: 5.1–8.36) compared to patients with the non‐COVID‐19 ACS.
Conclusion
COVID‐19 infection was present in 4% of patients hospitalized with an ACS in England and is associated with lower rates of guideline‐recommended treatment and significant mortality hazard.
Keywords: acute coronary syndrome, coronavirus disease 2019, England, mortality, pandemic
Introduction
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), has affected more than 17 million people resulting in almost 700 000 deaths worldwide [1]. Although COVID‐19 patients predominantly present with respiratory symptoms, various extra‐pulmonary manifestations including thrombotic events, myocardial injury and ischaemia, acute kidney injury and cardiac arrhythmias have also been reported [2, 3].
Acute coronary syndrome (ACS) in the context of viral infection may be related to atherosclerotic plaque rupture precipitated by endothelial cell damage, a cytokine storm and a heightened inflammatory state [4] Furthermore, admission with an ACS during the COVID‐19 pandemic in which large numbers of patients were hospitalized with COVID‐19 may increase the risk of nosocomial transmission of COVID‐19 in this vulnerable patient group. The management of patients presenting with ACS in the context of COVID‐19 remains a challenge [5]. There are limited data regarding the clinical characteristics, management strategies and post‐discharge mortality of patients hospitalized with a diagnosis of ACS and concomitant COVID‐19 infection [5]. Small case series of 18 and 28 patients presenting with ST‐elevation acute myocardial infarction (STEMI) and concomitant COVID‐19 infection have reported significant variability in presenting characteristics and in‐hospital survival of these patients [6, 7]. These reports lacked data around clinical presentation, pharmacological treatments and post‐discharge survival. Fewer data are available from a national or from a broader ACS perspective including non‐ST‐elevation myocardial infarction (NSTEMI). Such information could prove useful to devise optimal pathways of care in the event of a second wave of COVID‐19.
This study, using high‐resolution, multisource contemporary national data from England, systematically profiles the presenting and procedural characteristics, in‐hospital and 30‐day mortality in patients admitted with a diagnosis of ACS and concomitant COVID‐19 infection. The primary aim was to investigate the in‐hospital and 30‐day all‐cause mortality in patients hospitalized with COVID‐19 ACS diagnosis compared with those without COVID‐19 ACS. The second aim was to describe the differences in management and independent predictors of 30‐day mortality of those with COVID‐19 ACS.
Methods
Study data
An unselected, real‐world cohort of all patients hospitalized with a diagnosis of ACS in England was derived by linking patient records across four different sources of data, namely Hospital Episode Statistics (HES), the Myocardial Ischaemia National Audit Project (MINAP), the British Cardiovascular Intervention Society (BCIS) percutaneous coronary intervention registry and Civil Registration Death data. The full details about the validity, strengths, limitations and utility for research purposes have been described previously [8, 9, 10]. Briefly, HES contains International Statistical Classification of Disease – 10th Revision (ICD‐10) clinical, geographic, administrative and patient information of all patients hospitalized in any National Health Service (NHS) hospital in England [8]. MINAP is an exclusive ACS registry designed to collect information across 130 data fields about patient demographics, use of various pharmacological and invasive treatments and in‐hospital care of patients hospitalized with a diagnosis of ACS (type 1 myocardial infarction) in any NHS acute care hospital in England [10]. Similarly, almost over 98% of the PCI activity in England is captured in the BCIS PCI registry, which is designed to collect detailed procedural and clinical data of all patients undergoing PCI [9]. Finally, the civil registration of death register holds the mortality information of all deaths in England. All patient records in these datasets can be identified using a unique 10‐digit number. For this study, we used live reporting data from all hospitals in England submitting their data to these respective registries during the COVID‐19 pandemic.
Ethical approval
This study was conducted under the endorsement of the Chief Scientific Advisor to the UK government and Scientific Advisory Group for Emergencies (SAGE). The access to the data was granted under the Health and Social Care State Secretary’s notice issued under Regulation 3(4) of the NHS (Control of Patient Information Regulations) 2002 (COPI) to NHS Digital, allowing NHS Digital to share confidential patient information with organizations entitled to process this under COPI for COVID‐19 purposes. Furthermore, MINAP and BCIS data are collected and hosted by the National Institute of Cardiovascular Research (NICOR) and used for audit and research purposes without formal individual patient consent under section 251 of the NHS Act 2006 [11, 12, 13, 14]. The study complies with the Declaration of Helsinki.
Study cohort
The analytical cohort for this study consisted of consecutive patients aged ≥ 18 years hospitalized with a diagnosis of ACS in England and documented within the MINAP registry, between 1 March 2020 and 31 May 2020. The COVID‐19 status information for all patients was derived from HES using ICD‐10 codes ‘U071’ (confirmed) and ‘U072’ (clinical diagnosis) and linked with the MINAP record of the same patient matching across the two data sets based on their unique NHS number and admission date. In the second step, the records of all these patients were linked across to the records in the BCIS PCI registry using the same NHS number and admission week. Finally, the mortality information of all patients was tracked within 30 days beyond discharge or up to 10 July 2020 from the Civil Registration of Death Register. Figure S1 illustrates the cohort selection and data linkage steps of all patients in the study.
Final study data contained comprehensive detailed information about patient demographics, COVID‐19 status, clinical characteristics, comorbidities, pre‐hospital and in‐hospital pharmacological treatments, cardiac investigations, invasive coronary procedures, procedural characteristics and complications. Patients with missing or invalid NHS number and date of admission were excluded from the analysis. Patients with valid diagnosis codes of COVID‐19 captured in the HES data were defined as the ‘COVID‐19 ACS’ group, whilst all other ACS patients were defined as the ‘non‐COVID‐19 ACS’ group. Time to reperfusion therapy for STEMI was calculated from time of admission to time of receipt of reperfusion treatment where time of admission was defined as time of arrival into hospital. The primary outcomes of interest were in‐hospital and 30‐day mortality in ‘COVID‐19 ACS’ compared with ‘non‐COVID‐19 ACS’ patients.
Statistical analysis
Baseline characteristics were reported as number and percentages for categorical variables, means with standard deviation or median with interquartile ranges for continuous data. Statistical differences between the two groups were obtained using the chi‐square test or t‐test or the Kruskal–Wallis tests as appropriate. The daily counts of COVID‐19‐positive ACS patients were presented using a simple daily moving average from 1 March 2020 up to 31 May 2020, adjusted for seasonality. Multiple imputations using chained equation (MICE) techniques were used to impute missing values in all the study variables. Each of the imputation models included all the other variables used in the analyses as reported in the online supplement (Table S1). Linear regression for continuous variables, multinomial for nominal variables, ordinal logistic regression for ordered factors and logistic regression models for binary variables were used to generate 10 imputed data sets, and all subsequent analyses were performed on these and results were pooled using Robin’s rule [15, 16].
To study the association between ‘COVID‐19 ACS’ status and clinical outcomes, hierarchical logistic regression models with random intercept were constructed. The hospital ID was used as a random intercept to account for nesting of patients within the different hospitals. All models were adjusted for age, sex, ethnicity, body mass index, presenting characteristic (blood pressure, heart rate, cardiac arrest, clinical syndrome, creatinine, Killip class, left ventricular systolic function), cardiovascular comorbidities (previous PCI, previous CABG, previous AMI, previous cerebrovascular event, peripheral vascular disease, renal dysfunction, heart failure, hypercholesterolaemia, angina, diabetes, smoking status, asthma or chronic obstructive airway disease and family history of coronary heart disease), in‐hospital pharmacology (low molecular weight heparin, unfractionated heparin, warfarin, loop diuretic, glycoprotein IIb/IIIa inhibitor), discharge pharmacology (dual antiplatelet medication use, statin, angiotensin‐converting enzyme (ACE) or angiotensin receptor blocker use), receipt of coronary angiography or PCI and cardiology care. For patients undergoing PCI, the association between ‘COVID‐19 ACS’ status and mortality was estimated by constructing separate models adjusting all the procedural information reported in Table S1 and in addition to the aforementioned confounders. The independent predictors of 30‐day mortality were studied using multivariable logistic regression model. All tests were two‐sided, and statistical significance was considered as P < 0.05. Statistical analyses were performed in Stata MP 16.0 College Station, Texas, USA, via secure remote access on the NHS Digital servers hosting all the data sets.
Results
Between 1 March and 31 May 2020, 12 958 patients were hospitalized with ACS in England, of which 517 (4.0%) were COVID‐19‐positive. There was a steady increase in the number of daily COVID‐19 ACS hospitalizations during March, reaching a peak in the first week of April followed by a steady decline by the end of May (Fig. 1). Higher proportions of daily COVID‐19 ACS cases were admitted with a NSTEMI compared to STEMI throughout the study period.
Fig. 1.
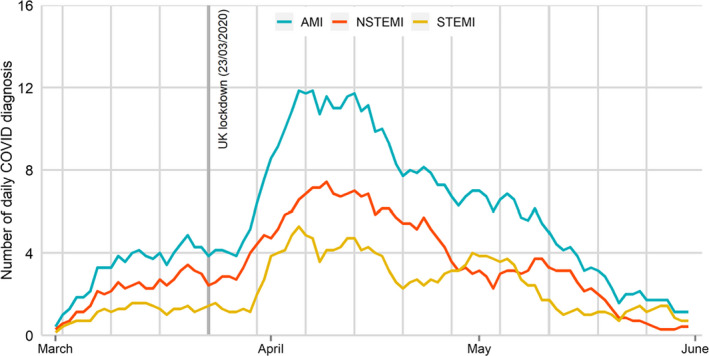
Daily cases of COVID‐19 ACS hospitalized during the study period. AMI = acute myocardial infarction, STEMI = ST‐elevation acute myocardial infarction, NSTEMI = non‐ST‐elevation acute myocardial infarction.
Patient characteristics
Patients in the COVID‐19 ACS group were older compared with the non‐COVID‐19 ACS group (72.8 years vs 67.0 years), and a greater proportion were from Black, Asian and Ethnic minority origin (20.2% vs 12.8%), and they had a lower body mass index (26.9 vs 28.2) and more likely to be hospitalized with NSTEMI (67.0% vs 62.0%). The COVID‐19 ACS group also exhibited an increased incidence of in‐hospital cardiac arrest (6.3% vs 3.0%), higher troponin levels and were more likely to have presented in pulmonary oedema (9.0% vs 3.4%) or cardiogenic shock (9.6% vs 3.9%). They had a higher prevalence of heart failure (23.7% vs 13.4%), cerebrovascular disease (15.7% vs 8.0%), insulin‐treated diabetes (13.6% vs 7.5%) and hypertension (69.4% vs 58.3%) (Table 1).
Table 1.
Baseline characteristics COVID‐19 ACS compared with non‐COVID‐19 ACS patients in the MINAP registry
| Variables | Non‐COVID‐19 ACS N = 12 441 |
COVID‐19 ACS N = 517 |
P value |
|---|---|---|---|
| Age, years mean (SD) | 67.0 (13.3) | 72.8 (13.9) | <0.001 |
| Male (%) | 8587 (69.1%) | 352 (68.1%) | 0.63 |
| BMI mean (SD) | 28.2 (5.5) | 26.9 (5.6) | <0.001 |
| Ethnicity | |||
| Whites | 9290 (87.2%) | 360 (79.8%) | <0.001 |
| BAME | 1359 (12.8%) | 91 (20.2%) | |
| Presenting characteristics | |||
| Heart rate, bpm, mean (SD) | 79.9 (19.9) | 86.7 (22.9) | <0.001 |
| Systolic blood pressure, mean (SD) | 140.7 (27.5) | 134.7 (29.9) | <0.001 |
| Cardiac arrest | |||
| Pre‐hospital cardiac arrest | 434 (3.7%) | 17 (3.5%) | <0.001 |
| In‐hospital cardiac arrest | 349 (3.0%) | 31 (6.3%) | |
| Clinical syndrome | |||
| STEMI | 4403 (38.0%) | 153 (33.0%) | 0.02 |
| NSTEMI | 7176 (62.0%) | 311 (67.0%) | |
| Peak troponin levels (median, IQR) | |||
| Troponin T | 99 (13–685) | 380 (63.7–20.45) | 0.002 |
| Troponin I | 399 (48.9–4036) | 431 (109–3396) | 0.83 |
| Highly sensitive troponin T | 231 (55–1149) | 245 (41–946) | 0.61 |
| Highly sensitive troponin I | 482 (53–4193) | 853 (144–4374) | 0.04 |
| Creatinine, mean (SD) | 94.9 (62.4) | 134.2 (115.2) | <0.001 |
| Killip class | |||
| No heart failure | 9114 (84.0%) | 280 (60.0%) | <0.001 |
| Basal crepitation | 952 (8.8%) | 100 (21.4%) | |
| Pulmonary oedema | 368 (3.4%) | 42 (9.0%) | |
| Cardiogenic shock | 421 (3.9%) | 45 (9.6%) | |
| LV systolic function | |||
| Good | 4593 (46.1%) | 132 (30.3%) | <0.001 |
| Moderate | 2665 (26.8%) | 110 (25.3%) | |
| Poor | 838 (8.4%) | 59 (13.6%) | |
| Not assessed | 1861 (18.7%) | 134 (30.8%) | |
| Comorbidities | |||
| Percutaneous coronary intervention | 2077 (19.5%) | 71 (15.1%) | 0.02 |
| Coronary artery bypass graft | 746 (7.1%) | 39 (8.3%) | 0.32 |
| Heart failure | 1419 (13.4%) | 112 (23.7%) | <0.001 |
| Hypercholesterolaemia | 4439 (40.8%) | 183 (39.0%) | 0.43 |
| Angina | 2004 (19.2%) | 83 (17.8%) | 0.47 |
| Cerebrovascular disease | 848 (8.0%) | 74 (15.7%) | <0.001 |
| Myocardial infarction | 2747 (25.5%) | 136 (28.6%) | 0.13 |
| Peripheral vascular disease | 560 (5.3%) | 31 (6.6%) | 0.22 |
| Chronic kidney disease | 884 (8.4%) | 112 (23.7%) | <0.001 |
| Diabetes | |||
| Not diabetic | 8547 (73.9%) | 298 (60.3%) | <0.001 |
| Diet controlled | 513 (4.4%) | 31 (6.3%) | |
| Oral medications | 1635 (14.1%) | 98 (19.8%) | |
| Insulin therapy | 868 (7.5%) | 67 (13.6%) | |
| Hypertension | 6474 (58.3%) | 335 (69.4%) | <0.001 |
| Smoking status | |||
| Never smoked | 3965 (37.6%) | 152 (43.1%) | <0.001 |
| Previous smoker | 3584 (34.0%) | 150 (42.5%) | |
| Current smoker | 3003 (28.5%) | 51 (14.4%) | |
| Asthma / COPD | 1753 (16.8%) | 95 (20.3%) | 0.04 |
| Family history of CHD | 2430 (27.8%) | 51 (14.4%) | <0.001 |
| In‐hospital pharmacology | |||
| LMWH/UFH | 6261 (72.7%) | 219 (59.7%) | <0.001 |
| Warfarin | 268 (3.1%) | 17 (4.6%) | 0.11 |
| Loop diuretic | 1801 (21.1%) | 166 (45.0%) | <0.001 |
| Glycoprotein IIb/IIIa inhibitor use | 1147 (12.5%) | 36 (9.1%) | 0.04 |
| Discharge pharmacology | |||
| Aspirin | 10951 (94.5%) | 428 (90.5%) | <0.001 |
| Any P2Y12 inhibitor | 10552 (92.8%) | 387 (84.1%) | <0.001 |
| Dual antiplatelet medications | 9874 (88.0%) | 345 (76.3%) | <0.001 |
| Statin | 9892 (95.1%) | 343 (89.1%) | <0.001 |
| ACEi / ARB | 9195 (88.6%) | 304 (80.4%) | <0.001 |
| Processes of care and clinical outcomes | |||
| Seen by cardiologist | 11436 (97.2%) | 445 (90.4%) | <0.001 |
| Percutaneous coronary intervention | 6708 (53.9%) | 156 (30.2%) | <0.001 |
| Why no PCI | |||
| Angiographically normal coronaries | 319 (4.8%) | 4 (1.7%) | <0.002 |
| PCI inappropriate | 356 (5.4%) | 22 (5.8%) | |
| Surgical disease | 135 (2.0%) | 4 (1.8%) | |
| Coronary angiography in NSTEMI | 5,259 (88.2%) | 107 (68.5%) | <0.001 |
| PCI in NSTEMI | 3,299 (46.0%) | 69 (22.2%) | <0.001 |
| Time to reperfusion for STEMI, hours median IQR | 0.76 (0.50–1.27) | 0.98 (0.65–1.52) | <0.001 |
| Call for help, hour median (IQR) | 1.45 (0.47–5.3) | 2.0 (0.40–6.8) | 0.26 |
| Time to coronary angiography, hour median (IQR) | 33.7 (14.4–128.8) | 38.1 (18.3–70.8) | <0.01 |
| Referral for cardiac rehabilitation | 8204 (75.9%) | 192 (42.9%) | <0.001 |
| Cardiology follow‐up | 8018 (87.1%) | 221 (73.9%) | <0.001 |
| In‐hospital mortality | 592 (5.1%) | 114 (24.2%) | <0.001 |
| 30‐day mortality | 843 (7.2%) | 207 (41.9%) | <0.001 |
Abbreviations: ACE, angiotensin‐converting enzyme; BMI, body mass index; bmp, beats per minute; CHD , coronary heart disease; COPD, chronic obstructive airway disease; IQR, interquartile range; LV, left ventricle; NSTEMI, non‐ST‐elevation myocardial infarction; SD, standard deviation; SD, standard deviation; STEMI, ST‐elevation myocardial infarction.
Out of 12 958 patients, 6,864 (53.0%) underwent PCI and were successfully linked from MINAP into the BCIS registry (Fig. S1). COVID‐19 ACS patients undergoing PCI were of similar age and had similar baseline characteristics to the overall ACS cohort (Table 2). The angiographic and procedural profiles of COVID‐19 ACS patients (such as number of lesions attempted, vessels attempted, multivessel PCI, number of stents used, use of intracoronary imaging such as IVUS, OCT and pressure wire use) were similar to the non‐COVID‐19 ACS cohort undergoing PCI (Table S2). From the patients who did not receive PCI, the proportions of angiographically normal coronaries or surgical disease were similar in both non‐COVID‐19 and COVID‐19 ACS groups.
Table 2.
Clinical characteristics of COVID‐19 ACS compared with non‐COVID‐19 ACS patients undergoing PCI in the BCIS registry
| Variables | Non‐COVID‐19 ACS N = 6708 |
COVID‐19 ACS N = 156 |
P value |
|---|---|---|---|
| Age, years mean (SD) | 64.4 (12.0) | 65.3 (12.5) | 0.38 |
| Male (%) | 5019 (74.9%) | 118 (75.6%) | 0.84 |
| BMI mean (SD) | 28.5 (5.2) | 27.9 (4.8) | 0.17 |
| Ethnicity | |||
| Whites | 5045 (85.5%) | 104 (72.7%) | <0.001 |
| BAME | 853 (14.5%) | 39 (27.3%) | |
| Presenting characteristics | |||
| Heart rate, bpm, mean (SD) | 78.1 (18.4) | 83.8 (20.9) | <0.001 |
| Systolic blood pressure, mean (SD) | 140.3 (27.1) | 137.1 (31.5) | 0.18 |
| Cardiac arrest | |||
| Pre‐hospital cardiac arrest | 274 (4.3%) | 9 (6.2%) | <0.001 |
| In‐hospital cardiac arrest | 179 (2.8%) | 14 (9.7%) | |
| Clinical syndrome | |||
| STEMI | 3394 (50.7%) | 87 (55.8%) | 0.21 |
| NSTEMI | 3299 (49.3%) | 69 (44.2%) | |
| Creatinine, mean (SD) | 89.2 (53.0) | 114.6 (105.2) | <0.001 |
| Killip class | |||
| No heart failure | 5224 (87.8%) | 95 (66.0%) | <0.001 |
| Basal crepitation | 336 (5.6%) | 18 (12.5%) | |
| Pulmonary oedema | 136 (2.3%) | 14 (9.7%) | |
| Cardiogenic shock | 251 (4.2%) | 17 (11.8%) | |
| LV systolic function | |||
| Good | 2750 (50.8%) | 58 (43.6%) | 0.21 |
| Moderate | 1704 (31.5%) | 50 (37.6%) | |
| Poor | 421 (7.8%) | 14 (10.5%) | |
| Not assessed | 539 (10.0%) | 11 (8.3%) | |
| Comorbidities | |||
| Percutaneous coronary intervention | 1265 (22.0%) | 31 (21.8%) | 0.96 |
| Coronary artery bypass graft | 303 (5.4%) | 11 (7.9%) | 0.22 |
| Heart failure | 542 (9.6%) | 20 (14.6%) | 0.05 |
| Hypercholesterolaemia | 2949 (49.5%) | 72 (51.1%) | 0.72 |
| Angina | 812 (14.6%) | 14 (10.4%) | 0.17 |
| Cerebrovascular disease | 361 (6.4%) | 15 (10.8%) | 0.03 |
| Myocardial infarction | 1412 (24.4%) | 42 (29.8%) | 0.14 |
| Peripheral vascular disease | 282 (5.0%) | 14 (10.1%) | 0.007 |
| Chronic kidney disease | 274 (4.9%) | 22 (16.1%) | <0.001 |
| Diabetes | |||
| Not diabetic | 4716 (75.5%) | 91 (60.7%) | <0.001 |
| Diet controlled | 274 (4.4%) | 6 (4.0%) | |
| Oral medications | 872 (14.0%) | 33 (22.0%) | |
| Insulin therapy | 384 (6.1%) | 20 (13.3%) | |
| Hypertension | 3678 (60.2%) | 104 (71.7%) | 0.005 |
| Smoking status | |||
| Never smoked | 2462 (38.3%) | 66 (46.2%) | 0.02 |
| Previous smoker | 2040 (31.7%) | 49 (34.3%) | |
| Current smoker | 1934 (30.0%) | 28 (19.6%) | |
| Asthma / COPD | 765 (13.7%) | 18 (13.3%) | 0.90 |
| Family history of CHD | 1542 (31.8%) | 28 (26.2%) | 0.21 |
| In‐hospital pharmacology | |||
| Low molecular weight heparin | 1867 (43.2%) | 39 (42.4%) | 0.88 |
| Unfractionated heparin | 2092 (47.2%) | 34 (36.2%) | 0.03 |
| Warfarin | 98 (2.2%) | 5 (5.4%) | 0.04 |
| Loop diuretic | 676 (15.6%) | 31 (33.7%) | <0.001 |
| Glycoprotein IIb/IIIa inhibitor use | 1015 (20.7%) | 32 (27.6%) | 0.07 |
| Discharge pharmacology | |||
| Aspirin | 6226 (98.2%) | 138 (97.9%) | 0.76 |
| Any P2Y12 inhibitor | 6013 (97.3%) | 121 (92.4%) | <0.001 |
| Dual antiplatelet medications | 5827 (95.6%) | 116 (89.9%) | 0.002 |
| Statin | 5645 (97.6%) | 107 (95.5%) | 0.17 |
| ACEi / ARB | 5391 (93.1%) | 110 (94.0%) | 0.70 |
| Processes of care and clinical outcomes | |||
| Referral for cardiac rehabilitation | 5068 (86.3%) | 84 (64.1%) | <0.001 |
| Cardiology follow‐up | 4662 (93.3%) | 87 (89.7%) | 0.16 |
| In‐hospital mortality | 180 (2.8%) | 19 (14.0%) | <0.001 |
| 30‐day mortality | 260 (4.1%) | 44 (30.8%) | <0.001 |
Abbreviations: ACE, angiotensin‐converting enzyme; BMI, body mass index; bmp, beats per minute; CHD , coronary heart disease; COPD, chronic obstructive airway disease; IQR, interquartile range; LV, left ventricle; NSTEMI, non‐ST‐elevation myocardial infarction; SD, standard deviation; SD, standard deviation; STEMI, ST‐elevation myocardial infarction.
Discharge medication and guideline‐indicated care
Patients in the COVID‐19 ACS group were less likely to receive optimal secondary prevention medication such as aspirin (90.5% vs 94.5%), dual antiplatelet medications (76.3% vs 88.0%), statin (89.1% vs 95.1%) and ACE/ARB (80.4% vs 88.6%) therapy compared with the non‐COVID‐19 ACS group. Overall, only one third (30.2% vs 53.9%) of COVID‐19 ACS patients received PCI compared with non‐COVID‐19 ACS patients. COVID‐19 NSTEMI patients were also less likely to undergo invasive coronary angiography (67.7% vs 81.0%) and PCI (22.2% vs 46.0%) compared with the non‐COVID‐19 NSTEMI cohort.
The median time from in‐hospital arrival to reperfusion for patients presenting with STEMI was 13.2 minutes longer in the COVID‐19 STEMI group compared with the non‐COVID‐19 STEMI group. The COVID‐19 NSTEMI group also experienced delays in time to coronary angiography (38.1 hours vs 33.7 hours) compared with the non‐COVID‐19 NSTEMI group. Less than half of the COVID‐19 ACS group were referred to the cardiac rehabilitation programme (42.9% vs 75.9%) or had cardiology follow‐up arranged following discharge from the hospital (73.9% vs 87.1%).
Clinical outcomes and predictors of mortality
In the MINAP cohort, the COVID‐19 ACS group had higher in‐hospital (24.2% vs 5.1%) and 30‐day mortality rates (41.9% vs 7.2%) compared with the non‐COVID‐19 ACS cohort. After adjustment for baseline differences in demographics, presenting characteristics, comorbidities and pharmacology, the hierarchical multilevel logistic regression model showed a significantly higher risk of in‐hospital (adjusted odds ratio: 3.27; 95% CI: 2.41–4.42) and 30‐day mortality (adjusted odds ratio: 6.53; 95% CI: 5.12–8.36) in the COVID‐19 ACS patients compared with the non‐COVID‐19 ACS patients (Fig. 2). In the subgroup analysis stratified according to type of ACS, although short‐term mortality was similar between the NSTEMI and STEMI groups, the NSTEMI group had higher mortality rates (adjusted odds ratio: 8.45; 95% CI: 6.03–11.83) at 30 days. Similarly, higher mortality rates were observed in COVID‐19 ACS patients undergoing PCI compared with non‐COVID‐19 ACS patients undergoing PCI. The multilevel regression model showed that increasing age per year, severe LVSD, in‐hospital cardiac arrest, peak troponin concentration levels, renal dysfunction and use of ACE/ARB on discharge were strong independent risk factors for 30‐day mortality in the COVID‐19 ACS group (Fig. 3).
Fig. 2.
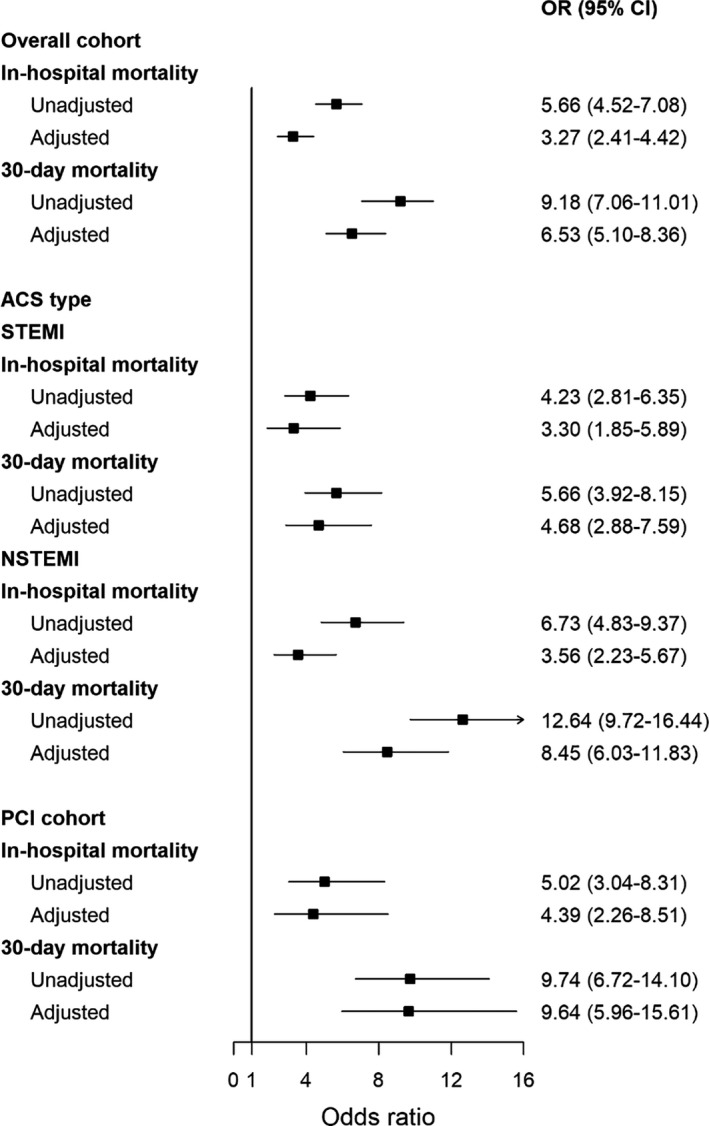
In‐hospital outcomes and 30‐day mortality of COVID‐19 ACS patient compared with non‐COVID‐19 ACS patients.
Fig. 3.
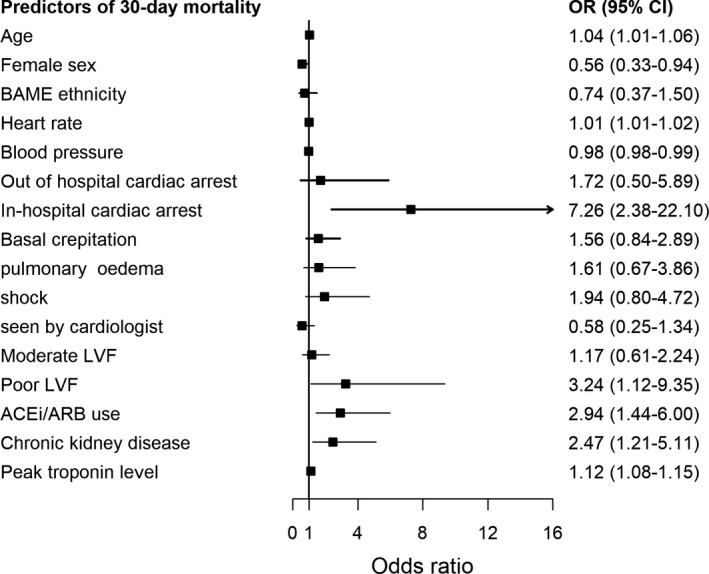
Independent predictors of 30‐day mortality in COVID‐positive AMI patients.
Discussion
This multisource, national report of contemporary data from all ACS‐related hospitalizations in England during the COVID‐19 period provides detailed information about the risk profile, clinical care and outcomes of ACS patients with COVID‐19 infection. Specifically, COVID‐19 ACS group had over sixfold increase in mortality within 30 days compared with non‐COVID‐19 ACS and were less frequently prescribed appropriate secondary prevention medications. They were older, more likely to be of BAME origin, had more comorbid features and exhibited high‐risk presenting characteristics such as higher Killip class, troponin concentrations, creatinine and evidence of LV systolic dysfunction.
A significant knowledge gap exists in the literature about incidence and profile of ACS patients with concomitant COVID‐19 diagnosis, and their associated clinical outcomes. We report that COVID‐19 infection in patients presenting with ACS is prevalent at a low level in a national cohort of patients. The higher prevalence of pulmonary oedema and shock at presentation, together with the higher troponin concentrations levels in the COVID‐19 ACS cohort, are suggestive of increased myocardial injury in this group corroborating findings from China and US cohorts [17, 18]. However, contrary to earlier small case series from New York and Italy [6, 7, 19], the angiographic characteristics of COVID‐19 ACS patients were not different from the non‐COVID‐19 ACS patients, with a very small prevalence of non‐obstructive coronary disease and similar procedural success.
Whilst previous studies have reported the incidence on myocardial injury based on cardiac biomarker levels in patients hospitalized with a confirmed COVID‐19 diagnosis [17, 18, 20, 21], none reported on management and outcomes of ACS patients with concomitant COVID‐19 diagnosis in a national, unselected cohort of type 1 myocardial infarction. The management of ACS patients during the COVID‐19 pandemic is based on expert clinical guidance, which lacks consensus on the optimal treatment strategies for patients presenting with ACS during the COVID‐19 outbreak [22, 23, 24, 25]. The Chinese Cardiac Society consensus statement proposed medical management for the majority of patients presenting with non‐ST‐elevation myocardial infarction (NSTEMI), and thrombolysis in those presenting with STEMI during the COVID‐19 pandemic [22]. In contrast, the North America and Canadian guidelines recommended the use of thrombolysis as an alternative to primary PCI for patients with STEMI, particularly where PCI services are restricted due to COVID‐19 [23, 25]. However, a joint statement from British Cardiovascular Society, BCIS and NHS England in the UK recommended that primary PCI should remain the default treatment for all STEMI, except in unusual circumstances [26]. In keeping with this national recommendation, we observed almost negligible use of thrombolysis for STEMI in the UK during the acute phase of the COVID‐19 pandemic. By contrast, there were significant differences in the care of COVID‐19 ACS patients in that only a third of COVID‐19 ACS patients received PCI, and they experienced greater delays in reperfusion treatment or an invasive strategy and had a significantly lower uptake of secondary prevention medications on discharge. Given that COVID‐19 ACS patients presented with more complex, high‐risk features, there is an urgent need to develop effective treatment pathways to align their care with guideline recommendations [27, 28]. For patients hospitalized with an ACS, especially with elevated cardiac troponin minor ECG changes, immediate COVID‐19 testing should be advocated in the current climate.
In the outcome analysis, COVID‐19 ACS patients had a poor prognosis compared with the non‐COVID‐19 ACS patients. One of four patients died in hospital with an over sixfold higher 30‐day mortality in the overall cohort and those undergoing PCI. Although we did not have information regarding the presenting COVID‐19 symptoms or respiratory complications in this cohort, we noted significantly higher levels of peak troponin, creatinine, a lower presenting blood pressure and tachycardia in this cohort. Troponin elevation three times the upper reference limit is known to be associated with worse in‐hospital outcomes in COVID‐19 patients [17, 18]. It remains unclear whether the rise in cardiac biomarkers is related to viral myocarditis [29, 30] or plaque rupture secondary to virus‐induced inflammatory response [31, 32, 33] or a type 1 AMI. In this cohort of type 1 myocardial infarction, there was a low prevalence of non‐obstructive coronary disease and the angiographic characteristics and procedural management of the COVID‐19 ACS were similar to the non‐COVID‐19 ACS group. We observed higher rates of COVID‐19 infection and subsequent 30‐day morality in NSTEMI compared with STEMI. Patients presenting with NSTEMI are generally older, more comorbid and less likely to present early during the COVID‐19 pandemic, which in conjunction with increased infection rates may be responsible for their poor outcomes.
The COVID‐19 ACS cohort were also less frequently prescribed low molecular weight or unfractionated heparin and statins. Patients with acute COVID‐19 are known to be at increased risk of thromboembolic complications and are therefore likely to benefit from appropriate anticoagulation to reduce thrombus burden and statins to stabilize plaque. In the risk factor analysis, elevated creatinine, peak troponin, heart rate, left ventricular systolic dysfunction and use of ACE inhibitor or ARBs were strong independent risk factors for 30‐day mortality in this report. Although recent data have negated earlier concerns of an increased risk of adverse outcomes in COVID‐19 patients with the use of ACE inhibitors or ARBs, further research is required to establish the role of these medications in patients presenting with COVID‐19 ACS [34, 35]. Patients hospitalized with ACS and concomitant COVID‐19 infection may also be focus on specific antiviral drug trials or immunosuppression therapy to establish the benefit of these therapies in this high‐risk cohort [5].
The interrogation of multisource, multihospital live data from England has given insight into the presenting characteristics, risk profile, treatment strategies and 30‐day mortality in an unselected cohort of patients hospitalized with COVID‐19 ACS. However, there are some study limitations, which must be kept in mind whilst interpreting these findings. First, the COVID‐19 diagnosis was based on the ICD‐10 codes and so it is unclear whether the diagnosis at the hospital was made on clinical grounds or using a formal polymerase chain reaction (PCR) test, and the timing of the tests leading to the diagnosis is unclear. Moreover, other ACS patients were not routinely tested at the start of the pandemic and we cannot say how many might have been carrying the virus, although the lack of a COVID‐19 code suggests they did not have a clinical syndrome related to the virus. Second, information around COVID‐19 symptoms, their duration and other organ involvement is not captured in the data sets used in this analysis, and hence, it is difficult to ascertain whether patients had COVID‐19 symptoms followed by an ACS or vice versa, and whether COVID‐19 infection occurred in the hospital or the community. Third, whilst the MINAP registry is the UK national ACS registry for type 1 AMI, we cannot rule out misclassification bias from misdiagnosis of myocarditis or type 2 AMI, although only a small proportion of cases that underwent angiography had non‐obstructive coronary artery disease. Finally, given the observational design of our study, the possibility of unmeasured confounders cannot be ruled out, as the registries do not capture markers of COVID severity such as admission to intensive care, the presence of hypoxia and the occurrence of non‐cardiac thromboembolic complications such as pulmonary embolus that are known to characterize severe infections.
Conclusion
COVID‐19 infection was present in one out of twenty‐five patients hospitalized with an ACS in England during the study period. It was associated with worst outcomes independent of the type of ACS; however, higher 30‐day mortality was observed in the NSTEMI group compared with the STEMI group. Elderly, comorbid and BAME patients with underlying cardiovascular disease are more likely to acquire COVID‐19 infection and often present with unfavourable presenting characteristics. These findings highlight the need to develop strategies for an efficient and equitable care for COVID ACS patients.
Conflicts of interest
None reported.
Author contributions
Dr M Rashid had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Rashid and Mamas conceived and designed the study. Rashid, Wu and Mamas acquired the data, analysed the data or interpreted the data. Rashid, Wu, Mamas and Gale drafted the manuscript. All other authors critically revised the manuscript for important intellectual content. Rashid involved in statistical analysis. NHS Digital provided administrative, technical or material support. Mamas involved in supervision.
Supporting information
Table S1 Summary data of missing variables included in the study.
Table S2 Angiographic characteristics and procedural outcomes of COVID19 ACS patients compared to non COVID19 ACS patients undergoing PCI in the BCIS registry.
Figure S1 STROBE study selection.
Acknowledgements
The authors acknowledge Chris Roebuck, Tom Denwood, Tony Burton and Courtney Stephenson and data support staff at NHS Digital for providing and creating the secure environment for data hosting and for analytical support, and Anil Gunesh and Julian Hains from the National Institute of Cardiovascular Outcomes Research for data transfer into the secure environment. This work uses data provided by patients and collected by the NHS as part of their care and support, and the authors would like to thank all for their contributions.
Rashid M, Wu J, Timmis A, Curzen N, Clarke S, Zaman A, Nolan J, Shoaib A, Mohamed MO, de Belder MA, Deanfield J, Gale CP, Mamas MA (School of Primary Care, Keele University, Keele; Royal Stoke Hospital, Stoke‐on‐Trent, UK; Leeds Institute for Data Analytics, University of Leeds; Queen Mary University London; University of Southampton, Southampton; Royal Papworth Hospital, Cambridge; Freemen Hospital, Newcastle Upon Tyne; Barts Health NHS Trust, London; University College London, London; Leeds Teaching Hospitals NHS Trust, Leeds, UK; Thomas Jefferson University, Philadelphia, PA, USA). Outcomes of COVID‐19‐positive acute coronary syndrome patients: A multisource electronic healthcare records study from England (Original Article). J Intern Med. 2021;290:88–100. 10.1111/joim.13246
References
- 1. CSSE J . Coronavirus COVID‐19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2020‐03‐15]. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. (2020).
- 2. Gulati A, Pomeranz C, Qamar Z, Thomas S, Frisch D, George G, et al. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID‐19 global pandemic. Am J Med Sci. 2020;360:5–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;26:1017–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montone RA, Iannaccone G, Meucci MC, Gurgoglione F, Niccoli G. Myocardial and microvascular injury due to coronavirus disease 2019. Eur Cardiol. 2020;15:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID‐19) with myocardial injury and mortality. JAMA Cardiol. 2020;5:751–3. [DOI] [PubMed] [Google Scholar]
- 6. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, et al. ST‐segment elevation in patients with covid‐19 ‐ A case series. N Engl J Med. 2020;382:2478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, et al. ST‐elevation myocardial infarction in patients with COVID‐19: clinical and angiographic outcomes. Circulation. 2020;141:2113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams JG, Mann RY. Hospital episode statistics: time for clinicians to get involved? Clin Med. 2002;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rashid M, Ludman PF, Mamas MA. British cardiovascular intervention society registry framework: a quality improvement initiative on behalf of the National Institute of Cardiovascular Outcomes Research (NICOR). Eur Heart J Qual Care Clin Outcomes. 2019;5:292–7. [DOI] [PubMed] [Google Scholar]
- 10. Wilkinson C, Weston C, Timmis A, Quinn T, Keys A, Gale CP. The myocardial ischaemia national audit project (MINAP). Eur Heart J Qual Care Clin Outcomes. 2020;6:19–22. [DOI] [PubMed] [Google Scholar]
- 11. Noman A, Balasubramaniam K, Alhous MHA, Lee K, Jesudason P, Rashid M, et al. Mortality after percutaneous coronary revascularization: prior cardiovascular risk factor control and improved outcomes in patients with diabetes mellitus. Catheter Cardiovasc Interv. 2017;89:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taxiarchi P, Kontopantelis E, Martin GP, Kinnaird T, Curzen N, Banning AP, et al. Same‐day discharge after elective percutaneous coronary intervention: insights from the British Cardiovascular Intervention Society. JACC: Cardiovas Int. 2019;12:1479–94. [DOI] [PubMed] [Google Scholar]
- 13. Rashid M, Curzen N, Kinnaird T, Lawson CA, Myint PK, Kontopantelis E, et al. Baseline risk, timing of invasive strategy and guideline compliance in NSTEMI: Nationwide analysis from MINAP. Int J Cardiol. 2020;301:7–13. [DOI] [PubMed] [Google Scholar]
- 14. Rashid M, Kontopantelis E, Kinnaird T, Curzen N, Gale CP, Mohamed MO, et al. Association between hospital cardiac catheter laboratory status, use of an invasive strategy, and outcomes after NSTEMI. Can J Cardiol. 2019;36:868–77. [DOI] [PubMed] [Google Scholar]
- 15. Rubin DB. Multiple imputation for nonresponse in surveys. New York: Wiley; 1987. [Google Scholar]
- 16. Rubin DB. Multiple imputation after 18 years. J Am Stat Assoc. 1996;91:473–89. [Google Scholar]
- 17. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID‐19 infection. J Am Coll Cardiol. 2020;76:533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamadeh A, Aldujeli A, Briedis K, Tecson KM, Sanz‐Sánchez J, Al Dujeili M, et al. Characteristics and outcomes in patients presenting With COVID‐19 and ST‐segment elevation myocardial infarction. Am J Cardiol. 2020;131:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Acute myocardial injury in patients hospitalized with COVID‐19 infection: A review. Prog Cardiovasc Dis. 2020;63:682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Han T, Woodward M, Anderson CS, Zhou H, Chen YD, et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta‐analysis. Prog Cardiovasc Dis. 2020;63:518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han Y, Zeng H, Jiang H, Yuan Z, Cheng X, Jing Z, et al. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID‐19 epidemic. Circulation. 2020;141:e810–16. [DOI] [PubMed] [Google Scholar]
- 23. Wood DA, Sathananthan J, Gin K, Mansour S, Ly HQ, Quraishi AU, et al. Precautions and procedures for coronary and structural cardiac interventions during the COVID‐19 pandemic: guidance from canadian association of interventional cardiology. Can J Cardiol. 2020;36:780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welt FG, Shah PB, Aronow HD, Bortnick AE, Henry TD, Sherwood MW, et al. Catheterization laboratory considerations during the coronavirus (COVID‐19) pandemic: from the ACC’s interventional council and SCAI. J Am Coll Cardiol. 2020;75:2372–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mahmud E, Dauerman HL, Welt FG, Messenger JC, Rao SV, Grines C, et al. Management of acute myocardial infarction during the COVID‐19 pandemic. J Am Coll Cardiol. 2020;96:336–45. [DOI] [PubMed] [Google Scholar]
- 26. Curzen N, Ray S. Cardiology services during the COVID‐19 pandemic. https://www.bcis.org.uk/news/cardiology‐services‐during‐the‐covid‐19‐pandemic/ (accessed 14 Apr 2020).
- 27. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the european society of cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 28. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:139. [DOI] [PubMed] [Google Scholar]
- 29. Zeng JH, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, et al. First case of COVID‐19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cappuccio FP, Siani A. Covid‐19 and cardiovascular risk: Susceptibility to infection to SARS‐CoV‐2, severity and prognosis of Covid‐19 and blockade of the renin‐angiotensin‐aldosterone system. An evidence‐based viewpoint. Nutr Metab Cardiovasc Dis. 2020;30:1227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marchetti M. COVID‐19‐driven endothelial damage: complement, HIF‐1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol. 2020;99:1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tahir F, Bin Arif T, Ahmed J, Malik F, Khalid M. Cardiac manifestations of coronavirus disease 2019 (COVID‐19): a comprehensive review. Cureus. 2020;12:e8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of covid‐19. N Engl J Med. 2020;382:2431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of covid‐19. N Engl J Med. 2020;382:2441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Summary data of missing variables included in the study.
Table S2 Angiographic characteristics and procedural outcomes of COVID19 ACS patients compared to non COVID19 ACS patients undergoing PCI in the BCIS registry.
Figure S1 STROBE study selection.


