Abstract
SARS‐CoV2 infection is a systemic disease that may involve multiple organs, including the central nervous system (CNS). Aims of our study are to describe prevalence and clinical features of neurological manifestations, mortality and hospital discharge in subjects hospitalized with COVID‐19. All individuals admitted for to our hospital COVID‐19 were retrospectively included. Patients were classified according to the symptoms at hospital entry in (1) isolated respiratory, (2) combined respiratory and neurologic, (3) isolated neurologic and (4) stroke manifestations. Descriptive statistics and nonparametric tests to compare the groups were calculated. Kaplan Meier probability curves and multivariable Cox regression models for survival and hospital discharge were applied. The analysis included 901 patients: 42.6% showed a severe or critical disease with an overall mortality of 21.2%. At least one neurological symptom or disease was observed in 30.2% of subjects ranging from dysgeusia/anosmia (9.1%) to postinfective diseases (0.8%). Patients with respiratory symptoms experienced a more severe disease and a higher in‐hospital mortality compared to those who showed only neurologic symptoms. Kaplan Meier estimates displayed a statistically significant different survival among groups (p = 0.003): subjects with stroke had the worst. After adjusting for risk factors such as age, sex and comorbidity, individuals with isolated neurologic manifestations exhibited a better survival (aHR 0.398, 95% CI [0.206, 0.769], p = 0.006). Neurologic manifestations in COVID‐19 are common but heterogeneous and mortality in subjects with isolated neurologic manifestations seems lower than in those with respiratory symptoms.
Keywords: COVID‐19, dizziness, Guillain‐Barré syndrome, seizures, stroke, syncope
At least one neurologic symptom was observed in 30.2% of subjects. Neurologic manifestations in COVID‐19 are common but heterogeneous. Patients with neurologic symptoms exhibited a less severe pulmonary disease and a lower in‐hospital mortality (aHR 0.398, 95% CI 0.206‐0.769, p = 0.006) compared to those showing respiratory symptoms.

1. INTRODUCTION
Because SARS‐CoV2 infection rapidly spread worldwide, COVID‐19 disease showed its complexity with different clinical manifestations including nervous system involvement. Although the exact mechanism by which SARS‐CoV‐2 penetrates the central nervous system (CNS) is not established, it has been proposed that the virus may reach the brain via cribriform plate and olfactory bulb (Baig et al., 2020), after systemic circulatory dissemination following infection of the lung (Netland et al., (2008)) or as a result in loss of involuntary control of breathing in acute respiratory insufficiency requiring assisted ventilation (Baig, 2020). Postmortem and neuroimaging studies showed evidence of cerebral inflammation, leukoencephalopathy, microthrombosis and bleeding phenomenon. Viral RNA is rarely found in cerebrospinal fluid (CSF) and postmortem brain samples, whereas specific anti‐SARS‐CoV‐2 antibodies have been detected in CSF. Other mechanisms leading to CNS dysfunction include cytokine‐driven inflammatory responses, abnormalities of the endothelial cells, blood‐brain barrier damage, autoimmune manifestations and coagulative disorders. Additionally, some issues such as the psychological trauma of life‐threatening illness and the pandemic‐related socio‐economic stressors may be relevant to psychiatric disorders development (Butler et al., 2020).
So far, a large number of case reports and some case series about the neurologic features of COVID‐19 have been published. The primary data suggested that the CNS involvement during SARS‐CoV‐2 infection was very common. In the first case series from Wuhan, China, Mao reported an incidence of 36% in their study population (78 out of 214), varying from nonspecific symptoms to more characteristic symptoms or disease (as encephalitis, seizures, consciousness disturbance, stroke), involvement of peripheral nervous system and neuromuscular disorders (Mao et al., 2020). Similarly, Romero‐Sánchez detected neurologic symptoms in 57.4% (483 out of 841) patients with COVID‐19 from the Spanish ALBACOVID registry (Romero‐Sánchez et al., 2020), and Karadaş found neurologic involvement in 34.7% (83 out of 239) patients in Ankara, Turkey (Karadaş et al., 2020). On the other hand, further studies suggested a lower occurrence: Xiong registered new‐onset specific neurologic events in 4.2% patients with COVID‐19 (39 out of 917) in 56 hospitals designated as COVID‐19 hubs in China (Xiong et al., 2020) whereas Pinna found CNS involvement in 7.7% patients (50 out of 650) hospitalized with COVID‐19 in Chicago, Illinois (Pinna et al., 2020). Taken together, these papers suggest a great diversity of clinical pictures that range from nonspecific symptoms (myopathy, dizziness or headache), to distinctive symptoms (like anosmia and ageusia) and more severe manifestations such as impaired consciousness, seizures or stroke (Pinzon et al., 2020; Wang et al., 2020). Some clinical manifestations could be considered as COVID‐19‐specific because of peculiar underlying mechanisms of development during SARS‐CoV‐2 infection: besides anosmia and ageusia, that are considered useful diagnostic markers, impaired consciousness, peripheral neuropathies such as Guillain‐Barré syndrome (GBS) and stroke could be enlisted here. Generally, some of the common nonspecific symptoms, as well as the distinctive anosmia and ageusia, appear early in the clinical course of the disease, whereas specific symptoms are more commonly reported in severely ill individuals (Pezzini & Padovani, 2020).
These studies provide essential information on the potential neurological manifestations related to SARS‐CoV‐2 infection, but several important limitations, such as methodological differences among the analyses, should be considered when interpreting the results. Moreover, a correct estimation of incidence and prevalence of neurologic involvement during COVID‐19 is difficult. Aims of our study are to describe prevalence of neurological symptoms and to analyse demographic characteristics, clinical features, mortality and hospital discharge rates in patients admitted for COVID‐19 in large a tertiary Hospital located in Milan, Northern Italy.
2. METHODS
2.1. Subjects
A retrospective, single centre analysis included all the patients consecutively admitted to our hospital who tested positive for SARS‐CoV‐2 at real‐time polymerase chain reaction (PCR) on rhino‐pharyngeal swab from February 23 to May 31, 2020. According to the local policies, the swab was collected in the Emergency Department not only in patients accessing for respiratory complaints but also in the presence of other warning factors (i.e., fever, diarrhea or other gastrointestinal symptoms, anosmia or dysgeusia, stroke, contact with infected individuals, living in “red zone” high risk areas, and so on) or in patients requiring hospital admission for other medical reasons.
2.2. Data collection
Demographic and clinical features were collected from the hospital electronic patient records, and the Charlson Comorbidity Index (CCI) was calculated for each subject. All the subjects who tested positive for SARS‐CoV‐2 underwent chest computed tomography (CT) scan to assess lung involvement. Individuals were then classified according to the World Health Organization severity classification (updated at the end of May 2020) in moderate, severe and critical disease ( 2021). A ‘moderate’ form included respiratory complaints and radiologic evidence of interstitial pneumonia but without respiratory failure. A ‘severe’ case was defined as the presence of respiratory distress (respiratory rate ≥30 per min) or oxygen saturation on room air at rest ≤90%. A ‘critical’ case was defined as the presence of respiratory failure due to bilateral pneumonia in association with either septic shock or acute respiratory distress syndrome observed in the first 72 hr from hospital admission.
Patients were stratified according to the presence of respiratory and/or neurologic disturbances at admittance in the Emergency Department. For postinfective encephalitis or peripheral neuropathy, symptoms were collected not only at admittance but also during the whole hospital stay since these clinical conditions may develop later. Patients were then classified according to the symptoms at hospital entry in (1) isolated respiratory, (2) combined respiratory and neurologic, (3) isolated neurologic and (4) stroke manifestations. The diagnosis of the neurologic disturbances relied on patients reports and clinical examination for the majority of complaints (headache, dysgeusia, anosmia, psychomotor agitation, mental confusion and dizziness). Patients underwent cerebral CT scan in case of stroke, seizure and syncope. Electrophysiology tests were performed in case of seizures and neuropathies. Lumbar puncture with CSF assessment was performed only in case of encephalitis and postinfective disease. Advanced neuroimaging, such as magnetic resonance (MR), could not be performed to reduce the risk of contamination and subsequent cross‐infection.
2.3. Ethical approval
The local Ethics Committee approved the protocol under the special conditions indicated by the Italian 648/96 law. All subjects provided written informed consent.
2.4. Statistical analyses
Descriptive statistics (median and interquartile range [IQR] for continuous variables, absolute and relative [%] values for categorical variables) and nonparametric tests (Kruskal‐Wallis for continuous and Chi‐square for categorical variables) were applied to compare the groups. Kaplan Meier probability curves and multivariable Cox regression models for survival and hospital discharge were used. Two‐tailed p values were calculated, and a value <0.05 was considered statistically significant. Data management and analysis were performed using SPSS version 25.
3. RESULTS
The analysis included 901 patients admitted for COVID‐19. Clinical characteristics are shown in Table 1: they were mainly Caucasian (81.7%) and with a median age of 64 years (IQR 52–77); both sexes were equally represented (males 51.7%). Although CCI was low (median 3 with IQR 1–5), 42.6% of patients showed a severe or critical disease with an overall in‐hospital mortality of 21.2%. The majority (629 individuals, 69.8%) presented only respiratory symptoms while 272 subjects (30.2%) showed at least one neurological complaint at admittance: they were classified according to the coexistence of respiratory and neurologic disturbances (111, 12.3%) or the presence of isolated neurologic symptoms (108, 12.0%). Given the differences in terms on demographic and clinical features (Table 2), 53 individuals presenting with stroke (5.9%) were grouped separately. The most common complaints were dysgeusia/anosmia (9.1%) and syncope (9%), followed by mental confusion/dizziness (6.8%). Among those presenting with stroke, 11 (20.8%) were hemorrhagic and 42 (79.2%) acute ischemic. Seventeen cases (32.1%) were considered eligible for treatment: 10 patients (18.9%) underwent thrombolysis and 7 (13.2%) mechanic endovascular thrombectomy. Mortality was 23.5% in those who were treated and 44.4% in those who were considered ineligible.
TABLE 1.
Clinical and demographic features of Study population
| Study population (N = 901) | |
|---|---|
| Male sex, N (%) | 556 (51.7) |
| Age (years), median (IQR) | 64 (52–77) |
| Ethnicity, N (%) | |
| Caucasian | 736 (81.7) |
| Latino | 88 (9.8) |
| MENA | 27 (3.0) |
| Asian | 39 (4.3) |
| Black African | 11 (.2) |
| Charlson comorbidity Index, median (IQR) | 3 (1–5) |
| Diabetes, N (%) | 176 (19.5) |
| Significant overweight/obesity, N (%) | 220 (24.4) |
| Blood hypertension, N (%) | 396 (44.0) |
| Ischemic heart disease, N (%) | 110 (12.2) |
| COPD, N (%) | 88 (9.8) |
| Advanced kidney disease*, N (%) | 66 (7.3) |
| Hemodialysis, N (%) | 3 (0.3) |
| Liver cirrhosis, N (%) | 4 (0.4) |
| Solid organ transplant recipient, N (%) | 15 (1.7) |
| Active neoplastic disease^, N (%) | 57 (6.3) |
| Autoimmune disease°, N (%) | 21 (2.3) |
| Time to Hospital Admittance (days), median (IQR) | 6 (3–9) |
| Disease severity, N (%) | |
| Moderate | 517 (57.4) |
| Severe | 126 (14.0) |
| Critical | 258 (28.6) |
| Overall in‐hospital mortality, N (%) | 191 (21.2) |
| Subjects with any neurological symptoms at admittance, N (%) | 272 (30.2) |
| Type of neurologic disturbance observed during hospital stay, N (%) | |
| Mental confusion/Dizziness | 61 (6.8) |
| Stroke | 53 (5.9) |
| Dysgeusia/Anosmia | 82 (9.1) |
| Seizure | 19 (2.1) |
| Syncope | 81 (9.0) |
| Headache | 39 (4.3) |
| Encephalitis | 5 (0.6) |
| Psychomotor agitation | 26 (2.9) |
| Post‐infective encephalitis/neuropathy | 7 (0.8) |
MENA, Middle‐East and North Africa; COPD, chronic obstructive pulmonary disease.
Stage III‐V according to the KDIGO classification; ^ either oncologic or hematologic active disease; ° including: rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, primary biliary cirrhosis, Crohn disease, Wegener granulomatosis, vasculitis, psoriasis.
TABLE 2.
Clinical and demographic features of Study population stratified according to baseline symptoms
| Respiratory only (N = 629) | Respiratory/ neurologic symptoms (N = 111) | Neurologic only (N = 108) | Stroke (N = 53) | P | |
|---|---|---|---|---|---|
| Male sex, N (%) | 409 (65.0) | 68 (61.3) | 56 (51.9) | 23 (43.4) | 0.001 |
| Age (years), median (IQR) | 63 (51–75) | 62 (52–76) | 72 (50–83) | 77 (68–85) | <0.001 |
| Ethnicity, N (%) | |||||
| Caucasian | 499 (79.3) | 91 (82.0) | 94 (87.0) | 52 (98.1) | 0.195 |
| Latino | 70 (11.1) | 15 (13.5) | 3 (2.8) | – | |
| MENA | 22 (3.5) | 1 (0.9) | 4 (3.7) | – | |
| Asian | 29 (4.6) | 4 (3.6) | 5 (4.6) | 1 (1.9) | |
| Black African | 9 (1.4) | – | 2 (1.9) | – | |
| Charlson Comorbidity Index, median (IQR) | 2 (1–4) | 2 (1–5) | 4 (1–5) | 5 (3–6) | <0.001 |
| Time to Hospital Admittance (days), median (IQR) | 7 (4–10) | 7 (5–10) | 1 (0–5) | 1 (0–5) | <0.001 |
| Body temperature at Hospital Admittance (°C), median (IQR) | 38.0 (37.1–38.5) | 38.0 (37.2–38.5) | 36.7 (36.0–37.6) | 36.3 (36.0–37.3) | 0.005 |
| Disease severity, N (%) | |||||
| Moderate | 325 (51.7) | 64 (57.7) | 88 (81.5) | 40 (75.5) | <0.001 |
| Severe | 88 (14.0) | 21 (18.9) | 12 (11.1) | 5 (9.4) | |
| Critical | 216 (34.3) | 26 (23.4) | 8 (7.4) | 8 (15.1) | |
| Overall in‐hospital mortality, N (%) | 133 (21.1) | 22 (19.8) | 16 (14.8) | 20 (37.7) | 0.010 |
Bold values refer to statistically significant factors in multivariate analysis.
Five patients (0.6%) who presented with mental confusion and psychomotor agitation developed overt encephalitis (or these, three underwent lumbar puncture). Seven individuals (0.7%) showed postinfective neurologic diseases (six with GBS and one with an anti‐NMDA receptor encephalitis) and they all underwent lumbar puncture. SARS‐CoV‐2 was detected in CSF only in one patient. The others had typical inflammatory CSF abnormalities (pleocytosis, high protein levels) but PCR for SARS‐CoV‐2 tested negative. No significant correlation was observed between CSF parameters and plasma inflammatory markers (data not shown).
As shown in Table 2, patients who developed respiratory symptoms were more commonly males, younger, with a lower CCI and a longer time to hospital admittance compared to those who showed neurologic symptoms. They experienced even a more severe lung involvement and a higher in‐hospital mortality compared to those who showed only neurologic symptoms.
If the stratification was performed according to the severity of COVID‐19, the presence of any neurologic involvement was higher among those with a moderate disease compared to those with severe or critical disease (37.1% versus 20.6% respectively, p < 0.001). No differences were observed in less specific clinical manifestation such as headache (4.6% versus 3.9%, p =.591) or syncope (10.1% versus 7.6%, p =.193). Indeed, patients with moderate SARS‐CoV‐2 infection had more commonly COVID‐19‐specific symptoms such as mental confusion/dizziness (9.5% versus 3.1%, p =.006) and dysgeusia/anosmia (11.0% versus 6.5%, p =.020). Other important manifestations were more common in the presence of a moderate disease like psychomotor agitation (5.0% versus 0%, p < 0.001) or seizures (3.1% versus 0.8%, p =.017). On the other hand, we failed to see differences in terms of encephalitis (1.0% versus 0%, p =.128) and postinfective diseases (1.2% versus 0.3%, p =.123).
The Kaplan Meier probability curves displayed a statistically significant different survival among the four groups (log rank 13.6, p =.003): those presenting with stroke had the worst outcome followed by those with respiratory symptoms, whereas individuals with isolated neurologic manifestations exhibited a better survival (Figure 1). The Cox model showed that all traditional factors (age, male sex, CCI, time to hospital admission) as well as the type of clinical manifestation were associated to the survival (Table 3). After adjusting for all these factors, age, male sex, CCI and time to hospital admission maintained their effect, whereas stroke did not show to have a different outcome compared to those presenting with respiratory symptoms. Moreover, patients presenting with isolated neurologic complaints upheld a better survival (aHR 0.398, 95% CI [0.206, 0.769], p =.006).
FIGURE 1.
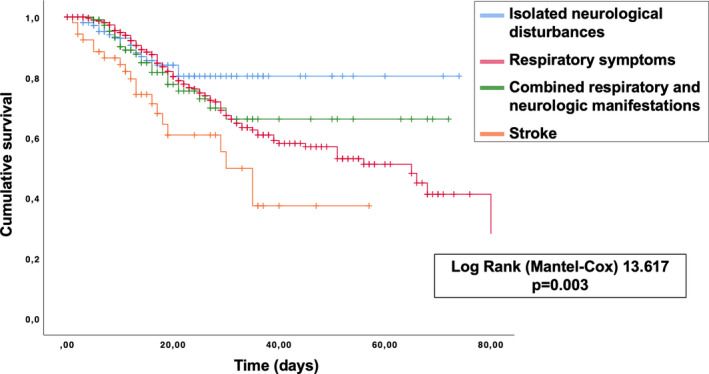
Kaplan Meier survival estimates curves stratified according to type of symptoms at hospital admittance.
TABLE 3.
Cox multivariable estimates for survival
| HR | 95% CI | P | aHR* | 95% CI | P | |
|---|---|---|---|---|---|---|
| Male sex | 1.401 | 1.027–1.911 | 0.033 | 1.958 | 1.414–2.712 | <0.001 |
| Age | 1.053 | 1.041–1.066 | <0.001 | 1.045 | 1.027–1.064 | <0.001 |
| Charlson comorbidity index | 1.309 | 1.236–1.386 | <0.001 | 1.114 | 1.016–1.222 | 0.022 |
| Time to hospital admittance | 0.969 | 0.944–0.995 | 0.020 | 0.974 | 0.950–1.000 | 0.049 |
| Respiratory only | 0.482 | 0.301–0.772 | 0.002 | 0.756 | 0.460–1.242 | 0.270 |
| Respiratory/Neurologic | 0.477 | 0.260–0.875 | 0.017 | 0.726 | 0.388–1.358 | 0.316 |
| Neurologic only | 0.325 | 0.168–0.627 | 0.001 | 0.398 | 0.206–0.769 | 0.006 |
| Stroke | 1 | ref | 1 | ref |
Adjusted according to male sex, age, CCI, time to hospital admittance and clinical presentation.
Bold values refer to statistically significant factors in multivariate analysis.
Conversely, the four subgroups did not show differences in terms of hospital stay and time to discharge (Log rank 1.0, p =.795, data not shown).
4. DISCUSSION
COVID‐19 appears to be a heterogenic disease: SARS‐CoV‐2 has an organotropism not limited to the respiratory system that involves also kidneys, liver, heart, skin and brain. The syndromic complexity includes very different clinical pictures also in terms of neurologic manifestations. Additionally, some reports of long‐lasting disturbances such as disabling fatigue (Halpin et al., 2021) and cognitive difficulties (Zhou et al., 2020) have been published. Thus, these so‐called ‘long COVID’ manifestations should be accounted in addition to acute neurologic symptoms.
CNS involvement was observed also with MERS (Kim et al., 2017) and SARS (Lau et al., 2004), but it seems more common in COVID‐19. In our cohort, 30.2% of patients described at least one neurological symptom, but prevalence is different among studies ranging from 4.2% to 57.4%. Table 4 summarizes the main findings regarding the frequency of neurologic symptoms in the published literature.
TABLE 4.
Comparison of incidence and type of neurologic manifestations in published case series.
| Present Study | Xiong | Romero‐Sánchez | Pinna | Chen | Karadaş | Mao | Varatharaj | Helms | Benussi | Paterson | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients enrolled | 901 | 917 | 841 | 650 | 274 | 239 | 214 | 153 | 58 | 56 | 43 |
| Number of subjects with neurologic symptoms, N (%) | 272 (30.2) | 39 (4.2) | 483 (57.4) | 50 (7.7) | 78 (28.4) | 83 (34.7) | 78 (36.4) | 153 (100) | 49 (84.4) | 56 (100) | 43 (100) |
| Geographical location | Italy | China | Spain | US | China | Turkey | China | UK | France | Italy | UK |
| Monocentric vs Multicentric | Mono‐ | Multi‐ | Multi‐ | Mono‐ | Mono‐ | Mono‐ | Multi‐ | Multi‐ | Mono‐ | Mono‐ | Multi‐ |
| Retrospective vs Prospective | Retro‐ | Retro‐ | Retro‐ | Retro‐ | Retro‐ | Prospect‐ | Retro‐ | Prospect‐ | Retro‐ | Retro‐ | Retro‐ |
| Cohort selection | Unselect* | Unselect* | Unselect* | Unselect* | Unselect* | Select^ | Unselect* | Select§ | Select° | Select§ | Select§ |
| Mental confusion/dizziness, N (%) | 61 (6.8) | 25 (2.7) | 165 (19.6) | 30 (4.6) | 48 (17.5) | 39 (16.3) | 52 (24.3) | 39 (25.5) | 26 (44.8) | No data | No data |
| Stroke, N (%) | 53 (5.9) | 10 (1.1) | 14 (1.7) | 10 (1.5) | No data | 9 (3.8) | 6 (2.8) | 74 (48.4) | 2 (3.4) | 43 (76.8) | 8 (18.6) |
| Dysgeusia/anosmia, N (%) | 82 (9.1) | No data | 93 (11.1) | 8 (1.2) | No data | 34 (14.2) | 23 (10.7) | No data | No data | No data | No data |
| Syncope, N (%) | 81 (9.0) | 3 (0.3) | 5 (0.6) | No data | No data | No data | No data | No data | No data | No data | No data |
| Seizures, N (%) | 19 (2.1) | 0 (0) | 6 (0.7) | 13 (2.0) | No data | 0 (0) | 1 (0.5) | 1 (0.7) | No data | 7 (12.5) | No data |
| Headache, N (%) | 39 (4.3) | 2 (0.2) | 119 (14.1) | 12 (1.8) | 31 (11.3) | 64 (26.8) | 28 (13.1) | No data | No data | No data | No data |
| Encephalitis, N (%) | 5 (0.6) | No data | 1 (0.1) | No data | No data | No data | No data | 7 (4.6) | 1 (1.7) | 0 (0) | 9 (20.9) |
| Psychomotor agitation, N (%) | 26 (2.9) | No data | 69 (8.2) | No data | No data | No data | No data | 23 (15.0) | 40 (69.0) | No data | No data |
| Post‐infective disease, N (%) | 7 (0.8) | No data | 1 (0.1) | 0 (0) | No data | 1 (0.4) | No data | 4 (2.6) | No data | 0 (0) | 18 (41.9) |
| Skeletal muscle injury, N (%) | No data | 2 (0.2) | 108 (12.8) | 6 (0.9) | No data | No data | 23 (10.7) | No data | No data | No data | No data |
Xiong W, et al. Neurology 2020; 95: e1479–e1487; Romero‐Sánchez CM, et al. Neurology 2020; 95: e1060–e1070; Pinna P, et al. J Neurol Sci 2020; 415: 116969; Chen T, et al. BMJ 2020; 368: m1091; Karadaş Ö, et al. Neurol Sci 2020; 41: 1991–1995; Mao L, et al. JAMA Neurol 2020; 77: 683–690; Varatharaj A, et al. Lancet Psychiatry 2020; 7: 875–882; Helms J, et al. N Engl J Med 2020; 382: 2268–2270; Benussi A, et al. Neurology 2020; 95: e910–e920; Paterson RW, et al. Brain 2020; 143: 3104–3120.
Unselected: the study included all subjects admitted to the hospital with a rhino‐pharyngeal swab that tested positive for SARS‐CoV‐2; ^ selected: the study included only patients able to communicate; § selected: the study included only patients with neurologic disturbances at admittance; ° selected: the study included only patients admitted to Intensive Care Unit.
The mentioned paper by Mao et al. (Mao et al., 2020) reported a more frequent CNS involvement in patients with severe pulmonary disease compared to non‐severe disease (31% versus 21%, p =.09), whereas in our cohort, patients with isolated neurologic symptoms at admittance had primarily a moderated lung disease (81.5%) and only small numbers were associated with severe and critical respiratory disease (9.4% and 15.1%, respectively). These patients also experienced a lower mortality, suggesting that a primary neurologic disease may have a different course compared to the respiratory syndrome. As a whole, neurologic involvement was higher among those with moderate COVID‐19 compared to those with a severe or critical disease (37.1% versus 20.6% respectively, p < 0.001). Patients with moderate SARS‐CoV‐2 infection had more commonly COVID‐19‐specific symptoms such as mental confusion/dizziness and dysgeusia/anosmia, whereas no differences were observed in less specific clinical manifestation such as headache or syncope. Encephalitis and postinfective diseases seemed to be more common in moderate COVID‐19 patients, but the small numbers hampered the potency of these analyses and the statistical significance was not met. These observations seem to confirm the model by Koralnik and Tyler (Koralnik & Tyler, 2020) who proposed that CNS invasion follows a different pathway of disease development compared to the classic respiratory illness. Mirfazeli et al. performed a hierarchical clustering analysis of COVID‐19‐related different clinical manifestations and found that respiratory, neurologic and gastroenterological symptoms cluster largely unconnectedly (Mirfazeli et al., 2020).
Anosmia and/or dysgeusia (recorded in 9.1% of individuals) and syncope (observed in 9.0%) were the most common neurologic manifestations. We observed seizures more frequently than what reported in the mentioned paper by Mao (Mao et al., 2020) (2.1% versus 0.5%, respectively). Additionally, some case reports describing seizures at presentation in adult and pediatric patients with SARS‐CoV‐2 infection have been published (Dugue et al., 2020; Sohal & Mossammat, 2020). As syncope may be a sign of partial epileptic seizures, the overall number of seizures may be underestimated.
In our cohort, we observed several cases of mental confusion (6.8%) and psychomotor agitation (2.9%), a possible expression of direct (Moriguchi et al., 2020) or postinfective (Panariello et al., 2020) CNS involvement. Unfortunately, we were unable to perform a complete neuroimaging assessment and CSF analysis in all cases and SARS‐CoV‐2 was isolated from CSF only in one patient. Moreover, Woo et al. reported that a large number of patients (78%) complained of persistent mild cognitive impairment after a median of 85 day from clinical recovery (Woo et al., 2020). It has been shown that inflammation continue after coronavirus clearance and several studies have demonstrated that inflammatory activation is linked to mild cognitive impairment (Shen et al., 2019), overt cognitive dysfunction (Chakrabarty et al., 2019) and dementia (Duarte et al., 2017). Therefore, a potential association between inflammatory status and persistent cognitive damage in patients even after COVID‐19 healing should be investigated. As a consequence, it is difficult to interpret these neurologic and psychiatric manifestation. SARS‐CoV‐2 infection should be considered as a differential diagnosis in psychiatric patients who present with symptoms of sudden onset to avoid wrong or delayed diagnosis. We propose to rule out SARS‐CoV2 during pandemic phases in subjects with these symptoms and no previous history of behavioural disturbances.
A peculiar manifestation of COVID‐19 is acute cerebrovascular disease. In our study, we observed 6% of strokes, a percentage similar to what reported by Li (Li et al., 2020) and slightly more than what described by Mao (2.8%) (Mao et al., 2020). As expected, our patients with stroke were older and with more comorbidities: these factors could partially explain the observed greater fatality rate. It should be noted also that a mortality of 37.7% is considerably higher that what reported in published literature, where it ranges from 11% to 19% (Saposnik et al., 2008; 2020), and it is higher than mortality in our centre (7.7% in 2019). Our data confirm the existing literature about a substantial superior mortality in individuals with both COVID‐19 and stroke than what observed in patients with stroke without SARS‐CoV‐2 infection (Ghasemiyeh et al., 2020; Jin et al., 2020). Oxley (Oxley et al., 2020) reported five large‐vessel strokes in COVID‐19 patients younger than 50 years, whereas we observed only four cases (0.4%) in such a young population, thus suggesting that role of the virus in stroke development in low‐risk population should be better investigated.
Stroke pathophysiology is complex and multifactorial including other cardiovascular risk factors such as diabetes, obesity, hypertension and previous coronary artery disease. Nevertheless, preliminary reports suggest that the risk factors and the underlying mechanisms of COVID‐19‐related stroke are different, with a lower prevalence of hypertension and a higher prevalence of cryptogenic stroke subtype (Yaghi et al., 2020). The perturbation of coagulative homeostasis has already been related to death in COVID‐19 patients (Zhang et al., 2020; Zhou et al., 2020). We previously showed that D‐dimer continued to increase despite the improvement in other inflammatory markers, suggesting a persistent alteration of the coagulation (Rossotti et al., 2020). The COVID‐19‐specific systemic inflammatory response usually involves endothelial dysfunction and microthrombosis with organ failure but without bleeding (Iba et al., 2019). As a consequence of this hypercoagulable state, SARS‐CoV‐2 can infect and damage endothelial cells leading to systemic microvascular and macrovascular complications (Wichmann et al., 2020). Other causal factors may be involved such as unrestricted angiotensin 2 action, increased production of adhesion molecules able to induce vascular inflammation and endothelial activation, complement stimulation, excessive production of neutrophil extracellular traps and increased platelet count (Allegra et al., 2020; Beyrouti et al., 2020).
Postinfective neurologic disturbances are a rare complication after viral or bacterial infections. Toscano et al. (Toscano et al., 2020) reported five cases of GBS in patients infected by SARS‐CoV‐2. Other GBS cases were reported from the Spanish ALBACOVID registry (Romero‐Sánchez et al., 2020), the Turkish cohort from Ankara (Karadaş et al., 2020), and many more from the British registry (Varatharaj et al., 2020) and multicentre specialistic consultation on neurologic events (Paterson et al., 2020). Reports of other immune‐mediated conditions during COVID‐19, such as acute necrotizing encelophaty (Dixon et al., 2020; Poyiadji et al., 2020) and acute disseminated encephalomyelitis (Parsons et al., 2020), have been recently published. In our cohort, the incidence of postinfective neurologic complication was low (0.8%), but it appeared considerably higher than what observed after other infective agents (generally below 0.002% each year in Europe) (Pithadia & Kakadia, 2010). Although this observation should be interpreted cautiously, these findings strengthen the hypothesis that SARS‐CoV‐2 might induce immune‐mediated neurologic damage after the infectious phase. The complex interplay between SARS‐CoV‐2 and the immune system could justify a high incidence of autoimmune or other inflammatory disturbances.
Our study has several limitations, first of all its retrospective, single centre study design with the typical drawbacks of this kind of analyses. Secondly, data were extracted from electronic medical records, so some symptoms—especially mild disturbances—might not have been recorded, leading to an underestimated prevalence of these complaints. Thirdly, advanced neuroimaging, such as MR, or invasive diagnostic procedures, such as lumbar puncture, were avoided or performed in a limited number of cases to reduce the risk of cross‐infection. As a consequence, most of the recorded symptoms could not be fully investigated and the definitive diagnosis could not be completely detailed. Finally, this study is hospital‐based; thus, it does not necessarily reflect the true incidence of neurologic manifestations in the general population. Additionally, our follow‐up is limited to in‐hospital stay so it could not define mortality rate after discharge and long‐term neurologic sequelae.
Despite these limitations, our study analysed one of the largest cohorts of COVID‐19 patients with neurologic disease and confirms how CNS involvement by COVID‐19 comprises different diseases, possible expression of different pathways of SARS‐CoV‐2 infection, as previously suggested. Larger multicentric studies and with a more homogeneous design are needed to better understand risk factors, pathophysiology and evolution of neurologic diseases during and after COVID‐19 to outline preventive strategies and specific treatment for each clinical manifestation.
CONFLICT OF INTERESTS
None of the Authors has conflicts of interests to declare.
AUTHOR CONTRIBUTIONS
RR collected, analysed, and interpreted the data; RR and GT wrote the paper; GG, AP, MEP, and ECA provided specialistic support for patients with neurologic and psychiatric symptoms; MM, SC, MV, AR, BN, OME, PT, FG, FC, RF were treating physicians; FDA, MC, MM, and CB helped in data collection and management; MP coordinated clinical and scientific activities during the first COVID‐19 pandemic. GT and RR equally contributed to this manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15159.
DATA AVAILABILTY STATEMENT
All the anonymized data used to perform this analysis will be available for any further revision.
ACKNOWLEDGEMENTS
NIGUARDA COVID‐19 WORKING GROUP: Donatella Bambacini, Fulvio Crippa, Maria Cristina Moioli, Davide Motta, Carloandrea Orcese, Annamaria Pazzi, Alice Sacco, Beniamino Piero Vigo, Nicola Ughi, Laura Ciceri, Silvia Colombo, Davide Ferrazzi, Claudia Galli, Linda Guarnieri, Enrica Silvia Periti, Lorenzo Porta, Valeria Tombini, Silvia Bondini, Alessandra Cernuschi, Anna Gandino, Marta Molaro, Alessandra Cernuschi, Lucrezia Rovati, Gabriele Bassi, Maurizio Bottiroli, Giampaolo Casella, Andrea Degasperi, Riccardo Giudici, Gianpaola Monti, Anna Rossi, Michele Mondino, Adriano Basile, Ruggero Ruggeri, Stefano Pastori, Claudia Alteri, Stefania Carta, Luna Colagrossi, Diana Fanti, Ester Mazzola, Alice Nava, Chiara Vismara.
Travi G, Rossotti R, Merli M, et al; on behalf of the Niguarda COVID‐19 Group . Neurological manifestations in patients hospitalized with COVID‐19: A retrospective analysis from a large cohort in Northern Italy. Eur J Neurosci. 2021;53:2912–2922. 10.1111/ejn.15159
Funding information
No specific funding support was planned for study design, data collection and analysis, and manuscript writing of this paper.
Edited by: Tara Spires‐Jones
References
- Allegra, A. , Innao, V. , Allegra, A. G. , & Musolino, C. (2020). Coagulopathy and thromboembolic events in patients with SARS‐CoV‐2 infection: Pathogenesis and management strategies. Annals of Hematology, 99, 1953–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig, A. M. (2020). Neurological manifestations in COVID‐19 caused by SARS‐CoV‐2. CNS Neuroscience and Therapeutics, 26, 499–501. 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig, A. M. , Khaleeq, A. , Ali, U. , & Syeda, H. (2020). Evidence of the COVID‐19 virus targeting the CNS: Tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chemical Neuroscience, 11, 995–998. [DOI] [PubMed] [Google Scholar]
- Beyrouti, R. , Adams, M. E. , Benjamin, L. , Cohen, H. , Farmer, S. F. , Goh, Y. Y. , Humphries, F. , Jäger, H. R. , Losseff, N. A. , Perry, R. J. , Shah, S. , Simister, R. J. , Turner, D. , Chandratheva, A. , & Werring, D. J. (2020). Characteristics of ischaemic stroke associated with COVID‐19. Journal of Neurology, Neurosurgery and Psychiatry, 91, 889–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, M. , Pollak, T. A. , Rooney, A. G. , Michael, B. D. , & Nicholson, T. R. (2020). Neuropsychiatric complications of covid‐19. BMJ, 371, m3871.– 10.1136/bmj.m3871. [DOI] [PubMed] [Google Scholar]
- Chakrabarty, T. , Torres, I. J. , Bond, D. J. , & Yatham, L. N. (2019). Inflammatory cytokines and cognitive functioning in early‐stage bipolar I disorder. Journal of Affective Disorders, 245, 679–685. [DOI] [PubMed] [Google Scholar]
- Dixon, L. , Varley, J. , Gontsarova, A. , Mallon, D. , Tona, F. , Muir, D. , Luqmani, A. , Jenkins, I. H. , Nicholas, R. , Jones, B. , & Everitt, A. (2020). COVID‐19‐related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurology ‐ Neuroimmunology Neuroinflammation, 7, e789.– 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, P. O. , Duarte, M. G. F. , Pelichek, A. , Pfrimer, K. , Ferriolli, E. , & Moriguti, J. C. (2017). Cardiovascular risk factors and inflammatory activity among centenarians with and without dementia. Aging Clinical and Experimental Research, 29, 411–417. [DOI] [PubMed] [Google Scholar]
- Dugue, R. , Cay‐Martínez, K. C. , Thakur, K. T. , Garcia, J.A. , Chauhan, L. V. , Williams, S. H. , Briese, T. , Jain, K. , Foca, M. , McBrian, D. K. , Bain, J. M. , Lipkin, W. I. , & Mishra, N. (2020). Neurologic manifestations in an infant with COVID‐19. Neurology, 94, 1100–1102. 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemiyeh, P. , Borhani‐Haghighi, A. , Karimzadeh, I. , Mohammadi‐Samani, S. , Vazin, A. , Safari, A. , & Qureshi, A. I. (2020). Major neurologic adverse drug reactions, potential drug‐drug interactions and pharmacokinetic aspects of drugs used in COVID‐19 patients with stroke: A narrative review. Therapeutics and Clinical Risk Management, 16, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin, S. J. , McIvor, C. , Whyatt, G. , Adams, A. , Harvey, O. , McLean, L. , Walshaw, C. , Kemp, S. , Corrado, J. , Singh, R. , Collins, T. , O'Connor, R. J. , & Sivan, M. (2021). Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: A cross‐sectional evaluation. Journal of Medical Virology, 93, 1013–1022. 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- Iba, T. , Levy, J. H. , Warkentin, T. E. , Thachil, J. , van der Poll, T. , & Levi, M. (2019). Diagnosis and management of sepsis‐induced coagulopathy and disseminated intravascular coagulation. Journal of Thrombosis and Haemostasis, 17, 1989–1994. [DOI] [PubMed] [Google Scholar]
- Jin, H. , Hong, C. , Chen, S. , Zhou, Y. , Wang, Y. , Mao, L. , Li, Y. , He, Q. , Li, M. , Su, Y. , Wang, D. , Wang, L. , & Hu, B. (2020). Consensus for prevention and management of coronavirus disease 2019 (COVID‐19) for neurologists. Stroke and Vascular Neurology, 5, 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadaş, Ö. , Öztürk, B. , & Sonkaya, A. R. (2020). A prospective clinical study of detailed neurological manifestations in patients with COVID‐19. Neurological Sciences, 41, 1991–1995. 10.1007/s10072-020-04547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. E. , Heo, J. H. , Kim, H. O. , Song, S. H. , Park, S. S. , Park, T. H. , Ahn, J. Y. , Kim, M. K. , & Choi, J. P. (2017). Neurological complications during treatment of Middle East respiratory syndrome. Journal of Clinical Neurology, 13, 227–233. 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralnik, I. J. , & Tyler, K. L. (2020). COVID‐19: A global threat to the nervous system. Annals of Neurology, 88, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, K. K. , Yu, W. C. , Chu, C. M. , Lau, S. –T. , Sheng, B. , & Yuen, K. ‐Y. (2004). Possible central nervous system infection by SARS coronavirus. Emerging Infectious Diseases, 10, 342–344. 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Li, M. , Wang, M. , Zhou, Y. , Chang, J. , Xian, Y. , Wang, D. , Mao, L. , Jin, H. , & Hu, B. (2020). Acute cerebrovascular disease following COVID‐19: A single center, retrospective, observational study. Stroke and Vascular Neurology, 5, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, L. , Jin, H. , Wang, M. , Hu, Yu , Chen, S. , He, Q. , Chang, J. , Hong, C. , Zhou, Y. , Wang, D. , Miao, X. , Li, Y. , & Hu, B. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan China. JAMA Neurology, 77, 683–690. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirfazeli, F. S. , Sarabi‐Jamab, A. , Jahanbakhshi, A. , Kordi, A. , Javadnia, P. , Shariat, S. V. , Aloosh, O. , Almasi‐Dooghaee, M. , & Faiz, S. H. R. (2020). Neuropsychiatric manifestations of COVID‐19 can be clustered in three distinct symptom categories. Scientific Reports, 10, 20957. 10.1038/s41598-020-78050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi, T. , Harii, N. , Goto, J. , Harada, D. , Sugawara, H. , Takamino, J. , Ueno, M. , Sakata, H. , Kondo, K. , Myose, N. , Nakao, A. , Takeda, M. , Haro, H. , Inoue, O. , Suzuki‐Inoue, K. , Kubokawa, K. , & Shimada, S. (2020). A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. International Journal of Infectious Diseases, 94, 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland, J. , Meyerholz, D. K. , Moore, S. , Cassell, M. , & Perlman, S. (2008). Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. Journal of Virology, 82, 7264–7275. 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley, T. J. , Mocco, J. , Majidi, S. , Kellner, C. P. , Shoirah, H. , Singh, I. P. , De Leacy, R. A. , Shigematsu, T. , Ladner, T. R. , Yaeger, K. A. , Skliut, M. , Weinberger, J. , Dangayach, N. S. , Bederson, J. B. , Tuhrim, S. , & Fifi, J. T. (2020). Large‐vessel stroke as a presenting feature of covid‐19 in the young. New England Journal of Medicine, 382, e60. 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panariello, A. , Bassetti, R. , Radice, A. , Rossotti, R. , Puoti, M. , Corradin, M. , Moreno, M. , & Percudani, M. (2020). Anti‐NMDA receptor encephalitis in a psychiatric Covid‐19 patient: A case report. Brain, Behavior, and Immunity, 87, 179–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, T. , Banks, S. , Bae, C. , Gelber, J. , Alahmadi, H. , & Tichuaer, M. (2020). COVID‐19‐associated acute disseminated encephalomyelitis (ADEM). Journal of Neurology, 267, 2799–2802. 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, R. W. , Brown, R. L. , Benjamin, L. , Nortley, R. , Wiethoff, S. , Bharucha, T. , Jayaseelan, D. L. , Kumar, G. , Raftopoulos, R. E. , Zambreanu, L. , Vivekanandam, V. , Khoo, A. , Geraldes, R. , Chinthapalli, K. , Boyd, E. , Tuzlali, H. , Price, G. , Christofi, G. , Morrow, J. , & Zandi, M. S. (2020). The emerging spectrum of COVID‐19 neurology: Clinical, radiological and laboratory findings. Brain, 143, 3104–3120. 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzini, A. , & Padovani, A. (2020). Lifting the mask on neurological manifestations of COVID‐19. Nature Reviews Neurology, 16(11), 636–644. 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna, P. , Grewal, P. , Hall, J. P. , Tavarez, T. , Dafer, R. M. , Garg, R. , Osteraas, N. D. , Pellack, D. R. , Asthana, A. , Fegan, K. , Patel, V. , Conners, J. J. , John, S. , & Silva, I. D. (2020). Neurological manifestations and COVID‐19: Experiences from a tertiary care center at the frontline. Journal of the Neurological Sciences, 415, 116969.– 10.1016/j.jns.2020.116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzon, R. T. , Wijaya, V. O. , Buana, R. B. , Al Jody, A. , & Nunsio, P. N. (2020). Neurologic characteristics in coronavirus disease 2019 (COVID‐19): A systematic review and meta‐analysis. Frontiers in Neurology, 11, 565. 10.3389/fneur.2020.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pithadia, A. B. , & Kakadia, N. (2010). Guillain‐Barré syndrome (GBS). Pharmacological Reports, 62, 220–232. 10.1016/S1734-1140(10)70261-9. [DOI] [PubMed] [Google Scholar]
- Poyiadji, N. , Shahin, G. , Noujaim, D. , Stone, M. , Patel, S. , & Griffith, B. (2020). COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: Imaging features. Radiology, 296, E119–E120. 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero‐Sánchez, C. M. , Díaz‐Maroto, I. , Fernández‐Díaz, E. , Sánchez‐Larsen, A. , Layos‐Romero, A. , García‐García, J. , González, E. , Redondo‐Peñas, I. , Perona‐Moratalla, A. B. , Del Valle‐Pérez, J. A. , Gracia‐Gil, J. , Rojas‐Bartolomé, L. , Feria‐Vilar, I. , Monteagudo, M. , Palao, M. , Palazón‐García, E. , Alcahut‐Rodríguez, C. , Sopelana‐Garay, D. , Moreno, Y. , & Segura, T. (2020). Neurologic manifestations in hospitalized patients with COVID‐19: The ALBACOVID registry. Neurology, 95, e1060–e1070. 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossotti, R. , Travi, G. , Ughi, N. , Corradin, M. , Baiguera, C. , Fumagalli, R. , Bottiroli, M. , Mondino, M. , Merli, M. , Bellone, A. , Basile, A. , Ruggeri, R. , Colombo, F. , Moreno, M. , Pastori, S. , Perno, C. F. , Tarsia, P. , Epis, O. M. , & Puoti, M. (2020). Safety and efficacy of anti‐il6‐receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: A comparative analysis. Journal of Infection, 81, e11–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saposnik, G. , Hill, M. D. , O'Donnell, M. , Fang, J. , Hachinski, V. , & Kapral, M. K. (2008). Variables associated with 7‐day, 30‐day, and 1‐year fatality after ischemic stroke. Stroke, 39, 2318–2324. [DOI] [PubMed] [Google Scholar]
- Shen, X. N. , Niu, L. D. , Wang, Y. J. , Cao, X. P. , Liu, Q. , & Tan, L. (2019). Inflammatory markers in Alzheimer's disease and mild cognitive impairment: A meta‐analysis and systematic review of 170 studies. Journal of Neurology, Neurosurgery, and Psychiatry, 90, 590–598. [DOI] [PubMed] [Google Scholar]
- Sohal, S. , & Mossammat, M. (2020). COVID‐19 presenting with seizures. ID Cases, 20, e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano, G. , Palmerini, F. , Ravaglia, S. , Ruiz, L. , Invernizzi, P. , Cuzzoni, M. G. , Franciotta, D. , Baldanti, F. , Daturi, R. , Postorino, P. , Cavallini, A. , & Micieli, G. (2020). Guillain‐Barré syndrome associated with SARS‐CoV‐2. New England Journal of Medicine, 382, 2574–2576. 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj, A. , Thomas, N. , Ellul, M. A. , Davies, N. W. S. , Pollak, T. A. , Tenorio, E. L. , Sultan, M. , Easton, A. , Breen, G. , Zandi, M. , Coles, J. P. , Manji, H. , Salman, R. A. , Menon, D. K. , Nicholson, T. R. , Benjamin, L. A. , Carson, A. , Smith, C. , Turner, M. R. , & Michael, B. D. (2020). Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: A UK‐wide surveillance study. Lancet Psychiatry, 7, 875–882. 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Shen, Y. , Li, M. , Chuang, H. , Ye, Y. , Zhao, H. , & Wang, H. (2020). Clinical manifestations and evidence of neurological involvement in 2019 novel coronavirus SARS‐CoV‐2: A systematic review and meta‐analysis. Journal of Neurology, 267, 2777–2789. 10.1007/s00415-020-09974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann, D. , Sperhake, J. P. , Lütgehetmann, M. , Steurer, S. , Edler, C. , Heinemann, A. , Heinrich, F. , Mushumba, H. , Kniep, I. , Schröder, A. S. , Burdelski, C. , de Heer, G. , Nierhaus, A. , Frings, D. , Pfefferle, S. , Becker, H. , Bredereke‐Wiedling, H. , de Weerth, A. , Paschen, H. R. , & Kluge, S. (2020). Autopsy findings and venous thromboembolism in patients with COVID‐19: A prospective cohort study. Annals of Internal Medicine, 173, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, M. S. , Malsy, J. , Pöttgen, J. , Seddiq Zai, S. , Ufer, F. , Hadjilaou, A. , Schmiedel, S. , Addo, M. M. , Gerloff, C. , Heesen, C. , Schulze Zur Wiesch, J. , & Friese, M. A. (2020). Frequent neurocognitive deficits after recovery from mild COVID‐19. Brain Communications, 2, fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Clinical Management of Covid‐19—Interim Guidance. (2021). Available online. https://www.who.int/publications/i/item/clinical‐management‐of‐covid‐19 (accessed on 2 January 2021).
- Xiong, W. , Mu, J. , Guo, J. , Liu, D. , Luo, J. , Li, N. , Liu, J. , Yang, D. , Gao, H. , Zhang, Y. , Lin, M. , Shen, S. , Zhang, H. , Chen, L. , Wang, G. , Luo, F. , Li, W. , Chen, S. , He, L. , & Zhou, D. (2020). New onset neurologic events in people with COVID‐19 infection in three regions in China. Neurology, 95, e1479–e1487. [DOI] [PubMed] [Google Scholar]
- Yaghi, S. , Ishida, K. , Torres, J. , Mac Grory, B. , Raz, E. , Humbert, K. , Henninger, N. , Trivedi, T. , Lillemoe, K. , Alam, S. , Sanger, M. , Kim, S. , Scher, E. , Dehkharghani, S. , Wachs, M. , Tanweer, O. , Volpicelli, F. , Bosworth, B. , Lord, A. , & Frontera, J. (2020). SARS‐CoV‐2 and stroke in a New York healthcare system. Stroke, 51, 2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Xiao, M. , Zhang, S. , Xia, P. , Cao, W. , Jiang, W. , Chen, H. , Ding, X. , Zhao, H. , Zhang, H. , Wang, C. , Zhao, J. , Sun, X. , Tian, R. , Wu, W. , Wu, D. , Ma, J. , Chen, Y. , Zhang, D. , & Zhang, S. (2020). Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. New England Journal of Medicine, 382, e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , Xiang, J. , Wang, Y. , Song, B. , Gu, X. , Guan, L. , Wei, Y. , Li, H. , Wu, X. , Xu, J. , Tu, S. , Zhang, Y. , Chen, H. , & Bin, C. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet, 395, 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Lu, S. , Chen, J. , Wei, N. , Wang, D. , Lyu, H. , Shi, C. , & Hu, S. (2020). The landscape of cognitive function in recovered COVID‐19 patients. Journal of Psychiatric Research, 129, 98–102. 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.nuffieldtrust.org.uk/resource/stroke‐and‐heart‐attack‐mortality (accessed on Sep 27, 2020), 2020.


