Abstract
Thrombotic complications occur at high rates in hospitalized patients with COVID‐19, yet the impact of intensive antithrombotic therapy on mortality is uncertain. We examined in‐hospital mortality with intermediate‐ compared to prophylactic‐dose anticoagulation, and separately with in‐hospital aspirin compared to no antiplatelet therapy, in a large, retrospective study of 2785 hospitalized adult COVID‐19 patients. In this analysis, we established two separate, nested cohorts of patients (a) who received intermediate‐ or prophylactic‐dose anticoagulation (“anticoagulation cohort”, N = 1624), or (b) who were not on home antiplatelet therapy and received either in‐hospital aspirin or no antiplatelet therapy (“aspirin cohort”, N = 1956). To minimize bias and adjust for confounding factors, we incorporated propensity score matching and multivariable regression utilizing various markers of illness severity and other patient‐specific covariates, yielding treatment groups with well‐balanced covariates in each cohort. The primary outcome was cumulative incidence of in‐hospital death. Among propensity score‐matched patients in the anticoagulation cohort (N = 382), in a multivariable regression model, intermediate‐ compared to prophylactic‐dose anticoagulation was associated with a significantly lower cumulative incidence of in‐hospital death (hazard ratio 0.518 [0.308–0.872]). Among propensity‐score matched patients in the aspirin cohort (N = 638), in a multivariable regression model, in‐hospital aspirin compared to no antiplatelet therapy was associated with a significantly lower cumulative incidence of in‐hospital death (hazard ratio 0.522 [0.336–0.812]). In this propensity score‐matched, observational study of COVID‐19, intermediate‐dose anticoagulation and aspirin were each associated with a lower cumulative incidence of in‐hospital death.
1. INTRODUCTION
Thrombosis is among the most devastating complications of COVID‐19. In multiple studies, venous thromboembolism (VTE), arterial thrombosis, and microvascular thrombosis have all been described. 1 , 2 , 3 , 4 , 5 , 6 High VTE rates have been reported in critically ill COVID‐19 patients despite the use of prophylactic anticoagulation. 1 , 6 , 7 The development of pulmonary microvascular thrombosis may be central to the pathogenesis of COVID‐19 in the lungs. 5 An elevated D‐dimer, a breakdown product of fibrin clots, is one of the strongest predictors of mortality from COVID‐19. 8 , 9
A common global practice has been to administer escalated intensities of antithrombotic therapy beyond standard prophylactic‐dose anticoagulation in hospitalized COVID‐19 patients. 10 , 11 , 12 To date, there has been little evidence to support this practice. 13 , 14 Some retrospective studies have observed lower mortality rates with therapeutic‐dose anticoagulation compared to either prophylactic‐dose anticoagulation or no anticoagulation, while others comparing therapeutic‐ and prophylactic‐dose anticoagulation have found no mortality difference. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Recent findings from the ACTIV‐4a randomized controlled trial showed that therapeutic‐ compared to prophylactic‐dose anticoagulation improved outcomes among non‐critically ill patients but not among critically ill COVID‐19 patients. 23 , 24 To date, however, no large‐scale study has compared the effects of intermediate‐ versus prophylactic‐dose anticoagulation.
Some investigators have also proposed a potential role for aspirin and other antiplatelet therapies in light of the high burden of microvascular thrombosis and emerging models of endotheliopathy, platelet activation, and immunothrombosis in COVID‐19. 5 , 25 , 26 , 27 , 28 , 29 One retrospective study reported improved outcomes with aspirin therapy but did not account for disease severity between treatment groups, making its conclusions difficult to interpret. 30
A major limitation in retrospective studies is bias in the likelihood of patients to receive the treatments being studied. In unadjusted observational studies, disease severity is a confounding factor affecting treatment decisions and outcomes, often precluding accurate analysis of potential treatment effects. To address this, propensity score matching for disease severity and other variables has been utilized in some observational studies, leading to findings compatible with those obtained from randomized controlled trials. 31 , 32 The use of propensity score matching in a few landmark observational studies in COVID‐19 has yielded key insights about potential treatment effects by enabling treatment groups with balanced covariates to be reliably compared. 33 , 34
We sought to examine the impact of intermediate‐dose anticoagulation and aspirin on in‐hospital mortality in COVID‐19. To account for variations in treatment, we utilized propensity score matching and multivariable regression analysis incorporating markers of disease severity and other clinical covariates. One scoring system for assessing disease severity in use at many hospitals is the Rothman Index (RI), a composite score of 26 distinct clinical, laboratory, and nursing variables, which has been shown to have prognostic value in some surgical and critical care studies, although its utility in COVID‐19 is presently unknown. 35 , 36 , 37 We hypothesized that the RI might be a useful tool for evaluating disease severity in COVID‐19, both for clinical care and for the purposes of propensity score matching. In this observational study, we first analyzed a large, multisite cohort of hospitalized COVID‐19 patients by multivariable regression analysis and found a novel prognostic role for the admission RI in predicting in‐hospital mortality. Then, incorporating the RI and other measures of illness severity, we performed propensity score matching and multivariable regression analyses and observed significant reductions in in‐hospital mortality among hospitalized COVID‐19 patients treated with intermediate‐dose anticoagulation or aspirin.
2. METHODS
2.1. Patients, data collection, and variables
This was a retrospective study of hospitalized adult patients with COVID‐19; Institutional Review Board approval was obtained for this study, and an approved Data Use Agreement between institutions permitted analysis. From March through June 2020, our hospital's Joint Data Analytics Team (JDAT) identified 4150 hospital encounters in the Yale New Haven Health System, consisting of a two‐campus tertiary care academic center (Yale New Haven Hospital and the St. Raphael Campus) and four community hospitals (Bridgeport Hospital, Greenwich Hospital, Lawrence & Memorial Hospital, and Westerly Hospital), with a diagnosis of COVID‐19 established via a nasopharyngeal polymerase chain reaction test. Patients were excluded if they were < 18 years of age (N = 35), had multiple inpatient hospital encounters due to transfer between hospitals or readmission (N = 1247; all such encounters were excluded in these cases), or had missing data (N = 83), yielding an overall study cohort size of 2785 unique patients.
Demographic, clinical, and laboratory data were extracted from each patient's medical record by JDAT. Established population health registries were used to identify patients with diabetes, hypertension, coronary artery disease (CAD), and congestive heart failure (CHF) (Table S1). Inclusion into a population health registry required an encounter diagnosis of the referenced disease state and at least a single, non‐abstract patient encounter within the health system in the preceding 3 years; in addition, either the patient problem list was required to contain the referenced diagnosis, or the patient had to have a minimum of two face‐to‐face encounters within the previous 12 months. For disease states without established population health registries, ICD‐10 codes were used. We defined cardiovascular disease as any of the following: hypertension, diabetes, CAD, myocardial infarction, CHF, atrial fibrillation, stroke, or transient ischemic attack. We categorized body mass index (BMI) according to the U.S. Centers for Disease Control definitions. 38 We categorized the first RI on admission into four quartiles (quartile 1, RI ‐33 to 43; quartile 2, RI 42 to 65; quartile 3, RI 66 to 79; quartile 4, RI 80 to 99), with the lowest and highest quartiles representing patients with the greatest and least illness severities, respectively.
2.2. Definitions of anticoagulation intensity
For our analysis, each patient was assigned to one anticoagulant group using the following criteria. First, the maximum dose of enoxaparin or heparin received during each patient's admission was determined. Patients who received a maximum enoxaparin dose of 30–40 mg at a weight‐adjusted concentration of < 0.7 mg/kg every 24 h, enoxaparin 30–40 mg at a weight‐adjusted concentration of < 0.4 mg/kg every 12 h, subcutaneous unfractionated heparin (UFH) 5000 units up to three times per day, or subcutaneous UFH 5000 or 7500 units up to three times per day with a BMI ≥40 kg/m2, and who did not receive any other type of documented anticoagulant during their hospitalization, were categorized as prophylactic‐dose anticoagulation. Patients who received a maximum enoxaparin dose of ≥0.4 and < 0.7 mg/kg every 12 h or subcutaneous UFH 7500 U at any frequency with a BMI < 40 kg/m2, and who did not receive any other type of anticoagulant during their hospitalization, were categorized as intermediate‐dose anticoagulation. Patients who received a maximum enoxaparin dose ≥0.7 mg/kg every 12 h, enoxaparin ≥0.7 mg/kg every 24 h with creatinine clearance < 30 mL/min, enoxaparin ≥1.4 mg/kg every 24 h, intravenous UFH, or intravenous bivalirudin were categorized as therapeutic‐dose anticoagulation. Patients who received any other dose of enoxaparin and who did not receive a direct oral anticoagulant (DOAC) or any other therapeutic‐dose anticoagulant were categorized as “Alternative enoxaparin dose”. Patients who received a DOAC and no other type of therapeutic‐dose anticoagulation were categorized as “DOAC”. All other patients were categorized as “No documented anticoagulation”. Manual chart review was performed in cases with ambiguous data regarding anticoagulation dosing.
2.3. Statistical analyses
The primary outcome in this study was in‐hospital death, measured as cumulative incidence of in‐hospital death, with cumulative incidence of hospital discharge as a competing risk. Univariable and multivariable regression modeling of subdistribution hazard functions for the primary outcome was performed in all cohorts; we also reported hazard ratios (HR) from competing risks regression. 39 Variables incorporated into the modeling included demographic factors, medical history, and clinical and laboratory features reflecting disease severity. Propensity score matching was performed on the different cohorts to achieve balance in covariates between patients treated with intermediate‐ versus prophylactic‐dose anticoagulation in the anticoagulation cohort, and separately between patients treated with in‐hospital aspirin versus no antiplatelet therapy in the aspirin cohort. Cumulative incidence curves were estimated for nonparametric visualization of in‐hospital death and discharge events and tested using Gray's test in the propensity score‐matched anticoagulation and aspirin cohorts 40 ; for clarity, only curves for in‐hospital death are displayed in the figures.
Propensity scores were calculated within each cohort using multivariable logistic regression models. Propensity scores included covariates that may affect both the likelihood of patients to receive the treatment of interest and the outcome of interest, and that were unbalanced between treatment groups before matching. These variables included a number of patient characteristics as well as markers of disease severity. Matching based on propensity scores incorporating different sets of covariates was performed using a 1:1 nearest‐neighbor algorithm, either with a caliper width of 0.25 (anticoagulation cohort) or without a caliper (aspirin cohort). In each analysis, the approach that yielded the best‐matched cohort was identified based on the most balanced distribution of propensity scores and the best balance in individual covariates between the two treatment groups.
3. RESULTS
In March 2020, our hospital system established antithrombotic guidelines for management of hospitalized COVID‐19 patients (Table S2). These guidelines recommended empiric prophylactic‐ or intermediate‐dose anticoagulation in all hospitalized COVID‐19 patients based on their D‐dimer level, which was measured once or twice daily throughout each patient's hospital admission; or therapeutic‐dose anticoagulation based on clinical suspicion for VTE. Ultimate decisions about anticoagulation dosing were left to the discretion of providers based on their assessments of individual patients.
In the initial version of the guidelines, hospitalized COVID‐19 patients with D‐dimer < 10 mg/L fibrinogen equivalent units (FEU) were recommended for prophylactic‐dose anticoagulation, while patients with D‐dimer > 10 mg/L FEU were recommended for intermediate‐dose anticoagulation. On April 13, 2020, following discussions with other peer institutions about their anticoagulation practices, the D‐dimer threshold for intermediate‐dose anticoagulation in our health system's guidelines was decreased to 5 mg/L FEU.
Early in the pandemic, studies performed at our institution and others supported a role for endotheliopathy and platelet activation in the development of severe COVID‐19. 27 , 29 Based on this, during the early phases of the pandemic, a number of providers at our institution routinely administered aspirin to COVID‐19 patients who were critically ill. On May 18, 2020, aspirin 81 mg daily was added to our hospital system's treatment guidelines as a recommendation for all hospitalized patients regardless of critical illness.
The overall study cohort consisted of 2785 patients (Table S3). Half of patients were male (50.1%; N = 1396). The majority were over 60 years old (58.4%; N = 1627). Among all patients, 13.8% (N = 383) died in the hospital; 83.7% (N = 2330) were discharged alive, while 2.6% (N = 72) remained in the hospital at the time of data abstraction. We sought to identify variables significantly associated with disease severity in COVID‐19 for use in propensity score matching. To achieve this, we performed multivariable analyses of the overall study cohort, examining associations of in‐hospital death with different variables (Table 1). We observed a novel association of low admission RI quartile with increased cumulative incidence of in‐hospital death in a model accounting for the competing risk of hospital discharge. Age > 60, male sex, obesity, and the maximum D‐dimer level during hospitalization (DDmax) were also significantly associated with in‐hospital death, in keeping with prior studies. 41 , 42
TABLE 1.
Multivariable analysis of in‐hospital death in the overall study cohort
| Cumulative incidence of in‐hospital death (competing risks model) | ||||
|---|---|---|---|---|
| HR for death | CI | p value | ||
| Age > 60 years | 3.545 | 2.599–4.836 | < .001 | |
| Male sex | 1.315 | 1.070–1.618 | .009 | |
| Obesity | 1.356 | 1.101–1.670 | .004 | |
| Cardiovascular disease | 1.014 | 0.799–1.286 | .91 | |
| African‐American | 0.850 | 0.670–1.077 | .18 | |
| DDmax | 1.040 | 1.030–1.051 | < .001 | |
| RI on admission | Quartile 1 | 6.713 | 4.860–9.274 | < .001 |
| Quartile 2 | 2.764 | 1.958–3.903 | < .001 | |
Note: Multivariable regression analysis was performed within the overall study cohort to examine the association of in‐hospital death with covariates. Cumulative incidence of in‐hospital death was evaluated in a competing risks model with hospital discharge, and hazard ratios (HR) for in‐hospital death were reported. For the maximum D‐dimer level during hospitalization (DDmax), the hazard ratio represents the effect of an increase of one fibrinogen equivalent unit.
Abbreviations: CI, 95% confidence interval; DDmax, maximum D‐dimer level during hospitalization; HR, hazard ratio; RI, Rothman Index.
3.1. Intermediate‐ versus prophylactic‐dose anticoagulation
To study the potential impact of intermediate‐ versus prophylactic‐dose anticoagulation, we created the “anticoagulation cohort”, a nested cohort of patients from the overall study cohort who were anticoagulated with either a maximum of prophylactic‐dose enoxaparin or unfractionated heparin, or a maximum of intermediate‐dose enoxaparin or unfractionated heparin (N = 1624). We then performed propensity score matching on patients in the anticoagulation cohort using a number of variables, including age, body mass index (BMI), DDmax, admission RI score, male sex, and African‐American race (Figure S1). Among all the different combinations of variables tested in patients in the anticoagulation cohort, propensity score matching with age, BMI, DDmax, admission RI score, and African‐American race achieved the most balanced distribution of covariates between patients treated with prophylactic‐ compared to intermediate‐dose anticoagulation (Table S4).
The final propensity score‐matched group of 382 patients from the anticoagulation cohort was well‐balanced between patients who received prophylactic‐ versus intermediate‐dose anticoagulation with respect to all variables analyzed except for DDmax, which was higher in patients who received intermediate‐dose anticoagulation, reflecting our hospital's treatment guidelines (Table S4). Using this group of propensity score‐matched patients, we fit a competing risks multivariable regression model adjusting for age, aspirin and antiplatelet therapy use, male sex, obesity, cardiovascular disease, African‐American race, DDmax, and admission RI. Treatment with intermediate‐ compared to prophylactic‐dose anticoagulation was associated with a significantly lower cumulative incidence of in‐hospital death on multivariable regression (HR 0.518 [0.308–0.872] (Table 2). Cumulative incidence curves also showed a significant reduction in in‐hospital death among propensity score‐matched patients in the anticoagulation cohort who were treated with intermediate‐ compared to prophylactic‐dose anticoagulation (Figure 1(A)).
TABLE 2.
Multivariable analysis of in‐hospital death in the propensity‐score matched anticoagulation cohort
| Cumulative incidence of in‐hospital death | ||||
|---|---|---|---|---|
| HR for death | CI | p value | ||
| Intermediate‐dose anticoagulation (compared to prophylactic‐dose) | 0.518 | 0.308–0.872 | .013 | |
| In‐hospital aspirin | 0.311 | 0.153–0.634 | .001 | |
| Home antiplatelet agent use prior to hospitalization | 2.663 | 1.335–5.313 | .006 | |
| Age > 60 years | 3.269 | 1.694–6.310 | < .001 | |
| Male sex | 2.255 | 1.283–3.963 | .005 | |
| Obesity | 2.096 | 1.217–3.608 | .008 | |
| Cardiovascular disease | 1.588 | 0.886–2.846 | .12 | |
| African‐American | 0.674 | 0.392–1.160 | .15 | |
| DDmax | 1.050 | 1.021–1.080 | < .001 | |
| RI on admission | Quartile 1 | 10.842 | 4.148–28.341 | < .001 |
| Quartile 2 | 6.518 | 2.394–17.751 | < .001 | |
Note: Multivariable regression analysis was performed among propensity score‐matched patients within the anticoagulation cohort to examine the association of in‐hospital death with covariates. Cumulative incidence of in‐hospital death was evaluated in a competing risks model with hospital discharge, and hazard ratios (HR) for in‐hospital death were reported. For the maximum D‐dimer level during hospitalization (DDmax), the hazard ratio represents the effect of an increase of one fibrinogen equivalent unit.
Abbreviations: CI, 95% confidence interval; DDmax, maximum D‐dimer level during hospitalization; HR, hazard ratio; RI, Rothman Index.
FIGURE 1.
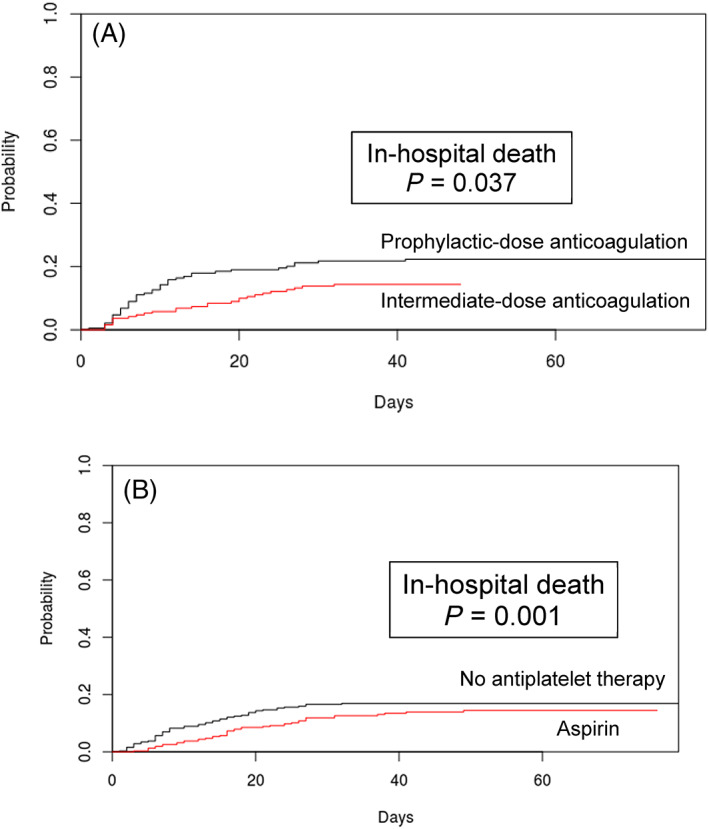
Cumulative incidence of in‐hospital death among propensity score‐matched patients (A) in the anticoagulation cohort, comparing intermediate‐ versus prophylactic‐dose anticoagulation, and (B) in the aspirin cohort admitted after May 18, 2020, comparing in‐hospital aspirin versus no antiplatelet therapy. (A) Patients in the anticoagulation cohort were propensity score matched for age, maximum D‐dimer level, admission Rothman Index score, body mass index, and African‐American race using a random number seed and a caliper width of 0.25. (B) Patients in the aspirin cohort admitted after May 18 were propensity score matched for age, maximum D‐dimer level, and admission Rothman Index score. In each panel, p values from Gray's test describe differences in cumulative incidence functions between treatment groups [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Aspirin versus no antiplatelet therapy
Next, we explored the effects of in‐hospital aspirin use. For this analysis, we established the “aspirin cohort”, a nested cohort of patients from the overall study cohort who were not on home antiplatelet therapy prior to admission and received either aspirin or no antiplatelet therapy during their hospitalization (N = 1956). Within the aspirin cohort, we performed propensity score matching for age, DDmax, admission RI, and male sex, which achieved the most balanced distribution of covariates between patients treated with in‐hospital aspirin compared to those who received no antiplatelet therapy (Figure S2; Table S5).
Using this propensity score‐matched group of 638 patients, we fit a competing risks multivariable regresson model adjusting for age, anticoagulation other than prophylactic dose, male sex, obesity, cardiovascular disease, African‐American race, DDmax, and admission RI; in addition, we included ICU admission as a covariate based on a tendency of providers at our institution to administer aspirin preferentially to critically ill patients earlier on in the pandemic, before aspirin was added onto our hospital's treatment guidelines. On multivariable analysis of propensity score‐matched patients in the aspirin cohort, the use of in‐hospital aspirin was associated with a lower cumulative incidence of in‐hospital death (HR 0.522 [0.336–0.812]) (Table 3).
TABLE 3.
Multivariable analysis of in‐hospital death in the propensity‐score matched aspirin cohort
| Cumulative incidence of in‐hospital death | ||||
|---|---|---|---|---|
| HR for death | CI | p value | ||
| In‐hospital aspirin | 0.522 | 0.336–0.812 | .004 | |
| Anticoagulation other than prophylactic‐dose (includes intermediate, therapeutic, DOAC, or other) | 2.034 | 1.016–4.074 | .045 | |
| ICU | 3.207 | 1.691–6.080 | < .001 | |
| Age > 60 years | 3.894 | 2.196–6.904 | < .001 | |
| Male sex | 1.227 | 0.777–1.938 | .38 | |
| Obesity | 1.342 | 0.873–2.063 | .18 | |
| Cardiovascular disease | 1.285 | 0.803–2.056 | .3 | |
| African‐American | 0.525 | 0.298–0.926 | .026 | |
| DDmax | 1.022 | 0.998–1.047 | .069 | |
| RI on admission | Quartile 1 | 3.333 | 1.774–6.264 | < .001 |
| Quartile 2 | 2.022 | 1.048–3.901 | .036 | |
Note: Multivariable regression analysis was performed among propensity score‐matched patients within the aspirin cohort to examine the association of in‐hospital death with covariates. Cumulative incidence of in‐hospital death was evaluated in a competing risks model with hospital discharge, and hazard ratios (HR) for in‐hospital death were reported. For the maximum D‐dimer level during hospitalization (DDmax), the hazard ratio represents the effect of an increase of one fibrinogen equivalent unit.
Abbreviations: CI, 95% confidence interval; DDmax, maximum D‐dimer level during hospitalization; DOAC, direct oral anticoagulant; ICU, intensive care unit; RI, Rothman Index.
Separately, we also analyzed outcomes of patients in the aspirin cohort who were admitted after May 18, the date on which our hospital system's antithrombotic guidelines added a recommendation to administer aspirin to all hospitalized COVID‐19 patients (Table S2). For this analysis, we applied propensity score matching for age, DDmax, and admission RI score, which together yielded the most balanced distribution of covariates between aspirin‐ and non‐aspirin‐treated patients admitted after May 18, among the different combination of variables tested (Figure S3). The final group of 140 propensity score‐matched patients was well‐balanced between aspirin‐ and non‐aspirin‐treated patients with respect to all variables except BMI (Table S6). Using this group of propensity score‐matched patients, we then fit a competing risks multivariable regression model adjusting for age, anticoagulation other than prophylactic dose, male sex, obesity, cardiovascular disease, African‐American race, DDmax, and admission RI. Once again, among patients admitted after May 18, the use of in‐hospital aspirin compared to no antiplatelet therapy was associated with a significantly lower cumulative incidence of in‐hospital death on multivariable regression (HR 0.036 [0.002–0.576] (Table S7). Cumulative incidence curves showed a significant reduction in in‐hospital death among propensity score‐matched patients in the aspirin cohort admitted after May 18 who were treated with in‐hospital aspirin compared to those who did not receive any antiplatelet therapy (Figure 1(B)).
4. DISCUSSION
In our large observational study of hospitalized COVID‐19 patients, we report, for the first time, a significantly lower cumulative incidence of in‐hospital death among patients who received intermediate‐ compared to prophylactic‐dose anticoagulation, and, separately, among patients who received in‐hospital aspirin compared to those who received no antiplatelet therapy. At present, consensus groups differ in their recommendations regarding the use of escalated‐intensity anticoagulation in COVID‐19 patients who are critically ill, highlighting the uncertainty that exists with this practice. 43 , 44 , 45 Retrospective studies of therapeutic‐ compared to prophylactic‐dose anticoagulation have reported mixed effects on mortality, while only two small studies have examined outcomes with intermediate‐ compared to prophylactic‐dose anticoagulation, largely focusing on venous thromboembolism rates, again with conflicting results. 7 , 15 , 16 , 18 , 19 , 20 , 21 , 22 , 46 , 47 More recently, the ACTIV‐4a randomized clinical trial showed efficacy of therapeutic‐ compared to prophylactic‐dose anticoagulation in non‐critically ill COVID‐19 patients but futility among critically ill patients. 23 , 24
Our analysis is the first large‐scale study to specifically examine intermediate‐ and prophylactic‐dose anticoagulation in COVID‐19. In contrast to many other studies, we utilized propensity score matching and multivariable regression analysis in order to diminish treatment selection bias by generating treatment and control groups with well‐balanced covariates, thereby allowing for a more reliable comparison of potential treatment effects. 32 Our findings suggest that there may be a beneficial role for intermediate‐dose anticoagulation in the treatment of hospitalized COVID‐19 patients, although we await the results of several randomized controlled trials to definitively address this question.
At present, no consensus guidelines are available regarding aspirin use in COVID‐19, reflecting a paucity of data in this regard. A biological rationale to support the use of aspirin in COVID‐19 may reside in the treatment of other microvascular thrombotic diseases such as thrombotic thrombocytopenic purpura, where antiplatelet agents may have a role. 48 Recently, one other retrospective study reported improved in‐hospital mortality in COVID‐19 patients who received aspirin within one week before or 24 h after admission. 30 Despite drawing similar conclusions, our study adopted a more rigorous methodological approach through the use of propensity score matching to account for differences in illness severity among patients, enabling us to more accurately compare different treatment effects. In addition, we excluded patients on home aspirin in order to minimize confounding effects from underlying cardiovascular disease. Presently, randomized controlled trials are underway to definitively address the question of whether aspirin may have an impact on outcomes in hospitalized COVID‐19 patients.
Our analysis also reveals a novel role for the admission RI as a prognostic tool for evaluating the risk of in‐hospital mortality in COVID‐19. The RI, which synthesizes multiple clinical, laboratory, and nursing assessment variables into a single score, has been shown to have predictive value for assessing mortality and readmission rates in some critical care and surgical studies, although its applicability to COVID‐19 has not been previously tested. 35 , 36 , 37 In our hospital system, the RI is calculated automatically by our electronic health record system upon admission and hourly throughout a patient's hospitalization, rendering it readily accessible to enable its use in real‐time clinical decision‐making. 37 Additional studies are warranted to further explore the potential role for the RI in assessing disease severity and guiding clinical interventions in COVID‐19.
Our study has several limitations, the most important being its retrospective nature. Overall provider adherence to our institution's COVID‐19 treatment guidelines was subject to provider preference, although many of the confounding factors arising from such bias would have been accounted for through our use of propensity score matching. Unobserved characteristics linked with patient outcomes may have introduced additional confounding variables despite our use of multivariable regression analysis. Heterogeneity in the number of doses of intermediate‐dose anticoagulation or aspirin that each patient received during their hospitalization may have biased our analysis against the detection of some significant associations by including patients in the intervention group who received limited exposure to the intervention. A possible improvement in clinical outcomes of hospitalized patients with COVID‐19 over time could have biased some of our findings, although in our analysis of patients in the aspirin cohort admitted after May 18, a significant reduction in in‐hospital mortality with aspirin use was still observed despite the later, shortened timeframe of the specific study population analyzed. We did not examine VTE rates, as only a small percentage of patients in our hospital system underwent VTE‐specific imaging in order to limit excess healthcare worker exposure to COVID‐19. The use of other therapies with potential disease‐modifying effects outside of those included in our analysis, such as remdesivir or dexamethasone, may also have impacted clinical outcomes, although during the three‐month span of our study, which represented the early phase of the pandemic, only a minority of patients in our overall study cohort would have been expected to receive either of these two therapies.
In summary, in our large, observational study of hospitalized patients with COVID‐19, using propensity score matching and multivariable regression analyses, we observed a mortality benefit with intermediate‐ compared to prophylactic‐dose anticoagulation and, separately, with in‐hospital aspirin compared to no antiplatelet therapy. Our findings suggest that increased‐intensity anticoagulation and antiplatelet therapy may be beneficial in the treatment of COVID‐19. We await the results of several randomized clinical trials to more definitively elucidate the impact of these therapies in COVID‐19.
AUTHOR CONTRIBUTIONS
G.G., M.L.M., Yiwen Liu, R.F., D.S.N., K.A.O., and A.I.L. designed the study. M.L.M., R.F., K.A., E.C., N.D., C.K., and Yuxin Liu performed chart abstractions. M.M., D.M., S.Y.W., C.P., R.D.B., C.I.O.C., H.J.C., J.M.S., and A.B.P. contributed valuable ideas. M.L.M., G.G., Yiwen Liu, R.F., D.S.N., K.A.O., and A.I.L. wrote the manuscript, and all authors participated in editing the manuscript. M.L.M., G.G., Yiwen Liu, and R.F. contributed equally as first authors. D.S.N., K.A.O., and A.I.L. contributed equally as senior investigators.
CONFLICT OF INTEREST
No conflict of interest exists for any author on this manuscript. This work was supported by a gift donation from Jack Levin and a separate anonymous donation to the Benign Hematology program at Yale, the DeLuca Foundation to fund hematology research at Yale, and the National Institutes of Health (grant HL142818 to H.J.C., and GM136651 and HL139116 to M.L.M.).
ETHICS APPROVAL STATEMENT
This study was approved by the Yale Institutional Review Board (HIC 2000027792).
PATIENT CONSENT STATEMENT
Patient consent was not mandated for this study. Permission to reproduce material from other sources: No material from other sources is included in this study.
CLINICAL TRIAL REGISTRATION
This study was not a clinical trial.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank all providers, health care workers, and staff for their tireless dedication to the care of patients with COVID‐19. We dedicate this work to all individuals afflicted by the pandemic. This study was presented at an Oral Session at the 62nd Annual Meeting of the American Society of Hematology (ASH) and was highlighted at a “Best of ASH” session hosted by the Hemostasis and Thrombosis Research Society. This work was supported by a gift donation from Jack Levin and a separate anonymous donation to the Benign Hematology program at Yale, the DeLuca Foundation to fund hematology research at Yale, and the National Institutes of Health (grant HL142818 to H.J.C., and GM136651 and HL139116 to M.L.M.).
Meizlish ML, Goshua G, Liu Y, et al. Intermediate‐dose anticoagulation, aspirin, and in‐hospital mortality in COVID‐19: A propensity score‐matched analysis. Am J Hematol. 2021;96:471–479. 10.1002/ajh.26102
Matthew L. Meizlish, George Goshua, Yiwen Liu and Rebecca Fine contributed equally as first authors.
Donna S. Neuberg, Kent A. Owusu and Alfred Ian Lee contributed equally as senior authors.
Funding information National Institutes of Health, Grant/Award Numbers: HL139116, GM136651, HL142818; Hemostasis and Thrombosis Research Society; American Society of Hematology
Contributor Information
Donna S. Neuberg, Email: neuberg@ds.dfci.harvard.edu.
Kent A. Owusu, Email: kent.owusu@ynhh.org.
Alfred Ian Lee, Email: alfred.lee@yale.edu.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Al‐Samkari H, Karp Leaf RS, Dzik WH, et al. COVID‐19 and coagulation: bleeding and thrombotic manifestations of SARS‐CoV‐2 infection. Blood. 2020;136:489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kashi M, Jacquin A, Dakhil B, et al. Severe arterial thrombosis associated with Covid‐19 infection. Thromb Res. 2020;192:75‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID‐19) vs patients with influenza. JAMA Neurol. 2020;77:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post‐mortem findings in a series of COVID‐19 cases from northern Italy: a two‐Centre descriptive study. Lancet Infect Dis. 2020;20:1135‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4:1178‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trigonis RA, Holt DB, Yuan R, et al. Incidence of venous thromboembolism in critically ill coronavirus disease 2019 patients receiving prophylactic anticoagulation. Crit Care Med. 2020;48:e805‐e808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrett CD, Moore HB, Yaffe MB, Moore EE. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19: a comment. J Thromb Haemost. 2020;18:2060‐2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033‐2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrandis R, Llau JV, Quintana M, et al. COVID‐19: opening a new paradigm in thromboprophylaxis for critically ill patients? Crit Care. 2020;24:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adam EH, Zacharowski K, Miesbach W. A comprehensive assessment of the coagulation profile in critically ill COVID‐19 patients. Thromb Res. 2020;194:42‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chowdhury JF, Moores LK, Connors JM. Anticoagulation in hospitalized patients with Covid‐19. N Engl J Med. 2020;383:1675‐1678. [DOI] [PubMed] [Google Scholar]
- 15. Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID‐19. J Am Coll Cardiol. 2020;76:1815‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paranjpe I, Fuster V, Lala A, et al. Association of Treatment Dose Anticoagulation with in‐Hospital Survival among Hospitalized Patients with COVID‐19. J Am Coll Cardiol. 2020;76:122‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferguson J, Volk S, Vondracek T, Flanigan J, Chernaik A. Empiric therapeutic anticoagulation and mortality in critically ill patients with respiratory failure from SARS‐CoV‐2: a retrospective cohort study. J Clin Pharmacol. 2020;60:1411‐1415. [DOI] [PubMed] [Google Scholar]
- 19. Pesavento R, Ceccato D, Pasquetto G, et al. The hazard of (sub)therapeutic doses of anticoagulants in non‐critically ill patients with Covid‐19: the Padua province experience. J Thromb Haemost. 2020;18:2629‐2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ionescu FJI, Nair GB, Konde AS, et al. Increasing doses of anticoagulation are associated with improved survival in hospitalized COVID‐19 patients [abstract]. Blood. 2020;136:22. [Google Scholar]
- 21. Al‐Samkari HGS, Karp Leaf R, Wang W, et al. Thrombosis, bleeding, and the effect of anticoagulation on survival in critically ill patients with COVID‐19 in the United States. Res Pract Thromb Haemost. 2020. 10.7326/M20-6739. [DOI] [Google Scholar]
- 22. Ho GD, Dusendang JR, Schmittdiel J, Kavecansky J, Tavakoli J, Pai A. Anticoagulant and antiplatelet use not associated with improvement in severe outcomes in COVID‐19 patients [abstract]. Blood. 2020;136:59. [Google Scholar]
- 23.NIH ACTIV Trial of blood thinners pauses enrollment of critically ill COVID‐19 patients. NIH News Releases https://www.nih.gov/news‐events/news‐releases/nih‐activ‐trial‐blood‐thinners‐pauses‐enrollment‐critically‐ill‐covid‐19‐patients.
- 24.Full‐dose blood thinners decreased need for life support and improved outcome in hospitalized COVID‐19 patients. NHLBI News Statement. https://www.nhlbi.nih.gov/news/2021/full‐dose‐blood‐thinners‐decreased‐need‐life‐support‐and‐improved‐outcome‐hospitalized.
- 25. Nakazawa D, Ishizu A. Immunothrombosis in severe COVID‐19. EBioMedicine. 2020;59:102942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowenstein CJ, Solomon SD. Severe COVID‐19 is a microvascular disease. Circulation. 2020;142:1609‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID‐19‐associated coagulopathy: evidence from a single‐centre, cross‐sectional study. Lancet Haematol. 2020;7:e575‐e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hottz ED, Azevedo‐Quintanilha IG, Palhinha L, et al. Platelet activation and platelet‐monocyte aggregate formation trigger tissue factor expression in patients with severe COVID‐19. Blood. 2020;136:1330‐1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID‐19. Blood. 2020;136:1317‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chow JH, Khanna AK, Kethireddy S, et al. Aspirin use is associated with decreased mechanical ventilation, ICU admission, and in‐hospital mortality in hospitalized patients with COVID‐19. Anesth Analg. 2020. 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 31. Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sturmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275:570‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid‐19. N Engl J Med. 2020;382:2411‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382:2441‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alarhayem AQ, Muir MT, Jenkins DJ, et al. Application of electronic medical record‐derived analytics in critical care: Rothman index predicts mortality and readmissions in surgical intensive care unit patients. J Trauma Acute Care Surg. 2019;86:635‐641. [DOI] [PubMed] [Google Scholar]
- 36. Wengerter BC, Pei KY, Asuzu D, Davis KA. Rothman index variability predicts clinical deterioration and rapid response activation. Am J Surg. 2018;215:37‐41. [DOI] [PubMed] [Google Scholar]
- 37. Rothman MJ, Rothman SI, Beals J. Development and validation of a continuous measure of patient condition using the electronic medical record. J Biomed Inform. 2013;46:837‐848. [DOI] [PubMed] [Google Scholar]
- 38.Defining Adult Overweight and Obesity. Centers for Disease Control and Prevention https://wwwcdcgov/obesity/adult/defininghtml.
- 39. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 40. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141‐1154. [Google Scholar]
- 41. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18:1324‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158:1143‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18:1859‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taccone FS, Gevenois PA, Peluso L, et al. Higher intensity Thromboprophylaxis regimens and pulmonary embolism in critically ill coronavirus disease 2019 patients. Crit Care Med. 2020;48:e1087‐e1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blombery P, Scully M. Management of thrombotic thrombocytopenic purpura: current perspectives. J Blood Med. 2014;5:15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


