Abstract
A 31‐year‐old Caucasian male developed reinfection with SARS‐CoV‐2, 2 ½ months after an initial episode of ICU admission for respiratory support due to COVID‐19. The second episode was in the form of malaise, aphthous gingival ulcer, and desquamating palmar lesion.
Keywords: coronavirus diseases 19, COVID‐19, pandemic, recurrence, SARS‐CoV‐2, symptomatology
A 31‐year‐old Caucasian male patient developed reinfection with SARS‐CoV‐2, 2 ½ months after an initial episode of ICU admission for respiratory support due to COVID‐19. The second episode was in the form of malaise, aphthous gingival ulcer, and desquamating palmar lesion.
![]()
1. INTRODUCTION
Reinfection with the severe acute respiratory syndrome novel coronavirus 2 (SARS‐CoV‐2) has been increasingly reported. However, no case report has described a different manifestation in the second episode from the initial one. Patient is a 31‐year‐old Caucasian male with a history of infection with SARS‐CoV‐2 resulting in admission to the intensive care unit for respiratory support due to coronavirus disease 19 (COVID‐19). Seventy‐nine days following discharge from the hospital, he developed malaise, aphthous gingival ulcer, and desquamating palmar lesion. He was confirmed, through a nasal swab PCR, to be reinfected with SARS‐CoV‐2. His recovery from reinfection was uneventful with no need to inpatient care and smooth return to daily routine. Despite controversy on the lasting immunity by the neutralizing antibody against SARS‐CoV‐2, reports of recurrent COVID‐19 are increasingly published with variable time intervals and presentations. This case report encourages the clinicians to remain alert about the potential risk of reinfection with SARS‐CoV‐2, even with a different symptomatology, while the vaccination efforts are soaring globally.
The pandemic of coronavirus diseases 19 (COVID‐19), caused by the severe acute respiratory syndrome novel virus 2 (SARS‐CoV‐2), continues despite initiation of the global vaccination. 1 Besides its exhausting length and overwhelming burden, the increasing number of patients with SARS‐CoV‐2 reinfection is concerning. 2 , 3 , 4 A systematic review of the literature estimated the incidence rate of the recurrent SARS‑CoV‑2 positivity to be 14.8%. 5
The majority of the case reports have described a more severe clinical presentation for reinfection with SARS‐CoV‐2 compared with the initial encounter. However, to the best of our knowledge, there is no report to describe a case of reinfection with a different clinical presentation from the initial episode.
2. CASE PRESENTATION
Patient is a 31‐year‐old Caucasian male, who serves as resident physician in a university hospital. He was diagnosed with a laboratory‐confirmed COVID‐19 in July 25, 2020. The patient initially presented with a 3‐day history of fever (oral temperature of 99.8°F), malaise, cough, shortness of breath, anosmia, and a dropped O2 saturation to 88% on room air. A computerized tomography (CT) of the chest showed bilateral ground‐glass opacity of the lungs (Figure 1). He was admitted to the intensive care unit (ICU) and received supportive treatment with supplemental oxygen 3‐4 liter/minute, hydroxy chloroquine tablet 200 mg twice a day, and dexamethasone vial, 6 mg intravenous daily. He recovered uneventfully in 1 week with an O2 saturation of 94% and resumed his daily routine gradually over the course of weeks. A follow‐up testing with polymerase chain reaction (PCR) on the nasal swab was negative for SARS‐CoV‐2, 2 weeks after discharge from the hospital.
FIGURE 1.

Chest CT scan showing diffuse bilateral ground‐glass opacities with preference in the lower lungs with foci of alveolar turbidity, suggestive for COVID‐19
In October 12, 2020 (79 days after the initial encounter), the patient developed malaise followed by painful submandibular lymphadenopathy and gingival aphthous ulcers (Figure 2). Two days later, he developed fever (oral temperature: 99.8°F) and myalgia. A PCR test of the nasopharyngeal swab was positive for COVID‐19, and the patient quarantined himself taking naproxen tablet 250 mg every 12 hours for 4 days. As he did not have any shortness of breath, he did not seek any medical care, and hence, no chest imaging was obtained. Although his symptoms improved over the next 3 days, he developed skin desquamation of the palms and fingers (Figure 3). The skin changes improved swiftly over the course of a week as did his other symptoms. The patient did not require any other medication. Follow‐up visit at 1 month and 6 months revealed complete resolution of the symptoms and return to daily routine.
FIGURE 2.
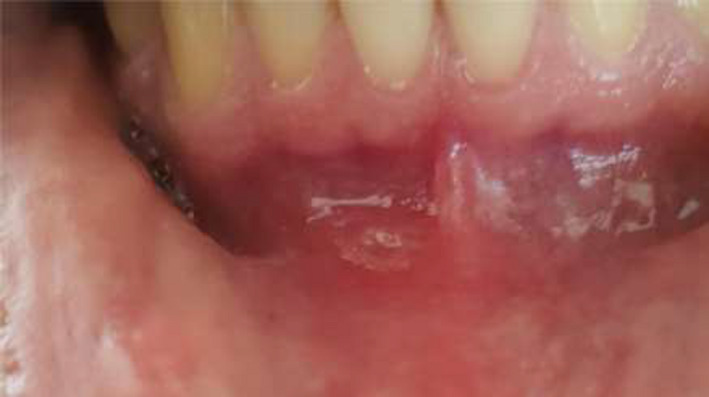
Ulcerative aphthous in lower gingiva during the second episode of COVID‐19
FIGURE 3.
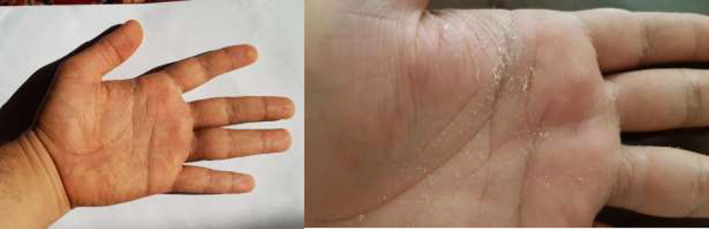
Skin desquamation of the palms and fingers during the recovery period in a patient with COVID‐19 recurrence
3. DISCUSSION
While our knowledge regarding the risk factors for COVID‐19 recurrence and other associated parameters is evolving, data are pointing out to the temporary immunity of anti–SARS‐CoV‐2 antibodies in unvaccinated individuals 6 and the emergence of viral escape mutants as potential mechanisms for recurrent cases. 7 Our case developed a reinfection with SARS‐CoV‐2 after an initial episode of severe disease and a 2‐month disease‐free interval. The second episode was significantly milder requiring no inpatient medical care but relatively different in presentation from the initial episode. In a surveillance study at the Oxford University Hospitals in the United Kingdom, Lumley et al measured anti‐spike and anti‐nucleocapsid IgG antibodies in 12 541 healthcare workers and followed them for a period of 31 weeks. 6 The authors found that out of 1265 seropositive cases, 88 had developed seroconversion during the follow‐up period. On the other hand, 223 seronegative subjects developed a positive PCR test (1.09 per 10 000 days at risk) of whom 44.84% were asymptomatic and 51.6% were symptomatic. This was significantly different from the only 2 seropositive cases, who became PCR‐positive during the follow‐up period (0.13 per 10 000 days at risk). While the anti–SARS‐CoV‐2 antibodies rendered, on average, an immunity against reinfection for a duration of 6 months, our case report along with others raises questions about the generalizability of such a short‐term protection. 4 , 5 However, none of these case reports have monitored the evolution of neutralizing antibodies or their titers against SARS‐CoV‐2 from the initial infection to the time of reinfection.
A genomic analysis of SARS‐CoV‐2 at two different times from a 25‐year‐old man from Washoe, Nevada, revealed genetically significant differences between the two species. 4 Unlike our patients, the second episode was more severe in terms of clinical symptomatology. Further case reports have also shown a declining antibody titer to coincide the reinfection with SARS‐CoV‐2. 2 , 3 , 8 The case report from Hong Kong showed that an initial mild infection with SARS‐CoV‐2 did not produce any effective neutralizing antibody, which 5 months later, although completely asymptomatic, resulted in reinfection with the virus. 2 Another case report from the Netherlands showed a more severe presentation of COVID‐19 recurrence compared with the index episode. 3 Although the latter patient was immunocompromised due to a recent B‐cell–depleting chemotherapy for Waldenström's macroglobulinemia, an effective innate immunity or T‐cell response might have acted as a savior. The same path can be imagined for the case report from Hong Kong in whom no effective neutralizing antibody was detected in either of the episodes. Unfortunately, our current laboratory setting did not permit measuring anti–SARS‐CoV‐2 serum antibody titers from the index infection to the recurrence of COVID‐19, nor did it allow the genomic analysis of the causative agents in these two different episodes.
The time interval between the initial infection with SARS‐CoV‐2 and the second episode has been variably reported in the literature. 3 , 4 , 5 , 8 While the duration of immunity rendered by anti‐spike or anti‐nucleocapsid IgG antibodies has been shown to be for a minimum of 6 months, a systematic review of the reported cases of reinfection with SARS‐CoV‐2 has estimated this interval to be 35.4 days. 5 The review has also found that a younger age and a longer time to become PCR‐negative are significantly associated with a higher risk of reinfection with SARS‐CoV‐2, while a severe disease might play a protective role.
Our case report supports the growing doubt about a lasting immunity against SARS‐CoV‐2. Although our patient presented differently in the second episode from the initial one, the clinical manifestation was clinically less severe. The time from initial infection to the recurrent episode was above the average time, which is reported in the literature. However, we could not examine the evolution of neutralizing antibody over this time interval as the titer was not measured in our case. While the current endeavors in global vaccination against SARS‐CoV‐2 are ongoing, clinicians should remain alert about variation in individuals' response to the infection and the potential risk of reinfection despite receiving the vaccine. This is especially important when we are reading the news about the emerging new variants of the SARS‐CoV‐2, which seem to be more contagious. 9
CONFLICT OF INTEREST
The authors have no conflict of interest to declare in any forms.
AUTHORS' CONTRIBUTION
SS: performed a literature review, developed the study structure, drafted the manuscript, and approved its final version for the intellectual content. SK: developed the study idea, performed a literature review, participated in drafting the manuscript, and approved its final version for the intellectual content. ET: participated in developing the study conception, collecting the patient data, and drafting the manuscript, and approved its final version for the intellectual content. SK: participated in developing the study conception, collecting the patient data, and drafting the manuscript, and approved its final version for the intellectual content. MN: participated in developing the study conception and revising the manuscript, and approved its final version for the intellectual content. NS: participated in collecting the patient data and revising the manuscript, and approved its final version for the intellectual content.
ETHICAL APPROVAL
We obtained patient consent to publish his clinical case report in an unidentified manner and without disclosing his personal information.
Shoar S, Khavandi S, Tabibzadeh E, Khavandi S, Naderan M, Shoar N. Recurrent coronavirus diseases 19 (COVID‐19): A different presentation from the first episode. Clin Case Rep. 2021;9:2149–2152. 10.1002/ccr3.3967
DATA AVAILABILITY STATEMENT
This study does not have any data repository or outsource to be accessed.
REFERENCES
- 1. Yeoh DK, Foley DA, Minney‐Smith CA, et al. The impact of COVID‐19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2020:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan PKS, Lui G, Hachim A, et al. Serologic Responses in Healthy Adult with SARS‐CoV‐2 Reinfection, Hong Kong, August 2020. Emerg Infect Dis. 2020;26(12):3076‐3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mulder M, van der Vegt D, Oude Munnink BB, et al. Reinfection of SARS‐CoV‐2 in an immunocompromised patient: a case report. Clin Infect Dis. 2020:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tillett RL, Sevinsky JR, Hartley PD, et al. Genomic evidence for reinfection with SARS‐CoV‐2: a case study. Lancet Infect Dis. 2020;21:52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azam M, Sulistiana R, Ratnawati M, et al. Recurrent SARS‐CoV‐2 RNA positivity after COVID‐19: a systematic review and meta‐analysis. Sci Rep. 2020;10(1):20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS‐CoV‐2 infection in health care workers. N Engl J Med. 2020;384(6):533‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeFrancesco L. COVID‐19 antibodies on trial. Nat Biotechnol. 2020;38(11):1242‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. To KK, Hung IF, Ip JD, et al. COVID‐19 re‐infection by a phylogenetically distinct SARS‐coronavirus‐2 strain confirmed by whole genome sequencing. Clin Infect Dis. 2020:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. New Covid‐19 Variants Centers for Disease Control and Prevention . https://www.cdc.gov/coronavirus/2019‐ncov/transmission/variant.html. Published 2021. Accessed March 1, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study does not have any data repository or outsource to be accessed.


