Abstract
Background
The efficacy of convalescent plasma (CP), an alternative for the treatment of COVID‐19, depends on high titers of neutralizing antibodies (nAbs), but assays for quantifying nAbs are not widely available. Our goal was to develop a strategy to predict high titers of nAbs based on the results of anti‐SARS‐CoV‐2 immunoassays and the clinical characteristics of CP donors.
Study Design and Methods
A total of 214 CP donors were enrolled and tested for the presence of anti‐SARS‐CoV‐2 antibodies (IgG) using two commercial immunoassays: EUROIMMUN (ELISA) and Abbott (Chemiluminescence). Quantification of nAbs was performed using the Cytopathic Effect‐based Virus Neutralization test. Three criteria for identifying donors with nAbs ≥ 1:160 were tested: – C1: Curve ROC; − C2: Conditional decision tree considering only the IA results and – C3: Conditional decision tree including both the IA results and the clinical variables.
Results
The performance of the immunoassays was similar referring to both S/CO and predictive value for identifying nAbs titers ≥1:160. Regarding the studied criteria for identifying CP donors with high nAbs titers: (a) C1 showed 76.1% accuracy if S/CO = 4.65, (b) C2 presented 76.1% accuracy if S/CO ≥4.57 and (c) C3 had 71.6% accuracy if S/CO was ≥4.57 or if S/CO was between 2.68‐4.57 and the last COVID‐19‐related symptoms were recent (within 19 days).
Conclusion
SARS‐CoV‐2 IgG immunoassays (S/CO) can be used to predict high anti‐SARS‐CoV‐2 nAbs titers. This study has proposed different criteria for identifying donors with ≥1:160 nAbs titers, all with high efficacy.
1. INTRODUCTION
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) represents an unprecedented challenge for the population, health workers, and government all over the world, becoming a global public health emergency with growing impact on the global economy. On March 11, 2020, the World Health Organization (WHO) declared SARS‐CoV‐2 a pandemic. As of August 23, 2020, SARS‐CoV‐2 infection reached about 23 million confirmed cases worldwide in more than 213 countries and caused more than 800 000 deaths (https://covid19.who.int). To date, no specific treatment has proved to be effective for SARS‐CoV‐2 infection, besides supportive care.
Passive antibody therapy with convalescent plasma (CP), a classic adaptive immunotherapy, has been applied to the prevention and treatment of many infectious diseases over many decades, from A/H1N1 Spanish Flu in 1917‐1918 to SARS in 2012. 1 The efficacy of passive antibody therapy has been associated with the concentration of neutralizing antibodies (nAbs) in plasma of recovered patients. 2 CP from patients who have recovered from viral infection can be used to improve clinical conditions and survival rate of patients with acute viral infections, including SARS‐CoV‐2, without severe adverse effects. Preliminary data showed a reduction of viral load, shorter hospital stay, and lower mortality in patients infected by SARS‐CoV‐2 treated with CP in comparison to those who were not. 3 , 4 , 5 , 6 , 7 , 8
Possible mechanisms related to the efficacy of CP therapy in SARS‐CoV‐2 include the passive transfusion of neutralizing antibodies and an immunomodulatory effect via amelioration of severe inflammatory response. 9 , 10 Patients infected with SARS‐CoV‐2 usually develop a primary immune response by days 10‐14, which is followed by virus clearance. 11 Therefore, theoretically, it should be more effective to administer the CP at the early stage of disease. A recent matched study suggested that non‐intubated patients may benefit more than those requiring mechanical ventilation. 12 However, other treatments might influence the relationship between CP and antibody level, including antiviral drugs, steroids, and intravenous immunoglobulin.
We are conducting a prospective randomized trial to evaluate the efficacy of CP for patients with moderate to severe SARS‐CoV‐2 disease. Convalescent donors have been recruited from the community. Pre‐requisite for plasma donation include age (>18 years old); no previous pregnancy; time elapsed from the last day of symptoms (>14 days); laboratorial evidence of prior infection by SARS‐CoV‐2; and screening negative for infectious diseases transmissible for blood (HIV 1+2, HTLV 1+2, hepatitis B and C, syphilis, and Chagas Disease). Moreover, we also have evaluated the level of neutralizing antibodies (nAbs) and the absence of RNAemia in a blood sample before plasma collection. As nAbs play important roles in virus clearance and have been considered as a key immune product for treatment against viral diseases, in concordance with Food and Drug Administration (FDA), we established that CP units for transfusion should contain nAbs with minimum titer of ≥1:160 (https://www.fda.gov/media/136798/download).
However, neutralization assays for SARS‐CoV‐2 are limited in availability and throughput, requiring biosafety level 3 facilities and skilled labor. Since such assay is often unavailable, one alternative is to perform the test later in a stored sample, or to perform another test to detect the presence of anti‐SARS‐CoV‐2 antibody prior to issuing the plasma unit for transfusion.
The correlation between immunoassays antibodies titers and neutralizing antibodies has not been thoroughly investigated and the knowledge of this association can help to make better therapeutic decisions.
The aim of this study is to evaluate the performance of criteria based on the results of anti‐SARS‐CoV‐2 immunoassays for the prediction of high nAbs titers in CP donors.
2. MATERIALS AND METHODS
2.1. Cohort recruiting
Two hundred sixty‐three convalescent individuals were evaluated in April 2020 for convalescent plasma donation by apheresis. The SARS‐Cov‐2
infection was previously confirmed by Real Time Reverse Transcription‐Polymerase Chain Reaction (RT‐PCR) of material collected from the upper
respiratory tract (nasopharynx or oropharynx). All candidates provided written informed consent and tested negative for SARS‐CoV‐2 by RT‐PCR. Blood samples were collected from all participants for performing the SARS‐CoV‐2 IgG immunoassay and blood RT‐PCR. Two hundred fourteen were tested for neutralizing antibodies.
2.2. Immunoglobulin G (IgG) immunoassays
Two commercial immunoassays comprising the structural protein of SARS‐CoV‐2 (S1 domain) were tested in parallel with all collected samples: Anti‐SARS‐CoV‐2 ELISA IgG EUROIMMUN (Lübeck, Germany) and Anti‐SARS‐CoV‐2 Chemiluminescence IgG Abbott (Chicago, US). Tests were performed in accordance with the manufacturer's instructions. The cutoff values for positive results were 1.1 and 1.4 for Euroimmun and Abbott assays, respectively.
2.3. Quantitative reverse‐transcriptase polymerase chain reaction (RT‐qPCR)
Blood samples with DO/CO ≥3 on the IgG immunoassay were subjected to SARS‐CoV‐2 RT‐qPCR using TaqMan method. A quantitative in house real‐time PCR assay amplifying the virus RdRp RNA‐dependent RNA polymerase and envelope was applied to determine the copy number of SARS‐CoV‐2. 13 The test had sensitivity of approximately 100 copies/mL. In all amplification reactions, positive and negative controls and an exogenous internal control were used.
2.4. Cytopathic effect‐based virus neutralization test (CPE‐VNT)
Two hundred fourteen samples were tested for neutralizing antibodies (nAbs) using the cytopathic effect‐based virus neutralization test (CPE‐VNT). The CPE‐VNT was adapted from Nurtop et al., 2018 14 and has already been described in Wendel et al., 2020. 15 Briefly, 5 × 104 cells/mL of Vero cells (ATCC CCL‐81) were seeded 24 hours before the infection in a 96‐well plate. Plasma samples were initially inactivated for 30 minutes at 56°C. We used 8 dilutions (2‐fold) of each plasma (1:20 to 1:2560). Subsequently, plasma was mixed vol/vol with 103 TCID50/mL of SARS‐CoV‐2/human/BRA/SP02cc/2020 strain virus (GenBanK access number: MT350282.1) 16 and pre‐incubated at 37°C for 1 hour to allow virus neutralization. Then, the plasma plus virus mixture was transferred onto the confluent cell monolayer and incubated for 3 days at 37°C, under 5% CO2. Virus neutralization titer referred to VNT100 is described as the highest dilution of serum that neutralized virus growth (absence of cytopathic effect). In each assay, a strong, assured internal positive control serum (RT‐qPCR positive + PRNT90 > 640) 17 was used, as well as a negative pre‐outbreak serum sample. All the procedures related to CPE‐VNT were performed in a biosafety level 3 laboratory, in accordance with WHO recommendations. 18
2.5. Statistical analysis
A descriptive analysis was carried out using frequencies, central tendency, and position measures. Mann‐Whitney and Kruskall‐Wallis non‐parametric tests were used to compare nAbs values in different groups and Bonferroni post‐hoc method was applied to adjust results for multiple comparisons. The variables age and days since last symptom were tested according to groups from tertiles of the distribution values.
Simple linear regression models were used to assess the relationship between ELISA S/CO values and the concentration of nAbs titers. The predictive value of immunoassay tests (Abbott and Euroimmun) for the identification of nAbs ≥160 was assessed using ROC curve graphs. 19 Then, the sensitivity, specificity, predictive values and accuracy of four cut‐off points obtained by different methods were calculated: (a) The Youden's index method which maximizes the sum between Sensitivity and Specificity; (b) The “Maximum Efficiency” method which is based on the maximization of the frequency of cases correctly classified (true positives or true negatives); (c) The “PROC01” method which is the point on the ROC curve closest to the point (0,1) or upper left corner of the graph; (d) a last method which established a fixed value for sensitivity (= 90%) and sought to maximize specificity. 19 , 20
To validate the proposed donation criteria, the total sample studied was divided into two parts (development sample and validation sample) according to the study enrollment date. For this analysis, the initial sample of 214 donors was divided into tertiles according to the date of the enrollment. The first two thirds of sample (development sample) were used to develop the criteria, while the last third of the sample (validation sample) was used to assess the performance of the proposed criteria. Three different criteria have been developed; (1) the first criterion established a S/CO cut‐off point of 4.65 based on the Youden's index method; (2) the second criterion was established based on the results of a simple conditional decision tree model using only the result of the ELISA test as an explanatory variable; and (3) the third criterion was established based on the results of a multivariate conditional decision tree model using the ELISA test result and the following variables: age, sex, need for hospitalization for treatment of SARS‐CoV‐2, and the time elapsed since the end of symptoms. All analyzes were performed in the R environment using RStudio software. 19 , 20
3. RESULTS
3.1. Studied population of donors
There were 263 potential CP donors evaluated, of whom 49 were excluded from the analysis either because nAbs titers were lacking (n = 35) or because the evaluation was performed less than 10 days after the symptom resolution (n = 14) (Figure 1 Supplementary Material, Figure S1).
FIGURE 1.
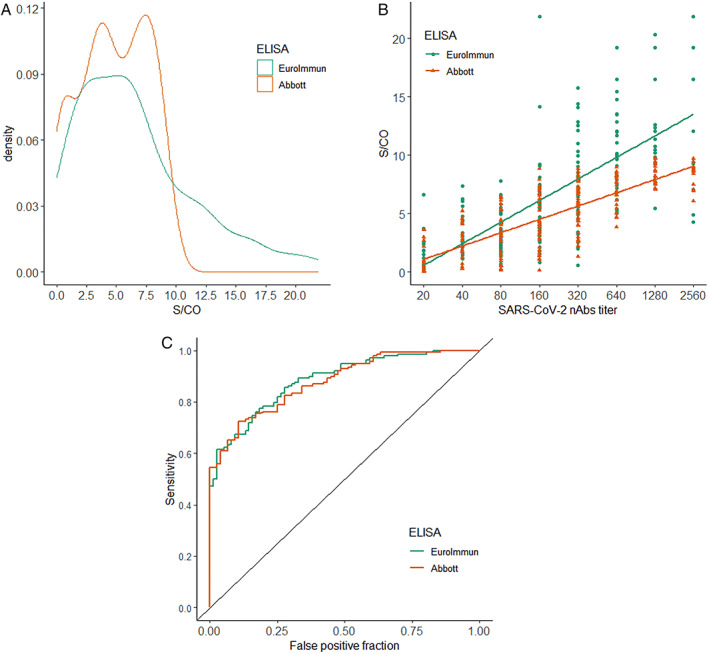
Comparison of the performance of the two studied immunoassay tests (Euroimmun and Abbott). A, Distribution density of S/CO values obtained from two immunoassays tests (n = 214); B, correlation of nAbs titers and S/CO values obtained from the two immunoassays methods; C, ROC curves for identifying nAbs titers ≥1:160 [Color figure can be viewed at wileyonlinelibrary.com]
Table 1 shows the descriptive data of the analyzed sample. Most donors were male (57.9%) and young (median age was 35 years/IQR = 15). The two most common clinical comorbidities were systemic arterial hypertension (8.7%) and pulmonary disease (6.5%).
TABLE 1.
Descriptive data of the studied cohort of donors (n=214)
| Male, n (%) | 124 (57.9) |
| Age years, median (IQR) | 35 (30‐45) |
| Hospitalization, n (%) | 15 (7.0) |
| Duration of symptoms (days), median (IQR) | 11 (7‐14) |
| Symptoms onset – Enrollment (days), median (IQR) | 31 (27‐39) |
| End of symptoms – Enrollment (days), median (IQR) | 20 (17‐26) |
| Comorbidities, n (%) | |
| Hypertension | 18 (8.4) |
| Diabetes mellitus | 2 (0.9) |
| Pulmonary disease | 14 (6.5) |
| Cardiac disease | 1 (0.5) |
| Tobacco use | 7 (3.3) |
Abbreviation: IQR, Interquartile range.
3.2. Correlation between nAbs titers and clinical/demographics factors
The titers of nAbs of the studied sample varied widely. Approximately 1 in each 5 donors (19.1%) had nAbs titers <1:80 (Figure 2 Supplementary Material, Figure S2). Titers were significantly higher among: (a) men (difference of median = 160 nAbs, P < .001), (b) individuals in the upper tertile of age (difference of median to lower tertile = 160 nAbs, P = .003), and (c) individuals who needed hospitalization to treat SARS‐CoV‐2 (difference of median = 1120 nAbs, P < .001). Donors with a shorter time between the end of symptoms and the enrollment had slightly higher nAbs titer, but without statistical significance (P = .067) (Figure 3 Supplementary Material, Figure S3).
FIGURE 2.
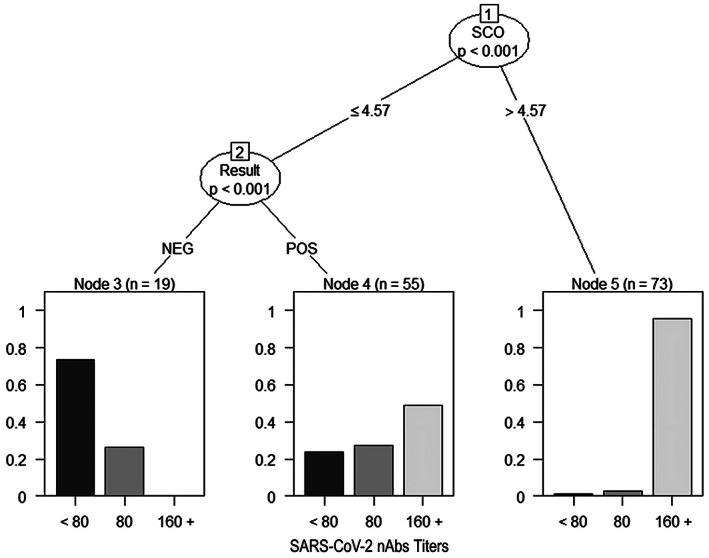
Conditional decision tree of criterion 1 for the prediction of high nAbs titers according to immunoassay result only
FIGURE 3.
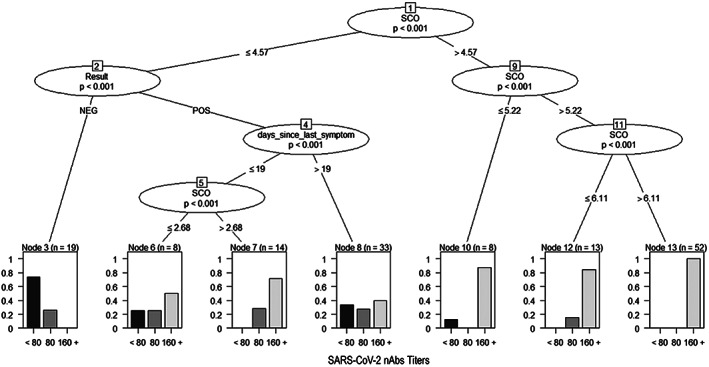
Conditional decision tree of criterion 2 for the prediction of high nAbs titers according to immunoassay result and the time (days) since last symptoms
3.3. Performance of the evaluated immunoassays
The distribution of the S/CO values obtained from the two evaluated immunoassays (Abbott and Euroimmun) is shown in Figure 1, letter A. A very similar distribution was noted. Median values of S/CO were 4.65 (IQR 2.70‐7.18) and 5.61 (IQR 2.72‐9.10) for Abbott and Euroimmun, respectively.
There was a positive correlation between S/CO and nAbs titers for both Abbott (R2 = 0.617; P < .001) and Euroimmun (R2 = 0.526; P < .001) assays (Figure 1, letter B). Also, the predictive value to identify nAbs titers ≥160 was similar between the two assays, with AUC value of 0.878 (IQR 0.83‐0.92) and 0.885 (IQR 0.84‐0.93) for Abbott and Euroimmun, respectively (P = .803) (Figure 1, letter C). Since both assays presented similar performance, the results were then analyzed using Abbott kit.
Table 2 shows the accuracy measurements of four different cut‐offs for the identification of nAbs values ≥160. The cut‐off points obtained by the Youden and Maximum Efficiency methods showed the highest values of accuracy and area under curve (AUC). The PROC01 and Sensitivity = 90% methods showed slightly lower AUC values despite the high sensitivity values found.
TABLE 2.
S/CO cut‐offs values nAbs titers ≥ 1:160 according to four methods. (n=214)
| Methods to find an optimal cut‐off | ||||
|---|---|---|---|---|
| Youden | Max efficiency | PROC01 | Sensitivity = 0.90 | |
| S/CO cut‐off | 4.65 | 3.81 | 1.05 | 2.8 |
| Below cut‐off | 50.5% | 37.4% | 14.0% | 26.6% |
| False positive | 3.7% | 9.8% | 22.4% | 15.9% |
| Accuracy | 77.5% | 78.5% | 70.5% | 77.1% |
| Sensitivity | 0.72 (0.64‐0.80) | 0.83 (0.75‐0.88) | 0.99 (0.96‐100) | 0.90 (0.83‐0.94) |
| Specificity | 0.89 (0.80‐0.95) | 0.72 (0.61‐0.82) | 0.37 (0.26‐0.49) | 0.55 (0.43‐0.67) |
| PPV | 0.92 (0.86‐0.95) | 0.84 (0.76‐0.90) | 0.74 (0.63‐0.99) | 0.78 (0.69‐0.87) |
| NPV | 0.64 (0.55‐0.81) | 0.69 (0.59‐0.80) | 0.96 (0.83‐0.98) | 0.75 (0.63‐0.83) |
| AUC | 0.80 (0.75‐0.85) | 0.77 (0.71‐0.83) | 0.67 (0.62‐0.73) | 0.72 (0.66‐0.78) |
Abbreviations: AUC, Area under the curve; NPV, Negative predictive value; PPV, Positive predictive value; PROC01, minimizes distance between ROC curve plot and point (0.1).
The next step was to determine the accuracy of different S/CO cut‐offs for predicting nAbs titers ≥160 (Table 2). The cut‐off points obtained by the Youden and Maximum Efficiency methods presented the highest values of accuracy and area under curve (AUC). The PROC01 and Sensitivity methods showed slightly lower AUC values despite the high sensitivity values found.
3.4. Validation of the S/CO cut‐off as criteria for selecting donors with high nAbs titers
For validating the S/CO optimal cut‐off, the initial sample of 214 donors was divided into tertiles from the date of the interview. The first two thirds (development sample) were used to develop the criteria, while the last third of the sample (validation sample) was used to validate the proposed criteria.
The clinical and laboratorial characteristics of the development sample (n = 147) and validation sample (n = 67) are presented in Table 3. The two samples had very similar characteristics regarding age, sex, nAbs titers, S/CO values, and need for hospitalization. Nevertheless, the time since last symptoms and recruitment was significantly longer in the validation sample (median 24, IQR 18‐29) days, when compared to the development sample (median 19, IQR 17‐24).
TABLE 3.
Clinical and laboratorial characteristics of development and validation samples
| Development (n=147) | Validation (n=67) | P | |
|---|---|---|---|
| Enrollment date | April 9‐ May 11 | May 13‐ June 1 | — |
| Male, n (%) | 91 (61.9) | 33 (49.3) | 0.112 |
| Age, median (IQR) | 35 (30‐43) | 37 (31‐46) | 0.308 |
| Hospitalization, n (%) | 9 (6.1) | 6 (9.0) | 0.564 |
| End of symptoms‐enrollment days, median (IQR) | 19 (17‐24) | 24 (18‐29) | <0.001 |
| SARS‐CoV‐2 nAbs titer, median (IQR) | 160 (80‐640) | 160 (80‐640) | 0.618 |
| SARS‐CoV‐2 nAbs titers ≥ 160, n (%) | 97 (66.0) | 41 (61.2) | 0.599 |
| Elisa Abbott positive test, n (%) | 123 (86.6) | 57 (85.1) | 0.931 |
| Elisa Abbott DO:CO, median (IQR) | 4.57 (2.72‐7.17) | 5.17 (2.72‐7.25) | 0.881 |
Abbreviation: IQR, Interquartile interval.
Three different criteria for the selection of donors were tested based on the results obtained in the development sample. Criterion 1 calculated the best cut‐off point for the identification of nAbs titers ≥160 according to the Youden's index method (S/CO > 4.65). Criteria 2 and 3 were elaborated from conditional decision trees. Figure 2 illustrates the result from conditional tree of criteria 2, which proposed a criterion for donation considering solely on the result of the immunoassay. In this model, nine out of ten potential donors (95.9%) with S/CO values >4.57 had nAbs titers ≥160. On the other hand, the rate of potential donors not eligible for donation with nAbs titers ≥80 was high in the group with positive ELISA 42/55 (76.4%).
The third criterion revealed that all donors with S/CO values >6.11 had nAbs titers ≥160 (sensitivity = 100%) and one third of the donors (11/33) with the time since the end of symptoms >19 days had nAbs titers <80. All donors with S/CO values >2.68 who reported more recent symptom resolution (between 10 and 19 days before recruitment) showed nAbs values ≥80. Thus, the third donation criterion tested in the validation sample provides for the donation based on S/CO values >4.57 and S/CO values between 2.68 and 4.57 as long as symptom resolution has been recent (between 10 and 19 days before recruitment) (Figure 3).
The performance analysis of the three criteria tested in the validation sample showed similar results. In general, better results were observed for the prediction of nAbs titers ≥1:80 when compared to the results of the prediction of nAbs ≥160. Criterion 3 demonstrated a reduction in the rate of potential donors discarded (40.3%) and, above all, in the false negative rate. The overall accuracy of the prediction of nAbs ≥1:80 increased from 71.6% to 76.1% (Table 4). On the other hand, the criterion 3 test in the validation sample also markedly increased the false positive rate for predicting nAbs ≥1:160 (Table 5).
TABLE 4.
Validation metrics of three criteria to predict nAbs titers ≥ 1:80 through Immunoassay S/CO value
| Criterion 1 | Criterion 2 | Criterion 3 | |
|---|---|---|---|
| DO/CO cut‐off | 4.65 | 4.57 | 4.57 or (2.68 + TSLS) |
| Below cut‐off | 32 (47.8%) | 32 (47.8%) | 27 (40.3%) |
| False positive fraction | 0 (—) | 0 (—) | 1 (1.5%) |
| False negative fraction | 19 (28.4%) | 19 (28.4%) | 15 (22.4%) |
| AUC | 0.82 (0.76‐0.88) | 0.82 (0.76‐0.89) | 0.82 (0.72‐0.92) |
| Global accuracy | 71.6% | 71.6% | 76.1% |
Abbreviations: AUC, Area under the curve; TSLS, Time since last symptom.
TABLE 5.
Validation metrics of three criteria to predict nAbs titers ≥ 1:160 through Immunoassay S/CO value. (n=67)
| Criterion 1 | Criterion 2 | Criterion 3 | |
|---|---|---|---|
| DO/CO cut‐off | 4.65 | 4.57 | 4.57 or (2.68 + TSLS) |
| Below cut‐off | 32 (47.8%) | 32 (47.8%) | 27 (40.3%) |
| False positive fraction | 5 (7.5%) | 5 (7.5%) | 9 (13.4%) |
| False negative fraction | 11 (16.4%) | 11 (16.4%) | 10 (14.9%) |
| AUC | 0.77 (0.66‐0.87) | 0.77 (0.66‐0.87) | 0.70 (0.59‐0.82) |
| Global accuracy | 76.1% | 76.1% | 71.6% |
Abbreviations: AUC, Area under the curve; TSLS, Time since last symptom.
4. DISCUSSION
Passive antibody therapy with convalescent plasma (CP), an adaptive immunotherapy, is an alternative for patients with SARS‐CoV‐2 until more definitive treatments such as monoclonal antibody, antiviral drugs, or vaccine are available. This treatment was used more than a century ago during the A/H1N1 Spanish Flu outbreak in 1917‐1918 and more recently for AIDS, MERS, SARS, and EBOLA viral epidemics. 1 , 21 , 22 , 23 It brings a patient suffering a severe and even lethal infection, immunoglobulins and possibly other immune regulatory factors obtained from plasma of immunized donors. The action mechanism of plasma therapy is not fully established and probably goes beyond administration of neutralizing antibodies. Especially in severe acute respiratory infections of viral etiology, an immunomodulatory effect through administration of anti‐inflammatory cytokines could be involved. 9
Up to now, there is no well‐designed prospective randomized clinical trial demonstrating the efficacy of CP against infectious disease. However, the Brazilian Ministry of Health is permitting the use of CP as an investigational treatment for patients with moderate or severe SARS‐CoV‐2 infection. 24 It is considered an investigational treatment because clinical studies have started but have not yet been completed. The success of CP is related to the presence of high titers of nAbs in the donated plasma. To our knowledge, the present study was the first to correlate the results obtained from two broadly available immunoassays designed to detect anti‐SARS‐CoV‐2 antibodies with the nAbs titers.
Our data show good correlation between nAbs titers and S/CO values obtained from two immunoassays analyzed, Abbott and Euroimmun (P < .001). These data have already been observed in other manuscripts that used an immunoassay. 25 , 26 , 27 , 28 One previous study has compared the performance of the Ortho and Abbot IgG immunoassays. The authors have found a better correlation between S/CO and nAbs for Ortho in comparison to Abbott when performing linear regression, but results were similar when using Spearman correlation test, reinforcing our results. 28 The present study is the first report showing a positive correlation between nAbs tested by an ELISA and a Chemiluminescence assay. This correlation is very important since many services do not have access to measuring nAbs, and, in this scenario, an immunoassay can be used as a screening to detect the presence of anti SARS‐CoV‐2 antibodies in the samples of convalescent donors.
Also, we have tested three criteria for identifying convalescent plasma donors with high nAbs titers. According to Youden method, the best cut‐off point for the identification of nAbs titers ≥160 is S/CO > 4.65. When a conditional decision tree model based solely on the result of the immunoassay was evaluated, 95.9% of potential donors with S/CO values >4.57 had nAbs titers ≥160. Finally, the conditional decision tree model based not only on the results of the immunoassay but also on the time of disappearance of the symptoms revealed that all donors with S/CO values >6.11 had nAbs titers ≥160 (sensitivity = 100%) and all donors with S/CO values >2.68 who reported more recent symptom resolution (between 10 and 19 days before recruitment) showed nAbs titers ≥80.
Our results confirm that S/CO values can be used to identify donors of CP with high probability to have therapeutic nAbs titers. These findings support an algorithm of screening in which donors with S/CO values above 4.57 or with cut‐off higher than 2.68 but with time of symptoms resolution under 19 days can be selected for CP donation with no need to perform nAbs titration. The nAbs analysis should, then, be restricted to the remaining donors with positive immunoassay results.
Finally, this study has also demonstrated the wide variability of nAbs titers among individuals recovered from COVID‐19 infection. The titers were higher among patients of male sex, older age, and requiring hospitalization for COVID‐19 care. This data reinforces previous information available in literature. 29
5. CONCLUSION
We evaluated the performance of Abbott and Euroimmun immunoassays for convalescent patient's IgG screening and the correlation between S/CO values with nAbs titers obtained by CPE‐VNT. Our results show that the S/CO cut‐off value of 4.57 for Abbott assay can be applied to identify CP units with high nAbs titers. These findings support a CCP screening algorithm in which immunoassay for IgG testing could be first performed as a qualification testing and, in the presence of S/CO > 4.57, CP units would be selected for transfusion. CP units with S/CO between 1.4 and 4.57 should be further tested using CPE‐VNT to titrate nAbs and be issued for transfusion based on this result.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Figure S1. Inclusion of the donors of convalescent plasma for the study.
Figure S2. Distribution of nAbs titers identified in the studied population of donors.
Figure S3. Variation of nAbs titers according to different clinical variables: gender, age, hospitalization and time for the end of symptoms.
ACKNOWLEDGMENTS
Funding was granted by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), projects no 2017/24769‐2 (RRGM), 2016/20045‐7 (ELD), 2020/06409‐1 (ELD) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), project no 88887.131387/2016‐00 (DBA).
Mendrone‐Junior A, Dinardo CL, Ferreira SC, et al. Correlation between SARS‐COV‐2 antibody screening by immunoassay and neutralizing antibody testing. Transfusion. 2021;61:1181–1190. 10.1111/trf.16268
Funding information Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Fundação de Amparo à Pesquisa do Estado de São Paulo
REFERENCES
- 1. Garraud O, Heshmati F, Pozzetto B, et al. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol. 2016;23:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of Convalescent Plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang B, Liu S, Tan T, et al. Treatment with Convalescent Plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:e9–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis. 2020;20:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (COVID‐19) patients with Convalescent Plasma. Am J Pathol. 2020;190:1680–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joyner M, Wright RS, Fairweather D, Senefeld J, Bruno K, Klassen S, Carter R, Klompas A, Wiggins C, Shepherd JR, Rea R, Whelan E, Clayburn A, Spiegel M, Johnson P, Lesser E, Baker S, Larson K, Ripoll Sanz J, Andersen K, Hodge D, Kunze K, Buras M, Vogt M, Herasevich V, Dennis J, Regimbal R, Bauer P, Blair J, van Buskirk C, Winters J, Stubbs J, Paneth N, Casadevall A. Early safety indicators of COVID‐19 Convalescent Plasma in 5,000 patients. medRxiv 2020. [DOI] [PMC free article] [PubMed]
- 9. Rojas M, Rodriguez Y, Monsalve DM, et al. Convalescent plasma in Covid‐19: Possible mechanisms of action. Autoimmun Rev. 2020;19:102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tong PB, Lin LY, Tran TH. Coronaviruses pandemics: Can neutralizing antibodies help? Life Sci. 2020;255:117836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu ST, Lin H‐M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, Rodriguez D, Tandon P, Bassily‐Marcus A, Bander J. Convalescent plasma treatment of severe COVID‐19: A matched control study. medRxiv 2020. [DOI] [PubMed]
- 13. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nurtop E, Villarroel PMS, Pastorino B, et al. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol J. 2018;15:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wendel S, Kutner JM, Machado R, et al. Screening for SARS‐CoV‐2 antibodies in convalescent plasma (CCP) in Brazil: Preliminary lessons from a voluntary convalescent donor program. Transfusion 2020;60(12):2938–2951. 10.1111/trf.16065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Araujo DB, Machado RRG, Amgarten DE, et al. SARS‐CoV‐2 isolation from the first reported patients in Brazil and establishment of a coordinated task network. Mem Inst Oswaldo Cruz. 2020;23(115):e200342. 10.1590/0074-02760200342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Almeida FJ, Olmos RD, Oliveira DBL, et al. Hematuria associated with SARS‐CoV‐2 infection in a child. Pediatr Infect Dis J. 2020;39(7):e161. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization (WHO) . Laboratory biosafety guidance related to the novel coronavirus ( 2019‐nCoV ). 1–12 (2020).
- 19. Robin X, Turck N, Hainard A, et al. pROC: An open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopez‐Raton M, Rodriguez‐Alvarez MX, Cadarso‐Suarez C, Gude‐Sampedro F. OptimalCutpoints: An R package for selecting optimal Cutpoints in diagnostic tests. J Stat Softw. 2014;61(8):1–36. [Google Scholar]
- 21. Morand‐Joubert L, Vittecoq D, Roudot‐Thoraval F, et al. Virological and immunological data of AIDS patients treated by passive immunotherapy (transfusions of plasma rich in HIV‐1 antibodies). Vox Sang. 1997;73:149–154. [DOI] [PubMed] [Google Scholar]
- 22. Wong VW, Dai D, Wu AK, Sung JJ. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9:199–201. [PubMed] [Google Scholar]
- 23. Colebunders RL, Cannon RO. Large‐scale convalescent blood and plasma transfusion therapy for Ebola virus disease. J Infect Dis. 2015;211:1208–1210. [DOI] [PubMed] [Google Scholar]
- 24. Brasil AVS. Nota TÉCNICA n° 19/2020/SEI/GSTCO/DIRE1/ANVISA, Agência Nacional de Vigilância Sanitária (ANVISA), Brazil, 2020. [Google Scholar]
- 25. Tan CW, Chia VN, Qin X, et al. SARS CoV 2 surrogate virus neutralization test based on antibody mediated blockage of ACE2 spike protein interaction. Nat Biotechnol. 2010;38:1073–1078. [DOI] [PubMed] [Google Scholar]
- 26. Oguntuyo KY, Stevens CS, Hung C‐T, et al. Quantifying absolute neutralization titers against SARS‐CoV‐2 by a standardized virus neutralization assay allows for cross‐cohort comparisons of COVID‐19 sera. doi: 10.1101/2020.08.13.20157222. [DOI] [PMC free article] [PubMed]
- 27. Fan Wu AW, Liu M, Wang Q, Chen J, Xia S, Ling Y, Zhang Y, Xun J, Lu L, Jiang S, Lu H, Wen Y, Huang J. Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. doi: 10.1101/2020.03.30.20047365 [DOI]
- 28. Luchsinger L, Ransegnola B, Jin D, Muecksch F, et al. Serological analysis of new York City COVID19 Convalescent Plasma donors. doi: 10.1101/2020.06.08.20124792 [DOI]
- 29. Wu F, Liu M, Wang A, et al. Evaluating the Association of Clinical Characteristics with Neutralizing Antibody Levels in patients who have recovered from mild COVID‐19 in Shanghai, China. JAMA Intern Med. 2020;180(10):1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Inclusion of the donors of convalescent plasma for the study.
Figure S2. Distribution of nAbs titers identified in the studied population of donors.
Figure S3. Variation of nAbs titers according to different clinical variables: gender, age, hospitalization and time for the end of symptoms.


