Abstract
Introduction
Our objective was to compare the fetal growth velocity and fetal hemodynamics in pregnancies complicated and in those not complicated by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection.
Material and methods
Prospective case‐control study of consecutive pregnancies complicated by SARS‐CoV‐2 infection during the second half of pregnancy matched with unaffected women. The z scores of head circumference, abdominal circumference, femur length, and estimated fetal weight were compared between the two groups. Fetal growth was assessed by analyzing the growth velocity of head circumference, abdominal circumference, femur length, and estimated fetal weight between the second‐ and third‐trimester scans. Similarly, changes in the pulsatility index of uterine, umbilical, and middle cerebral arteries, and their ratios were compared between the two study groups.
Results
Forty‐nine consecutive pregnancies complicated, and 98 not complicated, by SARS‐CoV‐2 infection were included. General baseline and pregnancy characteristics were similar between pregnant women with and those without SARS‐CoV‐2 infection. There was no difference in head circumference, abdominal circumference, femur length, and estimated fetal weight z scores between pregnancies complicated and those not complicated by SARS‐CoV‐2 infection at both the second‐ and third‐trimester scans. Likewise, there was no difference in the growth velocity of all these body parameters between the two study groups. Finally, there was no difference in the pulsatility index of both maternal and fetal Doppler scans throughout gestation between the two groups.
Conclusions
Pregnancies complicated by SARS‐CoV‐2 infection are not at higher risk of developing fetal growth restriction through impaired placental function. The findings from this study do not support a policy of increased fetal surveillance in these women.
Keywords: coronavirus disease 2019, fetal Doppler, fetal growth, growth velocity, SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2 infection, uterine artery Doppler
Abbreviations
- COVID‐19
coronavirus disease 2019
- MCA
middle cerebral artery
- PI
pulsatility index
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- UA
umbilical artery
Key message.
In pregnancies complicated by SARS‐CoV‐2 infection, fetal growth and growth velocity between the second and third trimesters of pregnancy were similar compared with pregnancies not exposed to the virus, not supporting a policy of increased fetal surveillance in these women.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has been a major issue in public health since the beginning of 2020, with new cases of infection, hospitalization, admission to intensive care units, and deaths increasing on a daily basis worldwide. 1 The impact of SARS‐CoV‐2 infection on pregnancy is a major concern for obstetrical care providers. 2 , 3 , 4 , 5 Since the beginning of the pandemic, pregnancy has been claimed to represent an independent risk factor for severe disease. Several systematic reviews and large observational cohorts have reported a higher risk of severe respiratory symptoms, need for mechanical ventilation, and admission to intensive care units in pregnant women with SARS‐CoV‐2 infection compared with non‐pregnant women. 6 , 7 , 8
The viral agent responsible for coronavirus disease 2019 (COVID‐19), SARS‐CoV‐2 enters host cells by interacting with the angiotensin‐converting‐enzyme receptor, the levels of which are increased in the pregnant uterus and placenta, making the latter a potential target for the infection. 9 , 10 This assumption has been subsequently strengthened by the reported increased prevalence of signs of decidual arteriopathy in pregnant women with SARS‐CoV‐2 infection, suggesting a potential connection between infection and impaired placental function. 11 , 12 , 13 On this basis, we hypothesized that placental changes due to SARS‐CoV‐2 infection may lead to impaired fetal growth in these pregnancies and alter fetal hemodynamics.
The primary aim of this study was to compare the fetal growth velocity in pregnancies complicated by, and in those not complicated by, SARS‐CoV‐2 infection. The secondary aim was to elucidate whether SARS‐CoV‐2 infection can alter maternal and fetal Doppler results.
2. MATERIAL AND METHODS
2.1. Study population
This is a prospective case‐control study including consecutive singleton pregnancies between 35 and 38 weeks of gestation complicated by SARS‐CoV‐2 infection earlier in gestation and receiving antenatal care at the Division of Maternal Fetal Medicine, Università di Roma Tor Vergata, Italy from September 2020 to November 2020. Further inclusion criteria were (a) gestational age confirmed by crown‐rump length at the 11‐14 weeks scan, (b) second‐trimester ultrasound assessment including uterine Doppler performed in our unit, and (c) delivery in our unit. Pregnancies complicated by fetal structural or chromosomal anomalies, maternal smoking, or medical complications potentially affecting fetal growth (ie diabetes, chronic hypertension, and autoimmune diseases) were excluded from the analysis. This cohort was compared with a control group of pregnancies not exposed to SARS‐CoV‐2 and managed in our center during the same time interval. The control group had the same exclusion criteria as the study group and was matched with the latter with regard to the main maternal and pregnancy characteristics with a 1:2 ratio.
SARS‐CoV‐2 infection was confirmed by the presence of a positive real‐time polymerase chain reaction result obtained by nasopharyngeal swab specimens during pregnancy. All women with confirmed SARS‐CoV‐2 infection experienced mild symptoms (fever, cough, sore throat, loss of smell and taste, diarrhea) and none required hospitalization.
2.2. Ultrasound assessment
Ultrasound assessment was performed at the time of the second‐trimester scan and then at 36 weeks of gestation. A third‐trimester scan is offered to all women to confirm fetal well‐being, ascertain fetal growth, and rule out impaired placental function and fetal growth restriction. Head circumference, abdominal circumference, and femur length were measured transabdominally according to the International Society of Ultrasound in Obstetrics and Gynecology guidelines. 14 Estimated fetal weight was calculated with the Hadlock‐4 formula. 15
Doppler velocity waveforms were obtained from the following vessels: uterine, umbilical (UA) and middle cerebral (MCA) arteries according to previously reported techniques. 16 Briefly, both uterine arteries were recorded at the apparent cross‐over with the external iliac artery and the mean pulsatility index (PI) was calculated as the average between the left and right sides. UA was recorded from a free‐floating cord loop and the MCA in an axial section of the fetal head at its origin from the circle of Willis. Cerebroplacental ratio and umbilical‐cerebral ratio were computed, respectively, by dividing MCA‐PI by UA‐PI and UA‐PI by MCA‐PI. All Doppler parameters were obtained according to the recommendations provided by the International Society of Ultrasound in Obstetrics and Gynecology, 17 with an angle of insonation <30°, in the absence of maternal and fetal movements and using an automated trace of at least three consecutive waveforms. Uterine artery velocity waveforms were recorded at both ultrasonographic recordings, whereas UA‐PI and MCA‐PI were evaluated only during the third‐trimester scan.
2.3. Data analysis
Biometric variables, estimated fetal weight, and Doppler indices change with gestational age, so data were expressed as the number of standard deviations (z score) by which they diverged from the expected mean difference obtained from previously constructed reference limits. 16 , 18 , 19
A sample size analysis was performed to evaluate the sample size necessary. Given a significance of .05 and power of −.80, a sample size of 47 in the study group and 94 in the control group is necessary to demonstrate differences of 0.5 z scores in the variables considered.
Growth velocity was calculated as the difference in the z scores between the measurements recorded at the time of second‐trimester scan and at 35‐38 weeks of gestation, divided by the time interval (expressed in days) between the two scans and multiplied by 100. 20 , 21 A similar approach was used for quantifying the Doppler changes in the PI of uterine arteries.
Categorical variables were presented as numbers (n) and percentages (%) and analyzed using chi‐squared test. Continuous variables were presented as median and interquartile range and analyzed using Mann‐Whitney U test.
Data were analyzed using SPSS version 23.0 (IBM Corp.) and medcalc Statistical softwares version 14.8 (MedCalc Software bvba). Two‐tailed p values lower than 0.05 were considered statistically significant.
2.4. Ethical approval
The study was approved by the institutional review board of our institution (#Ost4‐2020 on 30 July 2020) and all included women gave their written informed consent to participate.
3. RESULTS
Forty‐nine consecutive pregnancies complicated by, and 98 not complicated by, SARS‐CoV‐2 infection and managed at our center were included in the analysis. Median gestational age at SARS‐CoV‐2 infection was 30.6 weeks (interquartile range 28.9‐32.3 weeks) and all women were asymptomatic with negative real‐time polymerase‐chain reaction swabs at the time of the 36‐week scan. General characteristics of cases and controls are reported in Table 1. There was no difference in the main maternal and pregnancy characteristics, including maternal age, body mass index, parity, and gestational age at ultrasound and at birth.
TABLE 1.
General characteristics of study population stratified according to the exposure to SARS‐CoV‐2
| Characteristics |
Pregnancies complicated by SARS‐CoV‐2 infection (N = 49) |
Pregnancies not complicated by SARS‐CoV‐2 infection (N = 98) |
p value |
|---|---|---|---|
| Maternal age (years) | 30.4 (29.6–32.1) | 30.5 (29.25–32.2) | 0.712 |
| Maternal height (cm) | 160 (158–165) | 160 (158–166) | 0.829 |
| BMI (kg/m2) | 26.9 (24.3–29.1) | 26.2 (24.1–29.2) | 0.345 |
| Ethnicity | 0.784 | ||
| Caucasian | 47 (95.9%) | 93 (84.647%) | |
| Other | 2 (4.1%) | 5 (5.1%) | |
| Parity | 1 | ||
| Nulliparous | 35 (71.4%) | 70 (71.4%) | 0.797 |
| Assisted conception | 3 (6.1%) | 5 (5.1%) | 0.846 |
| Gestational age at first ultrasound examination (weeks) | 20.1 (19.3–22.2) | 20.2 (19.5–22.1) | 0.912 |
| Gestational age at second ultrasound examination (weeks) | 36.4 (35.4–36.9) | 36.4 (35.9.4–36.7) | |
| Gestational age at COVID‐19 infection | 30.2 (26.2–34.1) | ||
| Gestational age at delivery (weeks) | 39.9 (38.0–40.9) | 40.2 (38.1–40.7) | 0.224 |
| Birthweight (g) | 3322 (2830–3590) | 3424(3030–3780) | 0.377 |
| Head circumference at birth (mm) | 353 (344–369) | 355 (342–370) | 0.254 |
| Male | 24 (49.0%) | 50 (51.0.6%) | 0.816 |
Abbreviations: BMI, body mass index; COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Data are expressed as median (interquartile range) or as n (%).
There was no difference in head circumference, abdominal circumference, femur length and estimated fetal weight z score between pregnancies complicated and those not complicated by SARS‐CoV‐2 infection at either the second‐ or the third‐trimester scan (Table 2). Likewise, there was no difference in the growth velocity of head circumference (cases 0.14 vs control 0.02; p = 0.477), abdominal circumference (cases 0.12 vs control 0.01; p = 0.871), femur length (cases 0.23 vs control 0.16; p = 0.423) and estimated fetal weight (cases 0.54 vs control 0.02; p = 0.166) between the two study groups (Figure 1) .
TABLE 2.
Comparison of biometric and Doppler indices between the two groups obtained at the time of the two ultrasonographic examinations
| Characteristics |
Pregnancies complicated by SARS‐CoV‐2 infection (N = 49) |
Pregnancies not complicated by SARS‐CoV‐2 infection (N = 98) |
p value |
|---|---|---|---|
| Second trimester scan | |||
| HC z value | −0.07 (−0.63 to 0.56) | 0.18 (−0.34 to 0.76) | 0.229 |
| AC z value | −0,04 (−0.54 to 0.9) | 0.01 (−0.48 to 0.89) | 0.526 |
| FL z value | 0.36 (−0.15 to 095) | 0.23 (−0.48 to 0.70) | 0.163 |
| EFW z value | 0.19 (−0.65 to 0.68) | 0.25 (−0.24 to 0.69) | 0.168 |
| Mean uterine artery PI z value | 0.06 (−0.40 to 0.60) | 0.14 (−0.37 to 0.62) | 0.183 |
| 35–38 weeks scan | |||
| HC (mm) | 326 (320–336) | 328 (322–339) | 0.104 |
| HC z value | −0.16 (−0.97 to 0.48) | 0.18 (−0.40 to 0.89) | 0.133 |
| AC (mm) | 322 (312–351) | 323 (314–347) | 0.526 |
| AC z value | 0.01 (−0.58 to 0.82) | 0.06 (−0.53 to 0.70) | 0.957 |
| FL (mm) | 69 (66–70) | 68 (67–71) | 0.745 |
| FL z value | 0.27 (−0.97 to 0.87) | 0.21 (−0.70 to 0.91) | 0.942 |
| EFW (g) | 2812 (2640–2980) | 2829 (2640–2930) | 0.672 |
| EFW z value | 0.14 (−0.76 to 0.78) | 0.02 (−0.76 to 0.52) | 0.235 |
| Mean uterine artery PI z value | 0.04 (−0.65 to 0.74) | 0.06 (−0.52 to 0.62) | 0.309 |
| UA PI z value | −0.09 (−0.58 to 0.75) | 0.01 (−0.61 to 0.73) | 0.354 |
| MCA PI z value | 0.20 (−65 to 0.85) | 0.11 (−0.62 to 0.84) | 0.444 |
| CPR z value | −0.19 (−0.52 to 0.71) | 0.04 (−0.44 to 0.69) | 0.240 |
| UCR z value | −0.11 (−0.44 to 0.61) | 0.07 (−0.39 to 0.67) | 0.300 |
Abbreviations: AC, abdominal circumference; CPR, cerebroplacental ratio; EFW, estimated fetal weight; FL, femur length; HC, head circumference; MCA, middle cerebral artery; PI, pulsatility index; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; UA, umbilical artery; UCR, umbilical cerebral ratio.
Data are expressed as median (interquartile range).
FIGURE 1.
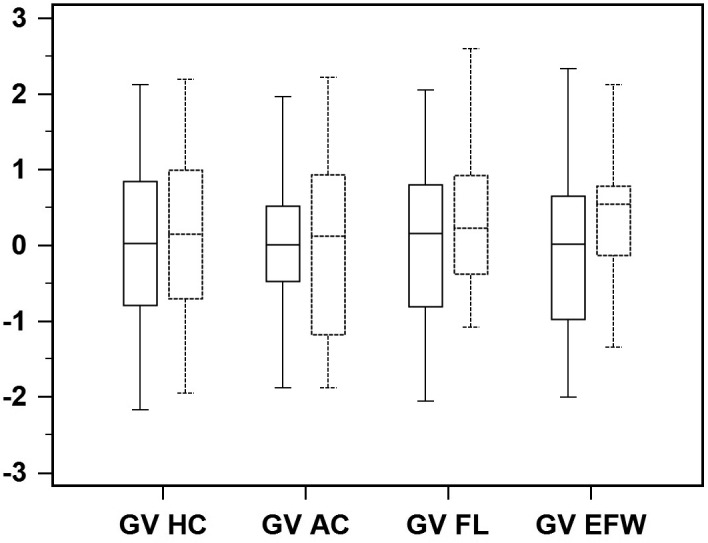
Box‐whisker plots of growth velocity (GV) of head circumference (HC), abdominal circumference (AC), femur length (FL) and estimated fetal weight (EFW) in fetuses of mothers affected by severe acute respiratory syndrome coronavirus 2 (dotted line) and in control fetuses (solid line)
Finally, there was no difference in the PI of both maternal and fetal Doppler scans during gestation between the two groups (Table 2).
4. DISCUSSION
The findings from this study show that in pregnancies complicated by SARS‐CoV‐2 infection during the second half of pregnancy, fetal growth and growth velocity between the second and third trimesters of pregnancy were similar compared with pregnancies not exposed to the virus. Likewise, there were no differences in the maternal and fetal Doppler findings between the two study groups. These data suggest that SARS‐CoV‐2 infection in pregnancy is unlikely to increase the risk of fetal growth restriction and that these pregnancies do not require additional scans to detect growth disorders.
To the best of our knowledge, this is the first study investigating the effects of SARS‐CoV‐2 infection on fetal growth. Prospective design, relatively large sample size, longitudinal assessment of fetal growth, and Doppler findings in the second and third trimesters of pregnancy represent the main strengths of this study. Furthermore, the two study populations were balanced as regards the main maternal and pregnancy variables potentially affecting fetal growth. The major limitation of the present study is that it relies on the inclusion of only mildly symptomatic cases. Severe SARS‐CoV‐2 infection has been associated with higher risk of vasculopathy and it may be entirely possible that the lack of association between SARS‐CoV‐2 infection and fetal growth disorders may be due to the fact that we included only pregnant women in the mild spectrum of the disease. However, about 92%‐95% of pregnant women with SARS‐CoV‐2 infection do not experience the severe spectrum of the disease, making the results for this study applicable to the large majority of infections in pregnancy. Further, we limited our observation to women who contracted the infection during the second half of pregnancy and we cannot exclude that the outcome could be different, if the infection had occurred earlier in pregnancy.
Despite the ongoing body of evidence that is rapidly accumulating on the course of the SARS‐CoV‐2 infection in pregnancy, several questions, including the potential effect of the virus on the fetus and placenta, remain unanswered. Among them, vertical transmission of SARS‐CoV‐2 infection is still a matter of debate. Mother‐to‐fetus transmission was reported as negligible at the beginning of the pandemic. 2 , 3 Conversely, more recent and large cohorts have reported a higher risk of vertical transmission. 22 , 23 , 24 , 25 A recent systematic review of 39 cohort or case‐series studies including 936 newborns from mothers affected by COVID‐19 showed that the pooled proportion of vertical transmission was 3.2%, with 27 neonates testing positive for SARS‐CoV‐2 infection by nasopharyngeal swab. 26 A subgroup analysis based on the study location showed a similar rate of vertical transmission when comparing studies from China with those from outside China (2.0% vs 2.7%). 26 Despite this, the actual risk of vertical transmission and its potential consequences on the fetus are currently largely unknown.
A recent report isolated SARS‐CoV‐2 exclusively from the placenta but not from newborns, so questioning the actual risk of transmission. 27 Features of fetal and maternal vascular malperfusion characterized by decidual arteriopathy with atherosis, fibrinoid necrosis, and mural hypertrophy of decidual arterioles have been described in placentas from pregnant women with SARS‐CoV‐2 infection. 11 , 12 , 13
In this study, placental pathology was not analyzed, so we cannot address this question. Moreover, outside SARS‐CoV‐2 infection, every condition leading to maternal vascular hypoperfusion is potentially associated with higher risk of impaired placental function, growth restriction, and stillbirth. 28 The findings from this study do not support this theory, but show that pregnancies complicated by SARS‐CoV‐2 infection are not at higher risk of fetal growth restriction; so, additional scans through pregnancy to rule out these disorders are not required.
A likely explanation for the lack of association between SARS‐CoV‐2 infection and fetal growth restriction may rely on the inclusion of women with mild symptoms, which may represent only the milder spectrum of COVID‐19. In this scenario, we cannot completely rule out that women experiencing more severe COVID‐19 may be at higher risk of fetal growth restriction. Furthermore, the time at maternal infection may change the risk of developing placental lesions. Finally, we did not consider whether SARS‐CoV‐2 infection represents an additional risk factor in women.
One of the more debated issues when managing pregnancies complicated by SARS‐CoV‐2 infection is whether more intensive fetal monitoring should be applied to these women. The findings from the present study do not support a policy of additional ultrasound scans to assess fetal well‐being in view of the lack of association between infection and impaired fetal growth. Furthermore, the risk of stillbirth in women with SARS‐CoV‐2 infection has been reported to be not significantly different from that of the baseline pregnant population not affected by infection. Therefore, women with SARS‐CoV‐2 infection should be reassured about the low risk of adverse fetal outcome. This is also important because pregnant women with SARS‐CoV‐2 infection experience increased levels of anxiety due to their specific concerns about the potential negative effect of the infection on their newborn. 29 , 30
5. CONCLUSION
Pregnancies complicated by SARS‐CoV‐2 infection during the second half of pregnancy are not at higher risk of developing fetal growth restriction. The findings from this study do not support a policy of increased fetal surveillance in these women.
CONFLICT OF INTEREST
None.
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC) . Data on COVID‐19 during pregnancy: weekly COVID‐19 pregnancy data (2021). Available at https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/special‐populations/pregnancy‐data‐on‐covid‐19.html. Accessed January 8, 2021.
- 2. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2:100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khalil A, Kalafat E, Benlioglu C, et al. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25:100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poon LC, Yang H, Dumont S, et al. ISUOG Interim Guidance on coronavirus disease 2019 (COVID‐19) during pregnancy and puerperium: information for healthcare professionals – an update. Ultrasound Obstet Gynecol. 2020;55:848‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang H, Acharya G. Novel coronavirus disease (COVID‐19) in pregnancy: what clinical recommendations to follow? Acta Obstet Gynecol Scand. 2020;99:439‐442. [DOI] [PubMed] [Google Scholar]
- 6. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99:823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WAPM (The world association of perinatal medicine) working group on COVID‐19 . Maternal and perinatal outcomes of pregnant women with SARS‐CoV‐2 infection. Ultrasound Obstet Gynecol. 2021;57:232‐241. [DOI] [PubMed] [Google Scholar]
- 8. Di Mascio D, Sen C, Saccone G, et al. Risk factors associated with adverse fetal outcomes in pregnancies affected by Coronavirus disease 2019 (COVID‐19): a secondary analysis of the WAPM study on COVID‐19. J Perinat Med. 2020;26:950‐958. [DOI] [PubMed] [Google Scholar]
- 9. Malinowski AK, Noureldin A, Othman M. COVID‐19 susceptibility in pregnancy: immune/inflammatory considerations, the role of placental ACE‐2 and research considerations. Reprod Biol. 2020;20:568‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhaundiyal A, Kumari P, Jawalekar SS, Chauhan G, Kalra S, Navik U. Is highly expressed ACE 2 in pregnant women "a curse" in times of COVID‐19 pandemic? Life Sci. 2021;264:118676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID‐19. Am J Clin Pathol. 2020;154:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baergen RN, Heller DS. Placental pathology in Covid‐19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23:177‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwartz DA, Baldewijns M, Benachi A, et al. Chronic histiocytic intervillositis with trophoblast necrosis are risk factors associated with placental infection from Coronavirus disease 19 (COVID‐19) and intrauterine maternal‐fetal systemic acute respiratory Coronavirus 2 (SARSCoV‐2) transmission in liveborn and stillborn infants. Arch Pathol Lab Med. 2020; in press. 10.5858/arpa.2020-0771-SA [DOI] [PubMed] [Google Scholar]
- 14. Salomon LJ, Alfirevic Z, Da Silva Costa F, et al. ISUOG Practice Guidelines: ultrasound assessment of fetal biometry and growth. Ultrasound Obstet Gynecol. 2019;53:715‐723. [DOI] [PubMed] [Google Scholar]
- 15. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am J Obstet Gynecol. 1985;151:333‐337. [DOI] [PubMed] [Google Scholar]
- 16. Rizzo G, Pietrolucci ME, Mappa I, et al. Modeling pulsatility index nomograms from different maternal and fetal vessels by quantile regression at 24–40 weeks of gestation: a prospective cross‐sectional study. J Matern Fetal Neonatal Med. 2020; in press. 10.1080/14767058.2020.1767060 [DOI] [PubMed] [Google Scholar]
- 17. Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol. 2013;41:233‐239. [DOI] [PubMed] [Google Scholar]
- 18. Rizzo G, Prefumo F, Ferrazzi E, et al. The effect of fetal sex on customized fetal growth charts. J Matern Fetal Neonatal Med. 2016;29:3768‐3775. [DOI] [PubMed] [Google Scholar]
- 19. Rizzo G, Pietrolucci ME, Mappa I, Maqina P, Makatsarya A, D'Antonio F. Modeling gestational age centiles for fetal umbilicocerebral ratio by quantile regression analysis: a secondary analysis of a prospective cross‐sectional study. J Matern Fetal Neonatal Med. 2020;23:1‐5. [DOI] [PubMed] [Google Scholar]
- 20. Cavallaro A, Veglia M, Svirko E, Vannuccini S, Volpe G, Impey L. Using fetal abdominal circumference growth velocity in the prediction of adverse outcome in near‐term small‐for‐gestational‐age fetuses. Ultrasound Obstet Gynecol. 2018;52:494‐500. [DOI] [PubMed] [Google Scholar]
- 21. Rizzo G, Mappa I, Bitsadze V, Khizroeva J, Makatsariya A, D'Antonio F. Administration of antenatal corticosteroid is associated with reduced fetal growth velocity: a longitudinal study. J Matern Fetal Neonatal Med. 2020;29:1‐6. [DOI] [PubMed] [Google Scholar]
- 22. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA. 2020;323:1846‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS‐CoV‐2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99:565‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zamaniyan M, Ebadi A, Aghajanpoor S, Rahmani Z, Haghshenas M, Azizi S. Preterm delivery, maternal death, and vertical transmission in a pregnant woman with COVID‐19 infection. Prenat Diagn. 2020;40:1759‐1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Algarroba GN, Rekawek P, Vahanian SA, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:275‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2021;224:35–53.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fillion A, Guerby P, Menzies D, et al. Pathological investigation of placentas in preeclampsia (the PEARL study). Hypertens Pregnancy. 2020;29:1‐7. [DOI] [PubMed] [Google Scholar]
- 29. Mappa I, Distefano FA, Rizzo G. Effects of coronavirus 19 pandemic on maternal anxiety during pregnancy: a prospective observational study. J Perinat Med. 2020;48:545‐550. [DOI] [PubMed] [Google Scholar]
- 30. Thapa SB, Mainali A, Schwank SE, Acharya G. Maternal mental health in the time of the COVID‐19 pandemic. Acta Obstet Gynecol Scand. 2020;99:817‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]


