Abstract
The novel coronavirus disease (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has resulted in an unprecedented public health crisis and economic losses. Although several cases of cats and dogs infected with SARS‐CoV‐2 have been reported during this outbreak, the prevalence of SARS‐CoV‐2 in dog and its transmission among other companion animals are still unknown. Here, we report an extensive serological study of SARS‐CoV‐2 infection in dogs in Wuhan and analyse the infection rates at different stages of the pandemic outbreak. A total of 946 dogs serum samples were collected from Wuhan, of which 36 samples were obtained prior to the pandemic outbreak. Indirect enzyme‐linked immunosorbent assay (ELISA) showed that 16 sera collected during the outbreak were detected as positive through the receptor‐binding domain (RBD) of SARS‐CoV‐2. Of these 16 sera, 10 exhibited measurable SARS‐CoV‐2‐specific neutralizing antibodies whose titres ranged from 1/20 to 1/180. No serological cross‐reactivity was detected between SARS‐CoV‐2 and canine coronavirus (CCV). Furthermore, with the effective control of the outbreak, a decrease in the SARS‐CoV‐2 seropositive dog number was observed. Our results suggest that SARS‐CoV‐2 has infected companion dogs during the outbreak, and that COVID‐19 patient families have a higher risk of dog infection. Our findings deepen our understanding of the infection of SARS‐CoV‐2 in dogs and provide an important reference for prevention of COVID‐19.
Keywords: dog, neutralizing antibody, SARS‐CoV‐2, serological investigation
1. INTRODUCTION
Coronaviruses, as members of the Orthocoronavirinae, carry positive‐sense single‐stranded RNA. Their genomes range from 27 to 32 kilobases in size, which are the largest among RNA viruses. Coronaviruses can infect a wide variety of hosts, and many vertebrates are susceptible to these viruses (Kindler et al., 2016). Three coronaviruses, namely, severe acute respiratory syndrome‐related coronavirus (SARS‐CoV), Middle East respiratory syndrome‐related coronavirus (MERS‐CoV) and severe acute respiratory syndrome‐related coronavirus‐2 (SARS‐CoV‐2), have caused pandemics in the first two decades of this century. In 2002–2003, SARS‐CoV infected 8,000 people worldwide with a mortality rate of 10%. This was followed by MERS‐CoV pandemics, which infected 2,500 people with a mortality rate of 36% since 2012 (de Wit et al., 2016). The first SARS‐CoV‐2 outbreak occurred in a local seafood market in Wuhan city, Hubei province, China (Liu et al., 2020; Lu et al., 2020). Subsequently, it turned into a global pandemic outbreak within a few months. The virus is considered to be one of the most virulent pathogens to threaten the public health across the globe (Hu et al., 2020).
SARS‐CoV‐2 uses the SARS‐CoV receptor (angiotensin‐converting enzyme 2, ACE2) and the serine protease (TMPRSS2) for S protein priming to gain entry into the host cell (Hoffmann et al., 2020). SARS‐CoV‐2 primarily targets lung epithelium cells, causing respiratory infection. The patients mainly exhibited such symptoms as fever, cough and shortness of breath (Chen et al., 2020). Severe cases develop acute respiratory distress syndrome and acute lung injury. By15 December 2020, the number of confirmed cases has grown up to 71,581,532 around the world including 1,618,374 deaths. Based on genome‐wide alignment analysis, the isolated SARS‐CoV‐2 strain is closest to the Bat‐CoV RaTG13 strain, isolated in Yunnan, China, with a nucleic acid identity of 96.2%. This finding suggests that SARS‐CoV‐2 might be evolved from bat coronavirus (Zhou et al., 2020). Furthermore, Malayan Pangolin‐CoV strains with high amino acid identity with SARS‐CoV‐2 S protein were also isolated. Although bats and Malayan pangolin may be the reservoir host for SARS‐CoV‐2, it remains unclear whether SARS‐CoV‐2 has other host species (Xiao et al., 2020). A series of animal models of SARS‐CoV‐2 infection have revealed that SARS‐CoV‐2 exhibits tissue tropism in cats and ferrets, and can replicate in their respiratory tract, but dogs show low susceptibility (Feng et al., 2011; Halfmann et al., 2020; Shi et al., 2020), implying that companion animals might get infected through contact with individuals carrying SARS‐CoV‐2. In our previous study, we conducted an extensive serology survey on cats in Wuhan and reported the prevalence and transmission of SARS‐CoV‐2 in cats(Q. Zhang et al., 2020). In this study, we investigated the SARS‐CoV‐2 infection in dogs by analysing the serum samples collected from dogs in Wuhan throughout outbreak and afterwards. The results provide a comprehensive serological evidence for the SARS‐CoV‐2 infection in dogs.
2. MATERIALS AND METHODS
2.1. Sample collection
The serum samples were collected from a total of 910 dogs in Wuhan between January and September 2020. The dogs belonged to the following four categories: (1) 31 abandoned dogs from animal shelters, (2) 851 dogs from pet hospitals, (3) 16 dogs from families with at least one COVID‐19 patient and (4) 12 dogs from the police dog base. The blood samples were collected via forelimb venipuncture, and sera were separated and stored at − 20°C until further detection. Nasopharyngeal and anal swabs were collected and put in tubes containing viral transport medium‐VTM (Copan Diagnostics, Brescia, Italy)(Haagmans et al., 2014). All samples were collected by personnel wearing full personal protective equipment, including head covers, goggles, N95 masks, gloves and disposable gowns.
2.2. Virus and cells
SARS‐CoV‐2 (IVCAS 6.7512) was isolated from a COVID‐19 patient as previously described (Zhou et al., 2020). Vero E6 was purchased from ATCC (ATCC® CRL‐1586™). All virus infection experiments were performed under animal biosafety level 3 (ABSL3) conditions.
2.3. Enzyme‐linked immunosorbent assay (ELISA)
Antibody was tested using indirect ELISA with the SARS‐CoV‐2 RBD protein (Sino Biological Inc, China) and peroxidase conjugated goat anti‐dog IgG (Sigma‐Aldrich, USA). Briefly, ELISA plates were coated overnight with RBD protein (1 μg/ml, 100 μl per well) at 4℃. After being blocked with PBS containing 5% skim milk for 2 hr at 37℃, the resultant ELISA plates were added with sera at a dilution of 1:40 and incubated. After 30‐min incubation at 37°C, the plates were washed 5 times with PBS buffer containing 0.05% Tween‐20. These plates were added with the diluted (1:8,000) peroxidase conjugated goat anti‐dog IgG and incubated for an additional 30 min. After another 5 times washes, the plates were added with Tetramethylbenzidine (TMB) substrate (Sigma‐Aldrich, USA) and incubated for 10 min. Then, the reaction was stopped, and optical density (OD) was measured at 450 nm. The sera were defined as positive if the OD values were twice ≥ the mean OD of the negative serum samples collected prior to outbreak.
2.4. Canine Coronavirus antibody ELISA
The kit was prepared by double‐resistant one‐step sandwich method. The canine coronavirus antigen was precoated onto the micropore plates. The plates were added successively with serum samples and HRP‐labelled detection antigen and incubated, and washed thoroughly. TMB substrate was added into the plates for rendering colour. The optical density (OD) value at the wavelength of 450 nm was measured with an enzyme marker and compared with the cut‐off value (negative control value + 0.15) to determine the presence or absence of coronavirus‐Ab in the serum samples.
2.5. Plaque reduction neutralization test (PRNT)
For virus neutralization test, serum samples were heat‐inactivated through incubation at 56°C for 30 min. Each serum sample was serially diluted with Dulbecco's Modified Eagle Medium (DMEM) by twofolds or threefolds according to the OD value. The diluted sample was mixed with equal volume of diluted virus and incubated at 37°C for 1 hr. Vero E6 cells in 24‐well plates were inoculated with the sera‐virus mixture at 37°C for 1 hr. Subsequently, the mixture was replaced with DMEM containing 2.5% FBS and 0.8% carboxymethylcellulose. After 3‐day culture, the plates were fixed with 8% paraformaldehyde and stained with 0.5% crystal violet. All the samples were tested in duplicate, and serum dilution titre that resulted in a plaque reduction by at least 50% was defined as neutralization titre (Davies et al., 2005).
2.6. Western blotting assay
The total protein concentration of purified and inactivated SARS‐CoV‐2 was determined using Bradford protein assay (Su et al., 2018). Four micrograms of protein was subjected to 12.5% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and was transferred on to 0.45 μ m nitrocellulose membrane. Then, the viral proteins were incubated with dog sera. After subsequent incubation with a peroxidase conjugated goat anti‐dog IgG, the protein bands were visualized and detected using the ECL System (Amersham Life Science, Arlington Heights, IL, USA).
3. RESULTS
3.1. Detection of anti‐SARS‐CoV‐2 antibody in dog sera
SARS‐CoV‐2‐RBD‐specific IgG titres of 946 dog sera were determined using ELISA, as described previously (Zhang et al., 2020). Among these 946 dog serum samples, 36 negative serum samples were collected prior to the pandemic outbreak, and their average OD value was set as cut‐off value (0.36). In terms of this cut‐off value, sera of 16 dog (1.75%) collected from January to September were detected as positive (Table 1 and Figure 1). One serum sample was strongly positive and showed an OD of 0.554, and the dog turned out to be in close contact with the COVID‐19 patients. In addition, the antibody specificity was confirmed by performing cross‐reaction test between SARS‐CoV‐2 positive dog sera and inactivated canine coronavirus (CCV) using ELISA (Figure 2).
TABLE 1.
Detection of the antibodies against SARS‐CoV‐2 in dogs
| Dog NO. | ELISA (OD450) | Neutralization Titre | Background of dogs |
|---|---|---|---|
| 1# | 0.55 | 1/180 | COVID−19 patient owner |
| 2# | 0.38 | 1/80 | COVID−19 patient owner |
| 3# | 0.44 | None | Stray dog |
| 4# | 0.38 | 1/20 | From pet hospital |
| 5# | 0.4 | 1/40 | From pet hospital |
| 6# | 0.42 | 1/20 | From pet hospital |
| 7# | 0.45 | 1/20 | COVID−19 patient owner |
| 8# | 0.45 | 1/20 | From pet hospital |
| 9# | 0.49 | 1/20 | From pet hospital |
| 10# | 0.4 | 1/20 | From pet hospital |
| 11# | 0.4 | None | From pet hospital |
| 12# | 0.37 | None | From pet hospital |
| 13# | 0.42 | 1/20 | From pet hospital |
| 14# | 0.38 | None | From pet hospital |
| 15# | 0.38 | None | From pet hospital |
| 16# | 0.39 | None | From pet hospital |
FIGURE 1.
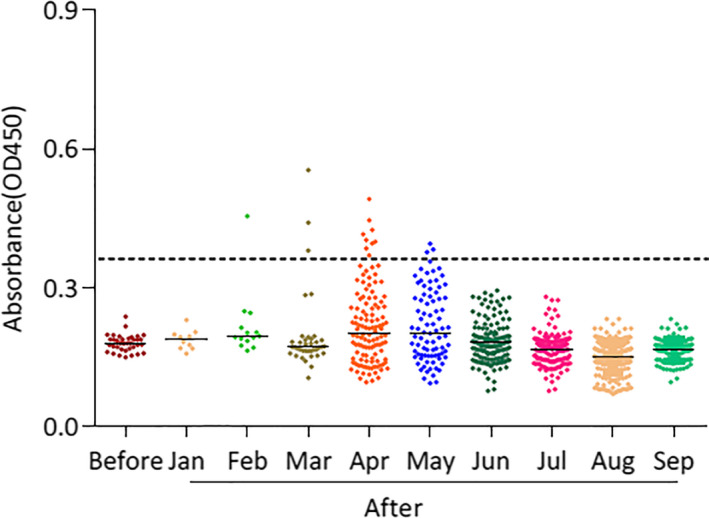
ELISA of dog serum samples against the recombinant receptor‐binding domain (RBD) of SARS‐CoV‐2 spike protein. The dashed line indicates the cut‐off. Each dot represents one individual sample within each antigen panel. Before: serum samples collected between June and December 2019 prior to COVID‐19 outbreak. After: serum samples collected between January to September 2020 after COVID‐19 outbreak
FIGURE 2.
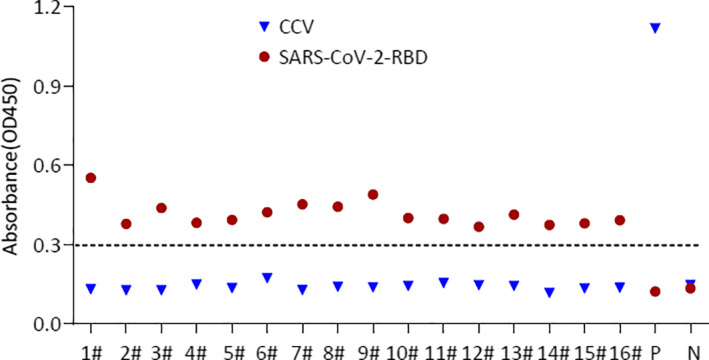
The cross‐reaction test between SARS‐CoV‐2 positive dogs serum samples with the Canine coronavirus (CCV) using ELISA. All sera were diluted 40‐folds. The dashed line indicates the cut‐off. P, hyperimmune serum against canine coronavirus. N, negative dog serum
3.2. Changes in serum antibody against SARS‐CoV‐2 in dogs with time
The ratio of SARS‐CoV‐RBD positive serum sample number to the total sample number per month from January to September was calculated. The positive rate was 7.14% (1/14), 7.89% (3/38), 7.37% (9/122) and 3.52% (3/85) in February, March, April and May, respectively. The results indicated that with passing time, the SARS‐CoV‐RBD positive serum rate peaked in Mar and April, and then decreased gradually, eventually disappeared by June. In the meanwhile, the outbreak in Wuhan was effectively under control (Figure 1).
3.3. Detected low neutralization titres and N protein‐specific binding in serum positive dogs
In order to verify the neutralization efficiency of ELISA positive sera, PRNT assay for SARS‐CoV‐2 was performed. Of the serum positive samples, 10 samples showed neutralization activity with titres ranging from 1:20 to 1:180 (Table 1 and Figure 3a), and most samples showed low neutralization titre of 1:20. Sample 1 and sample 2 collected from the dogs in COVID‐19 patient families showed relatively high titre of 1:180 and 1:80, respective. Neutralization activity was not detected in 6 samples, which might be attributed to the lack of specific neutralizing epitopes. Moreover, dogs with neutralization activity showed no respiratory symptoms. Furthermore, to verify the presence of SARS‐CoV‐2‐specific IgG in dog serum, Western blot assay was performed. The N protein of the purified inactivated SARS‐CoV‐2 was detected with positive dog serum diluted at 1:100 (Figure 3b). Positive and negative serum samples from human with the same dilution gradient were used as control.
FIGURE 3.
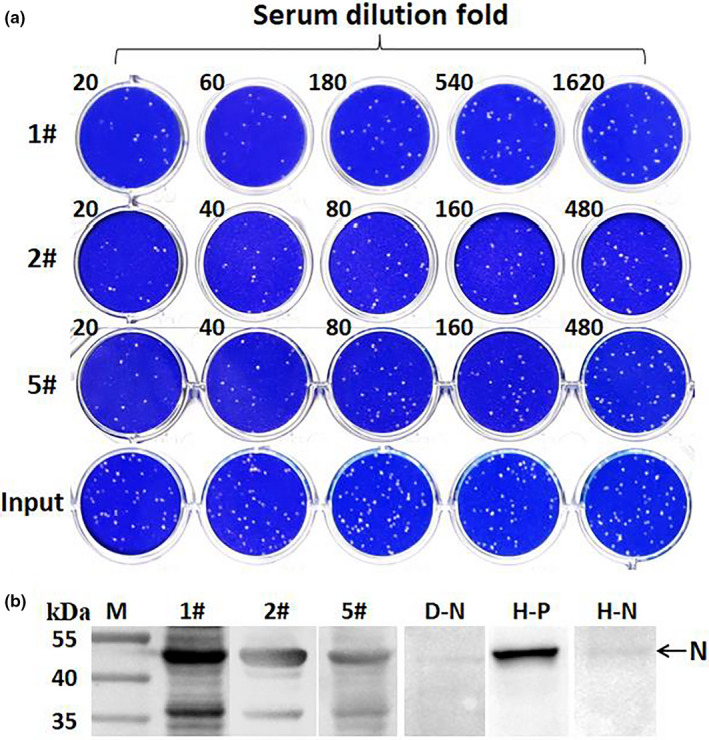
Virus neutralization test and Western blot assay of dog serum samples for SARS‐CoV‐2. (a) Dog#1, Dog#2 and Dog#5 sera were twofolds or threefolds serially diluted, respectively, and then mixed with SARS‐CoV‐2, input control at the same time; after incubation at 37°C for 1 hr, the mixture was used to infect Vero E6 cells, and replaced with Plaque Liquid media 1 hr later. The plates were fixed and stained 72 hr later. All samples were tested in duplicate. (b) Western blot of purified SARS‐CoV‐2 with dog or human sera. All sera were diluted 100‐fold. D‐N, negative dog serum. H‐P, human convalescent serum. H‐N, healthy human serum
4. DISCUSSION
In this study, we collected a large number of serum samples from dogs in Wuhan, China, and conducted an extensive investigation of SARS‐CoV‐2‐specific antibodies. To the best of our knowledge, this is first serum investigation of dog SARS‐CoV‐2 infection with a large number of samples over a large time span. Of 910 samples, 16 (1.75%) were detected to be positive using ELISA. Importantly, 10 (1.09%) serum samples were further confirmed to be positive by PRNT. Analysis of the background of all samples indicated that the dogs from relatively closed environments and healthy keepers, such as dogs from animal shelters or the police dog base, failed to test positive. On the contrary, the two dogs with the highest neutralization titres (1/80 and 1/180, respectively) were found to be from families with COVID‐19 patients. Our results suggested that the health status of the owner plays an important role in the seroconversion of the dogs.
Neutralizing antibodies are produced by B lymphocytes and play an important role in blocking new infection and virus clearance. Previous studies have indicated that neutralizing antibody titre of most patients exhibited a gradual decline two or three months after infection (Long et al., 2020). The present study revealed that the proportion of SARS‐CoV‐2 seropositive dogs peaked in March and then decreased gradually, and no positive serum was detected in June. Considering that the number of COVID‐19 patients in Wuhan gradually decreased to zero on 24th April, the reasons for the gradual decrease in the number of SARS‐CoV‐2 seropositive dogs could be the following: (1) the decrease in the infection source; (2) the lifetime of antibody in positive dogs is relatively short.
The SARS‐CoV‐2 was probably bat originated (Zhou et al., 2020). Although the intermediate host of SARS‐CoV‐2 remains unknown, a series of laboratory investigations of animal susceptibility such as cats, dogs and ferrets to SARS‐CoV‐2 were performed (Halfmann et al., 2020; Shi et al., 2020). The pet SARS‐CoV‐2 infection under natural conditions has drawn great concerns. Our data indicated that dogs could be infected with SARS‐CoV‐2 under natural conditions, but neutralizing antibody titres of seropositive dogs were relatively low, suggesting that dogs were less susceptible to SARS‐CoV‐2. Our results are consistent with serological studies of dogs in Italy reported earlier (Patterson et al., 2020). In addition, one previous study demonstrated that the positive dogs in Hong Kong were infected by COVID‐19 patient (Sit et al., 2020). Consistently, our data also suggested the possibility of human‐to‐dog transmission of SARS‐CoV‐2. However, so far, no evidence for dog‐to‐human transmission has been available. We obtained no information about COVID‐19‐like symptoms of positive dogs by communicating with the dog keepers. Therefore, we could determine neither the time when the dogs were infected nor the duration of the antibody in the body.
We also extracted RNA from oropharyngeal and rectal swabs from the dogs and conducted SARS‐CoV‐2‐specific qRT‐PCR using a commercial kit to detect ORF1ab and N genes according to the manufacturer's instruction with CT values less than 37 defined as positive. However, none of dogs were detected to be positive, which could be explained the results of the previous study of dog susceptibility to SARS‐CoV‐2 that the virus survives in dogs for no more than one week, even if they are infected with a high dose of the virus(Shi et al., 2020). Therefore, the main reasons for no detected RNA might be due to the low virus load and short shedding period of the virus in dogs. In addition, asymptomatic infection is hard to identify (Sit et al., 2020). Moreover, our Western blotting results indicated that antibody of SARS‐CoV‐2‐S protein in positive dog serum was less easily to be detected than that of SARS‐CoV‐2‐N protein. This may be due to the fact that the antibody of S protein tends to appear early and subside early, while the antibody of N protein has a relatively higher titre and a longer decay period. One previous study has reported that the N protein‐specific IgG is abundant in the sera of COVID‐19 patients (Liu et al., 2020).
Currently, there are more than million diagnosed cases of COVID‐19 worldwide, which greatly increases the probability of animals’ exposure to SARS‐CoV‐2 infection. At present, nine countries including China, the United States, Russia and Germany have reported the cases of SARS‐CoV‐2 infection in animals under natural conditions (Kiros et al., 2020). SARS‐CoV‐2 can infect minks and be transmitted efficiently among minks via respiratory droplets (Shuai et al., 2020). Even, the mink‐specific mutant strains have occurred under natural conditions and transmitted back to humans (Oude Munnink et al., 2020). In addition, recent studies have shown that SARS‐CoV‐2 can also adapt in mice within a short period of time (Wang et al., 2020). These findings suggest that SARS‐CoV‐2 tends to adapt to new host species. Therefore, it is also very necessary to reduce the transmission of SARS‐CoV‐2 from humans to animals and monitor the SARS‐CoV‐2 prevalence in susceptible animal species for controlling the COVID‐19 epidemic.
In conclusion, we carried out a long‐term serological investigation of SARS‐CoV‐2 infection in dogs in Wuhan. Our serological data show that some dogs have been exposed to the virus during COVID‐19 outbreak. However the positive cases have completely disappeared with the end of the epidemic. Since the role of the dog in the transmission of the SARS‐CoV‐2 is still unclear, the continuous serological monitoring in dogs is very necessary.
CONFLICT OF INTEREST
The authors declare no competing interests.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee.
AUTHOR CONTRIBUTIONS
Y. Z., Y. Y., Q. Z. and M. J. conceived and designed the study, wrote the report, drew the figures, and generated, analysed and interpreted data. J. G., C. H., X. H. and C. L. collected the samples. Y. Z., Y. Y., K. H., X. H., W. G., Y. Z., Y. Z. and C. L performed the experiments. H. C. coordinated the study. All authors critically revised the manuscript for important intellectual content and gave final approval for the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
We acknowledge Jiangxia Tongji hospital for providing the convalescent serum of COVID‐19 patient. We are particularly grateful to Wuhan National Biosafety Laboratory running team, including engineer, biosafety, biosecurity and administrative staff.
Zhao Y, Yang Y, Gao J, et al. A serological survey of severe acute respiratory syndrome coronavirus 2 in dogs in Wuhan. Transbound Emerg Dis.2022;69:591–597. 10.1111/tbed.14024
Ya Zhao and Yong Yang contributed equally to this work.
Contributor Information
Qiang Zhang, Email: jml8328@126.com, Email: 05yisan@163.com.
Meilin Jin, Email: jml8328@126.com, Email: 05yisan@163.com.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no data sets were generated or analysed during the current study.
REFERENCES
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , Qiu, Y. , Wang, J. , Liu, Y. , Wei, Y. , Xia, J. , Yu, T. , Zhang, X. , & Zhang, L. I. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet, 395(10223), 507–513. 10.1016/s0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, D. H. , McCausland, M. M. , Valdez, C. , Huynh, D. , Hernandez, J. E. , Mu, Y. , Hirst, S. , Villarreal, L. , Felgner, P. L. , & Crotty, S. (2005). Vaccinia Virus H3L Envelope Protein Is a Major Target of Neutralizing Antibodies in Humans and Elicits Protection against Lethal Challenge in Mice. Journal of Virology, 79(18), 11724–11733. 10.1128/jvi.79.18.11724-11733.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit, E. , van Doremalen, N. , Falzarano, D. , & Munster, V. J. (2016). SARS and MERS: Recent insights into emerging coronaviruses. Nature Reviews Microbiology, 14(8), 523–534. 10.1038/nrmicro.2016.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, J. , Billal, D. S. , Lupien, A. , Racine, G. , Winstall, E. , Legare, D. , & Ouellette, M. (2011). Proteomic and transcriptomic analysis of linezolid resistance in Streptococcus pneumoniae. Journal of Proteome Research, 10(10), 4439–4452. 10.1021/pr200221s [DOI] [PubMed] [Google Scholar]
- Haagmans, B. L. , Al Dhahiry, S. H. S. , Reusken, C. B. E. M. , Raj, V. S. , Galiano, M. , Myers, R. , Godeke, G.‐J. , Jonges, M. , Farag, E. , Diab, A. , Ghobashy, H. , Alhajri, F. , Al‐Thani, M. , Al‐Marri, S. A. , Al Romaihi, H. E. , Al Khal, A. , Bermingham, A. , Osterhaus, A. D. M. E. , AlHajri, M. M. , & Koopmans, M. P. G. (2014). Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. The Lancet Infectious Diseases, 14(2), 140–145. 10.1016/s1473-3099(13)70690-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann, P. J. , Hatta, M. , Chiba, S. , Maemura, T. , Fan, S. , Takeda, M. , Kinoshita, N. , Hattori, S.‐I. , Sakai‐Tagawa, Y. , Iwatsuki‐Horimoto, K. , Imai, M. , & Kawaoka, Y. (2020). Transmission of SARS‐CoV‐2 in Domestic Cats. The New England Journal of Medicine, 383(6), 592–594. 10.1056/NEJMc2013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N.‐H. , Nitsche, A. , Müller, M. A. , Drosten, C. , & Pöhlmann, S. (2020). SARS‐CoV‐2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 181(2), 271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, B. , Guo, H. , Zhou, P. , & Shi, Z. L. (2020). Characteristics of SARS‐CoV‐2 and COVID‐19. Nature Reviews Microbiology, 19(3), 141–154. 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler, E. , Thiel, V. , & Weber, F. (2016). Interaction of SARS and MERS Coronaviruses with the Antiviral Interferon Response. Advances in Virus Research, 96, 219–243. 10.1016/bs.aivir.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiros, M. , Andualem, H. , Kiros, T. , Hailemichael, W. , Getu, S. , Geteneh, A. , Alemu, D. , & Abegaz, W. E. (2020). COVID‐19 pandemic: Current knowledge about the role of pets and other animals in disease transmission. Virology Journal, 17(1), 143. 10.1186/s12985-020-01416-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Liu, W. , Zheng, Y. , Jiang, X. , Kou, G. , Ding, J. , & Zheng, S. (2020). A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in 238 admitted hospital patients. Microbes and Infection, 22(4–5), 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, Q.‐X. , Tang, X.‐J. , Shi, Q.‐L. , Li, Q. , Deng, H.‐J. , Yuan, J. , Hu, J.‐L. , Xu, W. , Zhang, Y. , Lv, F.‐J. , Su, K. , Zhang, F. , Gong, J. , Wu, B. O. , Liu, X.‐M. , Li, J.‐J. , Qiu, J.‐F. , Chen, J. , & Huang, A.‐L. (2020). Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nature Medicine, 26(8), 1200–1204. 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- Lu, H. , Stratton, C. W. , & Tang, Y. W. (2020). Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. Journal of Medical Virology, 92(4), 401–402. 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink, B. B. , Sikkema, R. S. , Nieuwenhuijse, D. F. , Molenaar, R. J. , Munger, E. , Molenkamp, R. , van der Spek, A. , Tolsma, P. , Rietveld, A. , Brouwer, M. , Bouwmeester‐Vincken, N. , Harders, F. , Hakze‐van der Honing, R. , Wegdam‐Blans, M. C. A. , Bouwstra, R. J. , GeurtsvanKessel, C. , van der Eijk, A. A. , Velkers, F. C. , Smit, L. A. M. , … Koopmans, M. P. G. (2020). Transmission of SARS‐CoV‐2 on mink farms between humans and mink and back to humans. Science, 371(6525), 172–177. 10.1126/science.abe5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, E. I. , Elia, G. , Grassi, A. , Giordano, A. , Desario, C. , Medardo, M. , Smith, S. L. , Anderson, E. R. , Prince, T. , Patterson, G. T. , Lorusso, E. , Lucente, M. S. , Lanave, G. , Lauzi, S. , Bonfanti, U. , Stranieri, A. , Martella, V. , Solari Basano, F. , Barrs, V. R. , … Decaro, N. (2020). Evidence of exposure to SARS‐CoV‐2 in cats and dogs from households in Italy. Nature Communications, 11(1), 6231. 10.1038/s41467-020-20097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , Liu, R. , He, X. , Shuai, L. , Sun, Z. , Zhao, Y. , Liu, P. , Liang, L. , Cui, P. , Wang, J. , Zhang, X. , Guan, Y. , Tan, W. , Wu, G. , … Bu, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science, 368(6494), 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai, L. , Zhong, G. , Yuan, Q. , Wen, Z. , Wang, C. , He, X. , Liu, R. , Wang, J. , Zhao, Q. , Liu, Y. , Huo, N. , Deng, J. , Bai, J. , Wu, H. , Guan, Y. , Shi, J. , Tian, K. , Xia, N. , Chen, H. , & Bu, Z. (2020). Replication, pathogenicity, and transmission of SARS‐CoV‐2 in minks. National Science Review, 8(3), 1–8. 10.1093/nsr/nwaa291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, T. H. C. , Brackman, C. J. , Ip, S. M. , Tam, K. W. S. , Law, P. Y. T. , To, E. M. W. , Yu, V. Y. T. , Sims, L. D. , Tsang, D. N. C. , Chu, D. K. W. , Perera, R. A. P. M. , Poon, L. L. M. , & Peiris, M. (2020). Infection of dogs with SARS‐CoV‐2. Nature, 586(7831), 776–778. 10.1038/s41586-020-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, X. , Tian, Y. , Zhou, H. , Li, Y. , Zhang, Z. , Jiang, B. , Yang, B. , Zhang, J. , & Fang, J. (2018). Inactivation Efficacy of Nonthermal Plasma‐Activated Solutions against Newcastle Disease Virus. Applied and Environment Microbiology, 84(9), 1–12. 10.1128/AEM.02836-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Shuai, L. , Wang, C. , Liu, R. , He, X. , Zhang, X. , Sun, Z. , Shan, D. , Ge, J. , Wang, X. , Hua, R. , Zhong, G. , Wen, Z. , & Bu, Z. (2020). Mouse‐adapted SARS‐CoV‐2 replicates efficiently in the upper and lower respiratory tract of BALB/c and C57BL/6J mice. Protein Cell, 11(10), 776–782. 10.1007/s13238-020-00767-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, K. , Zhai, J. , Feng, Y. , Zhou, N. , Zhang, X. U. , Zou, J.‐J. , Li, N. A. , Guo, Y. , Li, X. , Shen, X. , Zhang, Z. , Shu, F. , Huang, W. , Li, Y. U. , Zhang, Z. , Chen, R.‐A. , Wu, Y.‐J. , Peng, S.‐M. , Huang, M. , … Shen, Y. (2020). Isolation of SARS‐CoV‐2‐related coronavirus from Malayan pangolins. Nature, 583(7815), 286–289. 10.1038/s41586-020-2313-x [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Zhang, H. , Gao, J. , Huang, K. , Yang, Y. , Hui, X. , He, X. , Li, C. , Gong, W. , Zhang, Y. , Zhao, Y. A. , Peng, C. , Gao, X. , Chen, H. , Zou, Z. , Shi, Z.‐L. , & Jin, M. (2020). A serological survey of SARS‐CoV‐2 in cat in Wuhan. Emerging Microbes & Infections, 9(1), 2013–2019. 10.1080/22221751.2020.1817796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , Si, H.‐R. , Zhu, Y. , Li, B. , Huang, C.‐L. , Chen, H.‐D. , Chen, J. , Luo, Y. , Guo, H. , Jiang, R.‐D. , Liu, M.‐Q. , Chen, Y. , Shen, X.‐R. , Wang, X. I. , … Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no data sets were generated or analysed during the current study.


