Abstract
The impact of the newly discovered severe acute respiratory syndrome coronavirus 2 causing coronavirus disease‐19 (COVID‐19) in hemodialysis patients remains poorly characterized. Some hemodialysis techniques reduce systemic inflammation but their impact on COVID‐19 has not been addressed. The aim of this prospective study was to evaluate factors associated with mortality in COVID‐19 hemodialysis patients, including the impact of reducing interleukin‐6 using a cytokine adsorbent filter. This is a prospective single‐center study including 16 hemodialysis patients with COVID‐19. All were dialyzed using a polymethyl methacrylate (PMMA) filter. Interleukin‐6 levels were obtained before and after the first admission hemodialysis session and at 1 week. Baseline comorbidities, laboratory values, chest X‐ray, and treatments were recorded and compared between survivors and non‐survivors. Out of 16 patients (13 males, mean age 72 ± 15 years), 4 (25%) died. Factors associated with mortality were dialysis vintage (P = 0.01), chest X‐ray infiltrates (P = 0.032), serum C‐reactive protein (P = 0.05), and lactate dehydrogenase (P = 0.02) at 1 week, oxygen therapy requirement (P = 0.02) and anticoagulation (P < 0.01). At admission, non‐survivors had higher predialysis and postdialysis interleukin‐6 levels (P = 0.02 for both) and did not present the reduction of interleukin‐6 levels during the dialysis session with PMMA filter that was observed in survivors (survivors vs. non‐survivors: 25.0 [17.5–53.2]% vs. −2.8 [−109.4–12.8]% reduction, P = 0.04). A positive balance of interleukin‐6 during the admission dialysis was associated with mortality (P = 0.008). In conclusion, in hemodialysis COVID‐19 patients, a positive interleukin‐6 balance during the admission hemodialysis session was associated with higher mortality.
Keywords: COVID‐19, hemodialysis, interleukin‐6, mortality, polymethyl methacrylate, SARS‐CoV2
1. INTRODUCTION
A new disease caused by a coronavirus emerged in Wuhan (China) in 2019 named severe acute respiratory syndrome (SARS) coronavirus 2 (SARS‐CoV‐2) [1]. Although coronavirus disease‐19 (COVID‐19) has spread worldwide, there are still multiple unknown issues. It has a very wide spectrum of symptoms ranging from asymptomatic infection to severe respiratory damage with multi‐organ disorder [2]. Factors associated with poor outcomes include older age, chronic lung disease, cardiovascular disease, diabetes mellitus, obesity, immunocompromise, liver disease, and end‐stage renal disease [3]. Kidneys are frequently affected generating proteinuria, hematuria, and renal dysfunction but in a reversible mode if infection is resolved [4, 5]. However, renal impairment worsens the prognosis of COVID‐19, enhancing the risk of mortality [5]. Although the scarce information available in patients in dialysis suggests a high‐risk of complications, only case series have been published [6, 7, 8]. The largest study, by Goicoechea et al. demonstrated in a retrospective analysis of 36 hemodialysis patients, a higher mortality (30.5%) than in the general population [8]. The impaired immunity of patients with chronic kidney disease (especially those in dialysis), the chronic microinflammation status, and the presence of comorbidities probably contribute to this enhanced mortality [9, 10].
One of the most important issues in COVID‐19 is the lack of an effective therapy and a vaccine [11]. Patients with severe disease suffer an inflammatory reaction mediated by a cytokine storm, typically regulated by interferon gamma, tumor necrosis factor alpha, interleukin‐17, interleukin‐8, and interleukin‐6, leading to severe pneumonia with hypoxia and vascular injury. Ongoing clinical trials are evaluating some therapies to block these cytokines in COVID‐19 [12], while the anti‐interleukin‐6 agent tocilizumab has been used off‐label.
Some hemodialysis techniques remove inflammatory mediators including interleukins. On the one hand, convective transport is more effective in reducing systemic inflammation through clearance of middle‐size molecules [13]. On the other hand, some hemodialysis filters adsorb cytokines [14, 15]. However, until now no data are available on the effect of these filters on the cytokine storm of COVID‐19 and the potential impact on outcomes.
The aim of the present study is to evaluate the impact of COVID‐19 in a cohort of chronic hemodialysis patients and the relationship between hemodialysis interleukin‐6 dynamics and mortality.
2. PATIENTS AND METHODS
In this observational, single‐center, prospective study, we included 16 consecutive hemodialysis patients with COVID‐19 confirmed infection according to World Health Organization interim guidance, using positive real‐time reverse transcription‐polymerase chain reaction testing for SARS‐CoV‐2 in throat‐swab specimens from March 15 to April 28, 2020. Inclusion criteria were patients in hemodialysis program older than 18 years and clinically stable: absence of baseline chronic inflammation (defined as CRP in the last 3 months lower than 0.5 mg/dL), no hospitalization in the last 4 weeks and the absence of active neoplasia. In addition, we collected serum samples from eight patients of our unit as controls: four in online hemodiafiltration (OL‐HDF) and four in high‐flux hemodialysis. These control patients met the same inclusion and exclusion criteria as COVID‐19 patients.
Epidemiological, demographical, treatment, and clinical data were collected basally. Registered comorbidities were hypertension, diabetes, asthma, atrial fibrillation, history of coronary artery disease, and chronic obstructive pulmonary disease (COPD). Regarding treatment, we collected information on statins and renin‐angiotensin‐aldosterone system (RAAS) blockade.
Patients with confirmed COVID‐19 admitted to the hospital were prescribed thrice weekly high‐flux hemodialysis sessions during 4 h with a polymethyl methacrylate (PMMA) filter (Toray NF‐2.1H, effective surface area 2.1 m2, ultrafiltration coefficient 55 mL/h/mm Hg, inner diameter 200 μm) with adsorptive function [14]. At admission, we registered blood pressure, respiratory rate, and symptoms (fever defined as temperature higher than 37.5°C, diarrhea, anosmia, dysgeusia, myalgia, dyspnea, and cough). In the first dialysis session, we collected hemogram, CRP, procalcitonin, coagulation profile (with D‐dimer), interleukin‐6 (molecular weight 21 kDa), lactate dehydrogenase (LDH), and serum ferritin. After the session, a new blood sample was obtained to determine postdialysis interleukin‐6. All patients had a chest X‐ray at admission and, at least a second one 7 days later. In control patients, we determined interleukin‐6 before the hemodialysis session. Serum interleukin‐6 levels were quantified in duplicate with the human interleukin‐6 quantikine high sensitivity enzyme‐immune assay (R&D Systems Europe Ltd., Abingdon, UK). The intra‐assay and inter‐assay variability were 2.9% and 4.8% respectively. The parameter interleukin‐6 balance during dialysis was defined based on changes in interleukin‐6 levels between the predialysis and the postdialysis samples obtained at the admission hemodialysis session. The balance was considered to be positive if serum interleukin‐6 increased more than 2 pg/mL, neutral if the difference was ±2 pg/mL and negative if serum interleukin‐6 decreased more than 2 pg/mL. Reduction percentages for interleukin‐6 were calculated using the formulae: [(predialysis interleukin‐6 − post‐dialysis interleukin‐6)/value predialysis interleukin‐6] * 100, corrected using the method of Bergström and Wehle [16]. Negative balance was calculated using the average of the last three sessions before the admission.
Treatment for COVID‐19 was prescribed according to local guidelines and included hydroxychloroquine, azithromycin, lopinavir–ritonavir, methylprednisolone, and/or hydroxychloroquine, depending on evolution and severity of the disease.
Patients were followed during hospitalization and after discharge until COVID‐19 was recovered or until death. In the 7‐day hemodialysis session, we collected a new analysis including the same parameters as in the initial session.
All patients signed an informed consent. Local ethical committee approved the study with the reference BQG‐ALI‐2020‐01.
2.1. Statistics
Values are expressed as mean ± standard deviation or median (interquartile range) depending on their distribution, tested using the Kolmogorov–Smirnov test. Patients were divided into two groups regarding the vital situation at the end of the follow‐up (survival or non‐survival). Baseline information, data at admission and during follow‐up was compared in both groups using nonparametric test (Fisher exact test for categorical variables or Mann–Whitney test for continuous variables). Interleukin‐6 determinations were also compared in patients with COVID‐19 and in control patients. In the first hemodialysis session, we calculated the percentage reduction of interleukin‐6 to assess the capacity of the PMMA filter to eliminate cytokines and its association with mortality.
All statistical analyses were performed with SPSS 24.0 (SPSS, Inc., Chicago, IL, USA). Figures were drawn with GraphPad Prism 6.0 (GraphPad Software Inc, San Diego, CA, USA). P values <0.05 were considered statistically significant.
3. RESULTS
3.1. Baseline characteristics
A total of 16 COVID‐19 hemodialysis patients were included, 13 (81%) were male and the mean age was 72 ± 15 years (Table 1). Thirteen patients (81%) presented hypertension, 4 (25%) were on RAAS blockers; 7 (44%) were diabetic, and 3 (19%) had COPD.
TABLE 1.
Baseline characteristics
| Total (n = 16) | Survivors (n = 12) | Non‐survivors (n = 4) | p | |
|---|---|---|---|---|
| Sex (male) (n, %) | 13 (81) | 9 (75) | 4 (100) | 0.26 |
| Age (years) | 72 ± 15 | 69 ± 17 | 79 ± 4 | 0.27 |
| Hypertension (n, %) | 13 (81) | 11 (92) | 2 (50) | 0.06 |
| Dyslipidemia (n, %) | 9 (56) | 6 (50) | 3 (75) | 0.38 |
| Diabetes mellitus (n, %) | 7 (44) | 4 (33) | 3 (75) | 0.14 |
| History of coronary artery disease (n, %) | 2 (12) | 2 (17) | 0 (0) | 0.38 |
| Atrial fibrillation (n, %) | 2 (12) | 1 (8) | 1 (25) | 0.38 |
| Chronic obstructive pulmonary disease (n, %) | 3 (19) | 1 (8) | 2 (50) | 0.06 |
| Vascular access (fistulae, %) | 8 (50) | 6 (50) | 2 (50) | 1.00 |
| Hemodialysis technique (OL‐HDF) (n, %) | 16 (100) | 12 (100) | 4 (100) | — |
| Dialysis vintage (months) | 22 (8–43) | 18 (16–29) | 77 (13–171) | 0.01 |
| Renin‐angiotensin‐aldosterone system blockers (n, %) | 4 (25) | 4 (33) | 0 (0) | 0.18 |
| Statins (n, %) | 7 (44%) | 5 (42) | 2 (50) | 0.77 |
| Anticoagulants (n, %) | 2 (12%) | 1 (8) | 1 (25) | 0.38 |
| Immunosuppressive drugs (n, %) | 1 (6%) | 1 (8) | 0 (0) | 0.55 |
Note: Values are expressed as mean ± standard deviation or median (interquartile range).
Abbreviation: OL‐HDF, online hemodiafiltration.
Before admission, all the patients were receiving OL‐HDF with a 12‐h per week schedule and 8 (50%) had an arteriovenous fistulae as vascular access. The median dialysis vintage was 22 (8–43) months. The mean negative balance was 1.3 ± 0.8 L/session.
3.2. Initial clinical symptoms and laboratory values
Seven patients (44%) were asymptomatic and nine patients (56%) required hospitalization. As shown in Table 2, the most frequent symptom was fever (eight patients, 50%), followed by cough (five patients, 31%). None of the patients referred anosmia, dysgeusia, or myalgias. At admission, patients showed mild lymphopenia with elevation in inflammatory markers (CRP, procalcitonin, and ferritin) and D‐dimer. Being symptomatic was associated to more severe lymphopenia at admission (P = 0.04), lower oxygen partial pressure at admission (P = 0.04) and at first week (P = 0.01), more requirements of oxygen therapy (P = 0.04) and higher breathing frequency (P = 0.04) (Table 3). Before the first hemodialysis session following the diagnosis, interleukin‐6 was determined. COVID‐19 patients exhibited higher baseline interleukin‐6 levels than controls in OL‐HDF (P = 0.02) (Figure 1).
TABLE 2.
Clinical and laboratory data during follow‐up
| Total (n = 16) | Surviving (n = 12) | Non‐surviving (n = 4) | P | |
|---|---|---|---|---|
| Initial signs and symptoms a | ||||
| Oxygen saturation (%) | 93 ± 7 | 92 ± 8 | 95 ± 1 | 0.44 |
| Systolic blood pressure (mm Hg) | 136 ± 27 | 135 ± 24 | 139 ± 38 | 0.82 |
| Diastolic blood pressure (mm Hg) | 71 ± 14 | 72 ± 14 | 67 ± 16 | 0.54 |
| Breathing frequency (bpm) | 18 ± 7 | 18 ± 8 | 18 ± 2 | 0.97 |
| Cough (n, %) | 5 (31) | 3 (25) | 2 (50) | 0.35 |
| Fatigue (n, %) | 1 (6) | 1 (8) | 0 (0) | 0.55 |
| Fever (n, %) | 8 (50) | 6 (50) | 2 (50) | 1.00 |
| Diarrhea (n, %) | 1 (6) | 1 (8) | 0 (0) | 0.55 |
| Laboratory values at admission | ||||
| Leukocyte count (/mm3) | 7601 ± 4984 | 6248 ± 2987 | 11 660 ± 7888 | 0.05 |
| Neutrophils (%) | 74 ± 12 | 71 ± 11 | 84 ± 9 | 0.05 |
| Lymphocytes count (/mm3) | 927 ± 455 | 976 ± 483 | 780 ± 376 | 0.47 |
| Procalcitonin (ng/mL) | 0.75 (0.34–1.13) | 0.64 (0.29–1.41) | 0.77 (0.54–0.89) | 0.67 |
| C‐reactive protein (mg/dL) | 6.1 (0.9–12.1) | 4.6 (0.3–11.6) | 10.9 (6.6–12.4) | 0.26 |
| Lactate dehydrogenase (UI/L) | 215 (166–259) | 202 (154–255) | 235 (195–398) | 0.39 |
| D‐dimer (μg/mL) | 1.1 (0.8–1.7) | 1.0 (0.5–1.7) | 1.3 (0.9–2.4) | 0.97 |
| Ferritin (ng/mL) | 842 (384–1539) | 842 (543–1259) | 1232 (153–2471) | 0.53 |
| Oxygen partial pressure (mm Hg) | 77 ± 13 | 76 ± 14 | 80 ± 8 | 0.72 |
| Predialysis interleukin‐6 (pg/mL) | 13.9 (2.0–41.6) | 6.3 (0.5–35.3) | 45.7 (18.1–87.9) | 0.02 |
| Postdialysis interleukin‐6 (pg/mL) | 12.1 (2.2–34.2) | 3.8 (1.8–27.0) | 57.6 (14.8–140.7) | 0.02 |
| Reduction in interleukin‐6 (%) b | 23.6 (0.0–40.1) | 25.0 (17.5–53.2) | −2.8 (−109.4–12.8) | 0.04 |
| Chest X‐ray infiltrates at admission | ||||
| Normal (n, %) | 6 (37) | 5 (41) | 1 (25) | 0.03 |
| Unilateral (n, %) | 2 (13) | 0 (0) | 2 (50) | |
| Bilateral (n, %) | 8 (50) | 7 (59) | 1 (25) | |
| Treatment received | ||||
| Corticosteroids (n, %) | 4 (25) | 2 (17) | 2 (50) | 0.18 |
| Anticoagulation (n, %) | 4 (25) | 1 (8) | 3 (75) | <0.01 |
| Hydroxychloroquine (n, %) | 8 (50) | 6 (50) | 2 (50) | 1.00 |
| Azithromycin (n, %) | 9 (56) | 6 (50) | 3 (75) | 0.38 |
| Ceftriaxone (n, %) | 6 (37) | 4 (33) | 2 (50) | 0.55 |
| Lopinavir/ritonavir (n, %) | 1 (6) | 1 (8) | 0 (0) | 0.55 |
| Oxygen therapy (n, %) | 8 (50) | 4 (33) | 4 (100) | 0.02 |
| Laboratory values at 1 week | ||||
| Leukocyte count (/mm3) | 8460 ± 4298 | 6792 ± 2039 | 13 462 ± 5711 | <0.01 |
| Neutrophils (%) | 75 ± 10 | 71 ± 8 | 86 ± 3 | <0.01 |
| Lymphocytes count (/mm3) | 1021 ± 354 | 1044 ± 382 | 952 ± 285 | 0.67 |
| Procalcitonin (ng/mL) | 0.4 (0.2–0.9) | 0.3 (0.2–0.8) | 1.6 (0.4–12.7) | 0.13 |
| C‐reactive protein (mg/dL) | 3.6 (0.8–6.1) | 3.1 (0.3–5.0) | 5.4 (3.8–22.9) | 0.05 |
| Lactate dehydrogenase (UI/L) | 217 (156–309) | 198 (155–276) | 423 (179–624) | 0.02 |
| D‐dimer (μg/mL) | 1.6 (0.8–2.5) | 1.6 (0.8–2.5) | 1.6 (0.9–4.0) | 0.72 |
| Interleukin‐6 (pg/mL) | 8.2 (1.4–42.3) | 8.2 (0.2–12.3) | 34.1 (5.0–72.7) | 0.40 |
| Ferritin (ng/mL) | 1028 (480–1613) | 928 (480–1125) | 1868 (570–3417) | 0.19 |
| Oxygen partial pressure (mm Hg) | 79 ± 21 | 84 ± 20 | 65 ± 22 | 0.14 |
| Infiltrates in chest X‐ray at 1 week | ||||
| Normal (n, %) | 8 (50) | 8 (67) | 0 (0) | 0.03 |
| Unilateral (n, %) | 1 (6) | 0 (0) | 1 (25) | |
| Bilateral (n, %) | 7 (44) | 4 (33) | 3 (75) | |
Note: Values are expressed as mean ± standard deviation or median (interquartile range).
No patient presented anosmia, dysgeusia or myalgias.
During the dialysis session. A positive value means that interleukin‐6 levels were lower postdialysis session than predialysis.
TABLE 3.
Differences between symptomatic and asymptomatic patients
| Symptomatic (n = 9) | Asymptomatic (n = 7) | P | |
|---|---|---|---|
| Initial signs and symptoms a | |||
| Oxygen saturation (%) | 90 ± 9 | 96 ± 1 | 0.31 |
| Systolic blood pressure (mm Hg) | 140 ± 28 | 130 ± 25 | 1.00 |
| Diastolic blood pressure (mm Hg) | 73 ± 17 | 70 ± 9 | 1.00 |
| Breathing frequency (bpm) | 21 ± 9 | 14 ± 2 | 0.04 |
| Laboratory values at admission | |||
| Leukocyte count (/mm3) | 6417 ± 4663 | 9122 ± 5321 | 0.31 |
| Neutrophils (%) | 77 ± 12 | 71 ± 12 | 1.00 |
| Lymphocytes count (/mm3) | 716 ± 369 | 1198 ± 430 | 0.04 |
| Procalcitonin (ng/mL) | 0.9 (0.5–1.7) | 0.3 (0.2–0.8) | 1.00 |
| C‐reactive protein (mg/dL) | 9.6 (5.1–14.1) | 0.3 (0.2–10.8) | 0.31 |
| Lactate dehydrogenase (UI/L) | 229 (200–273) | 160 (138–228 | 0.31 |
| D‐dimer (μg/mL) | 1.5 (0.9–2.3) | 0.9 (0.4–1.4) | 0.31 |
| Ferritin (ng/mL) | 1166 (467–1948) | 564 (334–1135) | 0.31 |
| Oxygen partial pressure (mm Hg) | 67 ± 11 | 89 ± 4 | 0.01 |
| Predialysis interleukin‐6 (pg/mL) | 28.4 (10.9–52.6) | 3.0 (0.0–29.1) | 0.31 |
| Postdialysis interleukin‐6 (pg/mL) | 21.6 (7.2–86.2) | 3.0 (1.7–21.9) | 0.31 |
| Reduction in interleukin‐6 (%)b | 23 ([−36]–36) | 25 (0–44) | 1.00 |
| Chest X‐ray infiltrates at admission | |||
| Normal (n, %) | 2 (22) | 4 (57) | 0.22 |
| Unilateral (n, %) | 2 (22) | 0 (0) | |
| Bilateral (n, %) | 5 (56) | 3 (43) | |
| Treatment received | |||
| Corticosteroids (n, %) | 4 (45) | 0 (0) | 0.04 |
| Anticoagulation (n, %) | 2 (22) | 2 (29) | 0.77 |
| Hydroxychloroquine (n, %) | 5 (56) | 3 (43) | 0.61 |
| Azithromycin (n, %) | 6 (67) | 3 (43) | 0.34 |
| Ceftriaxone (n, %) | 6 (67) | 0 (0) | 0.01 |
| Lopinavir/ritonavir (n, %) | 1 (11) | 0 (0) | 1.00 |
| Oxygen therapy (n, %) | 7 (78) | 1 (14) | 0.04 |
| Laboratory values at 1 week | |||
| Leukocyte count (/mm3) | 8331 ± 4269 | 8625 ± 4672 | 1.00 |
| Neutrophils (%) | 76 ± 10 | 73 ± 10 | 1.00 |
| Lymphocytes count (/mm3) | 898 ± 248 | 1178 ± 423 | 0.31 |
| Procalcitonin (ng/mL) | 0.5 (0.3–1.7) | 0.4 (0.1–0.9) | 1.00 |
| C‐reactive protein (mg/dL) | 4.8 (2.8–8.4) | 1.9 (0.1–6.2) | 0.61 |
| Lactate dehydrogenase (UI/L) | 259 (173–354) | 173 (127–225) | 0.31 |
| D‐dimer (μg/mL) | 1.9 (1.2–3.1) | 0.8 (0.3–2.7) | 1.00 |
| Interleukin‐6 (pg/mL) | 16.6 (10.9–40.2) | 3.0 (0–29) | 0.04 |
| Ferritin (ng/mL) | 1107 (928–1868) | 645 (367–1131) | 0.31 |
| Oxygen partial pressure (mm Hg) | 69 ± 15 | 92 ± 22 | 0.04 |
| Infiltrates in chest X‐ray at 1 week | |||
| Normal (n, %) | 2 (22) | 6 (86) | 0.04 |
| Unilateral (n, %) | 1 (11) | 0 (0) | |
| Bilateral (n, %) | 6 (67) | 1 (14) | |
Note: Values are expressed as mean ± standard deviation or median (interquartile range).
During the dialysis session. A positive value means that interleukin‐6 levels were lower after the dialysis session than before.
FIGURE 1.
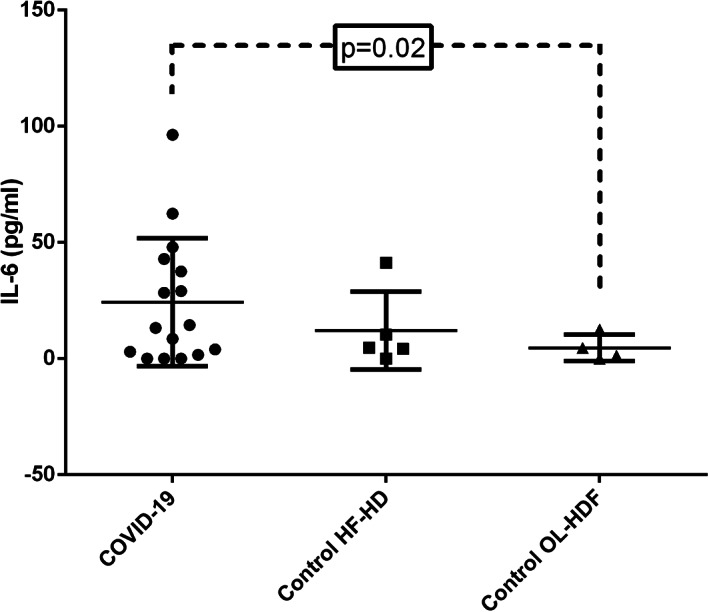
Baseline predialysis interleukin‐6 levels were higher in COVID‐19 than in control hemodialysis patients. COVID‐19, coronavirus disease‐19; HF‐HD, high‐flux hemodialysis; IL‐6, interleukin‐6; OL‐HDF, online hemodiafiltration
3.3. Radiological findings
Chest X‐ray was performed at admission and after 7 days. Ten (63%) patients had infiltrates that were bilateral in 8 (50%). After 1 week, infiltrates improved in two patients. The presence of symptoms at admission was associated with higher possibility of having chest X‐ray infiltrates at 1 week (P = 0.04) (Table 3).
3.4. Treatment
Treatment was based on local guidelines. Nine patients (56%) received azithromycin, eight (50%) received hydroxychloroquine, four (25%) received corticosteroids, and four (25%) were anticoagulated (two of them were previously receiving low molecular weight heparin and had severity criteria for continuing the therapy). Eight (50%) patients needed oxygen therapy. No patient received tocilizumab. Complete data are shown in Table 2. Being symptomatic at admission was associated to higher prescription of corticosteroids (P = 0.04) and ceftriaxone (P = 0.01) (Table 3).
3.5. Outcomes
Four patients (25%) died during follow‐up. Univariate analysis showed that dialysis vintage was the only baseline factor associated with higher mortality (P = 0.01). Importantly, symptoms at presentation did not differ between survivors and non‐survivors (Table 1).
Regarding in‐hospital factors, the presence of chest X‐ray infiltrates at admission (P = 0.032) and at 1 week (P = 0.032), and higher leukocyte and neutrophil counts, especially at 1 week (P < 0.01 for both) were associated to mortality (Table 2). In addition, LDH and C‐reactive protein values at 1 week were higher in patients who did not survive (P = 0.02 and P = 0.05, respectively) (Table 2). Non‐surviving patients needed more frequent oxygen therapy (P = 0.02) and anticoagulation (P < 0.01) (Table 2).
3.6. Serum interleukin‐6 values and outcomes
Serum interleukin‐6 levels before and after the admission dialysis session were higher in patients who did not survive (Figure 2). As shown in Figure 3, serum interleukin‐6 decreased during the first dialysis session using PMMA filters at admission by a median of 25.0 (17.5–53.2)% in survivors, while this decrease was not observed in patients who died (median −2.8 [−109.4 to 12.8]%) (P = 0.04). A positive balance of interleukin‐6 during the admission dialysis was associated with mortality (P = 0.008).
FIGURE 2.
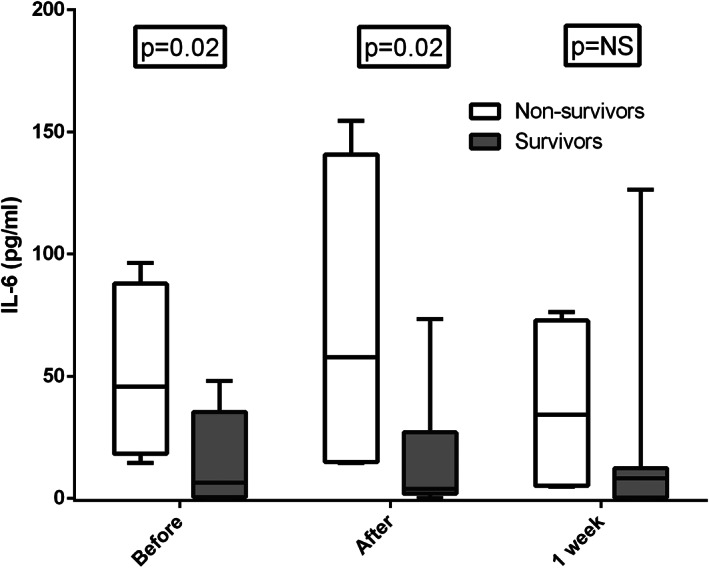
Predialysis and postdialysis interleukin‐6 levels at the admission hemodialysis session and predialysis values at 1 week, comparing surviving and non‐surviving COVID‐19 patients. COVID‐19, coronavirus disease‐19; IL‐6, interleukin‐6
FIGURE 3.

Median reduction in serum interleukin‐6 during the first hemodialysis session with a PMMA filter at admission was higher in surviving than in non‐surviving COVID‐19 patients. A negative value means that serum interleukin‐6 increased during dialysis. COVID‐19, coronavirus disease‐19; PMMA, polymethyl methacrylate
4. DISCUSSION
The main finding of this prospective study is that in hemodialysis COVID‐19 patients, a positive balance of interleukin‐6 during the admission hemodialysis session using PMMA membranes was associated with higher mortality. As this was observed at the first hemodialysis session after hospital admission, this parameter may help to guide a concrete therapy or even inclusion into clinical trials. In this regard, the mortality of COVID‐19 in hemodialysis patients is high: 25% in our cohort, in line with previous literature in hemodialysis patients and higher than in the general population [17, 18]. However, the lack of differences in initial symptoms and most other disease parameters at presentation between survivors and non‐survivors complicates clinical decision‐making.
This is the first report of an extracorporeal technique that can reduce cytokines. In addition, the evaluation of the dynamic changes of cytokines with this technique may also stratify the risk of death [19, 20]. Thus, our study further supports the association between inflammation and mortality. Increased serum interleukin‐6 in COVID‐19 has been established as a marker but also as an effector of the disease. Interleukin‐6 levels at the beginning and at the end of the hemodialysis session were higher in those patients who finally died. Interestingly, we prescribed PMMA, a filter able to clear plasma cytokines. Despite this, in some COVID‐19 hemodialysis patients interleukin‐6 levels were higher after the dialysis session, suggesting that cytokine production exceeded the filter clearance [14]. These findings were not interfered by the use of tocilizumab, an interleukin‐6 blocker, which was not prescribed to these patients. In this regard, pending the results of clinical trials, the successful use of tocilizumab for severe COVID‐19 has been reported, especially if used early in the disease [21]. In addition, only one patient presented residual renal function, a well‐known mechanism for clearing cytokines [22]. The analysis of the dynamic response of serum interleukin‐6 during PMMA hemodialysis may help identify patients at higher risk of death and also potentially more likely to benefit from anti‐interleukin‐6 strategies. This may be of special interest in situations where health services are overwhelmed, limiting tocilizumab availability, or as inclusion criteria for trials of anti‐inflammatory therapy.
Other factors related to poor outcomes were dialysis vintage and more severe pulmonary disease in chest X‐ray with higher requirements of oxygen therapy. These were expected associations, based on the known natural history of end stage renal disease on dialysis and of COVID‐19. There was a dissociation between the presence of shortness of breath at presentation and the radiological severity and outcomes, as previously observed for COVID‐19 [23].
Some inflammatory parameters were also associated with mortality, including higher leukocyte account, C‐reactive protein, and LDH, but this was mainly observed after 1 week of disease course, which is later than the differences in interleukin‐6 dynamic changes during dialysis and potentially too late for prescription of life‐saving therapy. However, other markers that have been systematically associated to mortality as ferritin did not show this association [24]. Hemodialysis patients have an especial iron metabolism interfered by baseline inflammation, iron therapy and erythropoiesis stimulating agents [25].
Until now, the optimal treatment for COVID‐19 is unknown. Only one antiviral drug has been approved recently in the United States and there are reports on the efficacy of anti‐inflammatory, antiviral or antibiotic drugs with controversial results [26]. However, clinical trials are still recruiting. In our series, the association of certain therapies with outcomes was likely associated to developing more severe disease rather than to an adverse impact of therapy.
Early assessment of risk is difficult in COVID‐19 hemodialysis patients. Despite the high mortality, only 56% had symptoms at diagnosis. The only published study that included hemodialysis patients irrespective of their clinical status observed an incidence of SARS‐CoV‐2 infection of 21% [7]. The most common symptoms in our series were fever and cough, in accordance with recently published data [7, 8]. The low frequency of these symptoms, especially fever, in comparison to general population could be explained by the dysregulation of the immune system of the end‐stage‐renal disease patients [27]. As in a Wuhan retrospective study, lymphopenia was associated to the lack of symptoms in hemodialysis patients [7].
It should be noted that the use of cytokine adsorbent filters is not limited to chronic hemodialysis patients and can be used in critically ill patients prescribed continuous renal replacement therapy. A recent retrospective study of 5449 COVID‐19 patients disclosed that 36% developed acute kidney injury and 5% required renal replacement therapy evidencing the importance of support therapies in intensive care units [28].
Our study has some limitations. As it is the case for other COVID‐19 reports in dialysis patients, the sample size is small, and it is a single‐center study. For these reasons, some differences did not reach statistical significance although seemed to be clinically relevant. However, the results provide an important insight into risk stratification in an unstudied population, even with these limitations. Second, we were not able to assess other inflammatory markers such as interferon gamma, tumor necrosis factor alpha, interleukin‐17, or interleukin‐8. In COVID‐19, interleukin‐6 seems to be the most important mediator in the cytokine storm and it is assessed in routine clinical practice. Thus, our findings may have a direct clinical application. Third, we could not compare results in HF‐HD with OL‐HDF due to technical reasons in our COVID hemodialysis unit. The use of convection may have achieved additional clearance of cytokines. Despite these limitations, we understand that the description of the associated factors with mortality in the hemodialysis population and observations regarding the dynamic assessment of the best characterized cytokine in COVID‐19 is clinically relevant in diagnosis, risk stratification and treatment.
5. CONCLUSIONS
In conclusion, in COVID‐19 hemodialysis patients, a positive balance of interleukin‐6, despite the use of a polymethyl methacrylate filter, during the first hemodialysis session at admission was associated with higher mortality. This observation has implications for risk stratification and potentially for the early prescription of anti‐inflammatory therapy.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
We would like to thank the Nephrology Department of the Hospital Universitario de La Princesa (Madrid, Spain) for their hard work during this pandemic.
Quiroga B, Muñoz Ramos P, Giorgi M, et al. Dynamic assessment of interleukin‐6 during hemodialysis and mortality in coronavirus disease‐19. Ther Apher Dial. 2021;25:908–916. 10.1111/1744-9987.13626
REFERENCES
- 1. Jin H, Liu J, Cui M, Lu L. Novel coronavirus pneumonia emergency in Zhuhai: impact and challenges. J Hosp Infect. 2020;104:452–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020;383:1757–66. [DOI] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID‐19 pneumonia. J Am Soc Nephrol. 2020;31:1157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sánchez‐Álvarez JE, Pérez Fontán M, Jiménez Martín C, Blasco Pelícano M, Cabezas Reina CJ, Sevillano Prieto ÁM, et al. SARS‐CoV‐2 infection in patients on renal replacement therapy. Report of the COVID‐19 Registry of the Spanish Society of Nephrology (SEN). Situación de la infección por SARS‐CoV‐2 en pacientes en tratamiento renal sustitutivo. Informe del Registro COVID‐19 de la Sociedad Española de Nefrología (SEN). Nefrologia. 2020;40:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiong F, Tang H, Liu L, Tu C, Tian JB, Lei CT, et al. Clinical characteristics of and medical interventions for COVID‐19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31:1387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goicoechea M, Sánchez Cámara LA, Macías N, Muñoz de Morales A, Rojas ÁG, Bascuñana A, et al. COVID‐19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goicoechea M, Quiroga B, García de Vinuesa S, Verdalles Ú, Reque J, Panizo N, et al. Intraindividual interleukin‐6 variations on the cardiovascular prognosis of patients with chronic renal disease. Ren Fail. 2012;34:1002–9. [DOI] [PubMed] [Google Scholar]
- 10. Stefoni S, DeSanctis LB, Nanni‐Costa A, Iannelli S, Borgnino LC, Buscaroli A, et al. Dialysis and the immune system. Contrib Nephrol. 1995;113:80–91. [DOI] [PubMed] [Google Scholar]
- 11. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;323:1824. [DOI] [PubMed] [Google Scholar]
- 12. Arnaldez FI, O'Day SJ, Drake CG, et al. The Society for Immunotherapy of Cancer perspective on regulation of interleukin‐6 signaling in COVID‐19‐related systemic inflammatory response. J Immunother Cancer. 2020;8:e000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. den Hoedt CH, Bots ML, Grooteman MP, van der Weerd NC, Mazairac AHA, Penne EL, et al. Online hemodiafiltration reduces systemic inflammation compared to low‐flux hemodialysis. Kidney Int. 2014;86:423–32. [DOI] [PubMed] [Google Scholar]
- 14. Nakada TA, Oda S, Matsuda K, Sadahiro T, Nakamura M, Abe R, et al. Continuous hemodiafiltration with PMMA hemofilter in the treatment of patients with septic shock. Mol Med. 2008;14:257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pickkers P, Vassiliou T, Liguts V, Prato F, Tissieres P, Kloesel S, et al. Sepsis management with a blood purification membrane: European experience. Blood Purif. 2019;47(Suppl 3):1–9. [DOI] [PubMed] [Google Scholar]
- 16. Bergström J, Wehle B. No change in corrected beta 2‐microglobulin concentration after cuprophane haemodialysis. Lancet. 1987;1:628–9. [DOI] [PubMed] [Google Scholar]
- 17. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med. 2020;382:e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Scarpioni R, Manini A, Valsania T, De Amicis S, Albertazzi V, Melfa L, et al. Covid‐19 and its impact on nephropathic patients: the experience at Ospedale “Guglielmo da Saliceto” in Piacenza. G Ital Nefrol. 2020;37:2020. [PubMed] [Google Scholar]
- 19. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID‐19: interleukin‐6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID‐19 related pneumonia. Eur J Intern Med. 2020;76:31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leong SC, Sao JN, Taussig A, Plummer NS, Meyer TW, Sirich TL. Residual function effectively controls plasma concentrations of secreted solutes in patients on twice weekly hemodialysis. J Am Soc Nephrol. 2018;29:1992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macdonald JH, Fearn L, Jibani M, Marcora SM. Exertional fatigue in patients with CKD. Am J Kidney Dis. 2012;60:930–9. [DOI] [PubMed] [Google Scholar]
- 24. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakanishi T, Kimura T, Kuragano T. The hepcidin–anemia axis: pathogenesis of anemia in chronic kidney disease. Contrib Nephrol. 2019;198:124–34. [DOI] [PubMed] [Google Scholar]
- 26. Alijotas‐Reig J, Esteve‐Valverde E, Belizna C, Selva‐O'Callaghan A, Pardos‐Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID‐19. Beyond the anti‐viral therapy: a comprehensive review. Autoimmun Rev. 2020;19:102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim KW, Chung BH, Jeon EJ, Kim BM, Choi BS, Park CW, et al. B cell‐associated immune profiles in patients with end‐stage renal disease (ESRD). Exp Mol Med. 2012;44:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID‐19. Kidney Int. 2020;98:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


