Abstract
Pulmonary embolism (PE) is a major cause of cardiovascular morbidity and mortality. Obstructive sleep apnea (OSA) is increasingly recognized in the aging population, especially with the rising obesity epidemic. The impact of OSA on inpatient mortality in PE is not well understood. We used the Nationwide Inpatient Sample databases from 2005 to 2016 to identify 755,532 acute PE patients (age≥18 years). Among these, 61,050 (8.1%) were OSA+. Temporal trends in length of stay, inpatient mortality, and its association with OSA in PE patients were analyzed. The proportion of PE patients who were OSA+ increased from 2005 to 2016. OSA+ PE patients were younger and predominantly men. Despite a higher prevalence of traditional risk factors for inpatient mortality in OSA+ patients, OSA was associated with a lower risk of mortality in PE patients (odds ratio, 95% confidence interval; p: unadjusted 0.56, 0.53–0.58; p < 0.0001 and adjusted 0.55, 0.52–0.58; p < 0.0001). Overall mortality and length of stay in PE patients decreased over time. Relative to OSA– patients, there was a slight increase in mortality among OSA+ PE patients over time, although the length of stay remained unchanged between the two groups. In conclusion, OSA+ PE patients had a lower inpatient mortality compared to OSA– patients despite a higher prevalence of traditional mortality risk factors. Secondary pulmonary hypertension related to OSA with preconditioning of the right ventricle to elevated afterload may potentially explain the protective effect of OSA on mortality in PE. However, mechanistic studies need to further elucidate the links behind this association.
Keywords: pulmonary embolism, in-hospital mortality, obstructive sleep apnea
Acute pulmonary embolism (PE) is a leading cause of cardiovascular mortality second only to stroke and myocardial infarction.1,2 PE is a frequently encountered clinical syndrome with estimated inpatient admissions for PE in the United States rising from 23 per 100,000 in 1993 to 65 per 100,000 in 2012.3,4 Mortality risk in PE patients increases further if there is a delay in recognition or treatment. In the presence of right ventricular (RV) dysfunction, acute PE can cause significant hemodynamic compromise eventually leading to acute right heart failure, cardiogenic shock, and ultimately death. Even in appropriately treated PE patients, mortality is as high as 10% to 15% at one year.5
With the epidemic of obesity, obstructive sleep apnea (OSA) has been recognized as a potential, treatable risk factor for cardiovascular disease.6 Some reports indicate that OSA may be associated with PE and recurrent venous thromboembolism.7,8 OSA is also associated with the development of secondary pulmonary hypertension (PH), World Health Organization Groups II and III, through a variety of mechanisms. Nocturnal hypoxia is commonly associated with untreated OSA, resulting in hypoxic pulmonary vasoconstriction and elevated RV afterload. Chronic carbon dioxide retention and obesity hypoventilation syndromes are also associated with secondary PH. Patients with OSA often have risk factors for left ventricular diastolic dysfunction or have frank diastolic heart failure, and OSA can exacerbate sodium and volume retention in these patients if left untreated.6 Thus, secondary PH and elevated RV afterload is common in patients with OSA, and this may lead to some component of gradual RV preconditioning by which OSA patients may be able to generate higher RV systolic pressures, which may in turn make them less susceptible to acute RV failure in the context of an acute PE. Thus, prevalent OSA may lead to lower mortality from acute PE.
However, whether OSA is associated with mortality in PE patients is not well understood. We utilized a large national database to assess the association between OSA and inpatient mortality in PE patients. We also analyzed the temporal trends in inpatient hospitalizations for PE by prevalent OSA over a 12-year study period from 2005 to 2016. We hypothesized that patients with prevalent OSA presenting with acute PE would have a lower inpatient mortality compared to those without OSA.
Methods
Data source
We utilized the NIS inpatient databases from 2005 to 2016 for this study. NIS, which is a database developed by Healthcare Cost and Utilization Project—a Federal-State-Industry partnership sponsored by Agency Healthcare Research and Quality, contains data on inpatient hospital stays from all participating states under the project (n = 47 in 2016). NIS is the largest, all-payer inclusive inpatient database that is publicly available, and each year contains data on approximately 8 million discharges from more than 1000 hospitals to approximate a 20% stratified and weighted sample of inpatient discharges from all US community hospitals (defined as all non-federal, short-term, general, and other subspecialty hospital, excluding rehabilitation and long-term acute care hospitals). NIS is representative of more than 97% of the US population. Inpatient stay records in the NIS comprise of clinical and other resource utilization information available from discharge abstracts derived from state-mandated discharge reports.9 Discharge weights provided with the NIS files can be used to derive national estimates.10 Of note, beginning 1 October 2015, as hospital administrative data in the US started using International Classification of Diseases, Tenth Edition, Clinical Modification/Procedure Coding System (ICD-10 CM/PCS), the last three months of the 2015 and entire 2016 NIS contained data based on ICD-10-CM/PCS codes, whereas ICD, Ninth Edition (ICD-9-CM/PCS) codes were used before. These changes were accounted for during structuring the NIS databases for these years.11
Study population
From 2005 to 2016, a total of 76,742,940 discharge records were included in the NIS for US adult population (age ≥18), corresponding to a national estimate of 371,006,179 hospitalizations across the country. We identified all patients aged ≥18 hospitalized for PE using ICD-9-CM diagnosis codes of 415.x and ICD-10-CM diagnosis codes of I26.x. We excluded patients with history of PE, PE complicating abortion, pregnancy, childbirth, PE due to trauma, and PE due to complications of surgical and medical care (Supplementary Table 1). Thus, our final study cohort comprised 755,532 patients with PE. The study population was stratified by presence or absence of OSA, defined by multiple ICD-9 or ICD-10 codes seen in Supplementary Table 4. Furthermore, continuous positive airway pressure (CPAP) use was defined using ICD-9-PCS code 93.90 and ICD-10-PCS code 5A09357. Further details regarding patient characteristics are provided in the Supplement.
Outcome measures
Our primary outcome was inpatient mortality. Secondary outcome of interest was length of stay (LOS) during index hospitalization for PE. We also studied temporal trends of mortality in PE patients by prevalent OSA and also compared mortality between patients with and without OSA (OSA+ and OSA–, respectively).
Statistical analysis
Weighted data were used for statistical analyses. We accounted for the weighting, clustering, and stratification needed in the NIS design in all analyses by using STATA’s SVY suite of commands with hospital used as the primary sampling unit. Temporal trends in mortality were assessed using Cochran-Armitage trend test. LOS trend was assessed by multivariable linear regression using log-transformed LOS as the dependent variable and year as a continuous variable. All trend analyses were carried out using NIS provided trend weights to account for NIS redesign in 2012. Association between OSA and inpatient mortality in PE patients was analyzed using multivariable logistic regression. All multivariable regression models were created utilizing generalized estimating equations. All statistical analyses were performed using Stata 15.1 (StataCorp, LP, College Station, TX). To account for multiple testing, Holm–Bonferroni correction was applied such that p < 0.001 was considered for statistical significance. More detailed statistical analysis methods are presented in the Supplement.
Results
Baseline characteristics of the study population
A total of 755,532 patients with hospitalization for PE in the NIS databases were identified from 2005 to 2016. Out of the total study population, 694,482 were OSA– and 61,050 were OSA+ (Table 1). OSA+ patients were younger compared to the OSA– patients (median age, interquartile range (IQR): 61, 51–71 vs. 65, 52–77; p < 0.0001) and less likely to be women (41.1% vs. 53.7%, p < 0.0005). Ethnicities differed between the two groups (p < 0.0005) largely due to differences in the proportion of Caucasian patients (OSA– vs. OSA+: 73.5% vs. 77.2%) and Hispanics (6% vs. 4.5%). Primary payer was more likely to be private insurance in OSA+ patients (34.8% vs. 29.3%) and less likely to be Medicare (49.3% vs. 52.8%) (p < 0.0005). The distribution of patients in income quartiles also differed, with the highest proportion of both subgroups being in the lowest income quartile.
Table 1.
Baseline characteristics of study population stratified by presence or absence of obstructive sleep apnea.
| Parameter | Total population | PE without OSA | PE with OSA | p |
|---|---|---|---|---|
| Total number of cases (weighted) | 755,532 | 694,482 | 61,050 | |
| Age, years | 65 (52–77) | 65 (52–77) | 61 (51–71) | <0.0001 |
| Females | 398,058 (52.7) | 372,950 (53.7) | 25,108 (41.1) | <0.0005 |
| Race | ||||
| White | 485, 194 (73.8) | 443,207 (73.5) | 41,971 (77.2) | <0.001 |
| African American | 107,897 (16.4) | 99,417 (16.5) | 8480 (15.6) | |
| Hispanic | 38,850 (5.9) | 36,414 (6) | 2436 (4.5) | |
| Asian or Pacific Islander | 7412 (1.1) | 7105 (1.2) | 307 (0.6) | |
| Native American | 2925 (0.5) | 2692 (0.5) | 233 (0.4) | |
| Other | 14,984 (2.3) | 14,055 (2.3) | 929 (1.7) | |
| Primary expected payer | ||||
| Medicare | 396,195 (52.5) | 366,139 (52.8) | 30,056 (49.3) | <0.0005 |
| Medicaid | 75,092 (10.0) | 69,247 (10) | 5845 (9.6) | |
| Private insurance | 224,584 (29.8) | 203,352 (29.3) | 21,232 (34.8) | |
| Self-pay | 32,077 (4.2) | 30,287 (4.4) | 1790 (3) | |
| No charge | 3496 (0.5) | 3301 (0.5) | 195 (0.3) | |
| Other | 22,637 (3.0) | 20,789 (3) | 1832 (3) | |
| Median household income | ||||
| 0 to 25th percentile | 220,511 (29.2) | 188,361 (27.7) | 15,832 (26.4) | <0.0005 |
| 26th to 50th percentile | 189,685 (25.1) | 173,966 (25.6) | 15,715 (26.2) | |
| 51st to 75th percentile | 182,085 (24.1) | 166,435 (24.5) | 15,646 (26.1) | |
| 76th to 100th percentile | 163,251 (21.6) | 150,518 (22.2) | 12,729 (21.2) | |
| Clinical history | ||||
| Atrial fibrillations/flutter | 105,727 (14.0) | 95,996 (13.8) | 9731 (15.9) | <0.0001 |
| Alcohol use | 24,837 (3.3) | 23,390 (3.4) | 1447 (2.4) | <0.0005 |
| Catheter-directed thrombolysis | 1709 (0.2) | 1508 (0.2) | 201 (0.3) | <0.0001 |
| Chronic blood loss anemia | 12,242 (1.6) | 11,478 (1.7) | 764 (1.3) | 0.001 |
| Chronic pulmonary disease | 186,436 (24.7) | 163,944 (23.6) | 22,492 (36.8) | <0.0001 |
| Coagulopathy | 56,541 (7.5) | 52,365 (7.5) | 4176 (6.8) | 0.004 |
| Coronary artery disease | 156,448 (20.7) | 140,603 (20.3) | 15,845 (26.0) | <0.0001 |
| Deficiency anemia | 154,719 (20.5) | 143,335 (20.6) | 11,384 (18.7) | <0.0005 |
| Depression | 84,196 (11.1) | 74,135 (10.7) | 10,061 (16.5) | <0.0001 |
| Dyslipidemia | 212,157 (28.1) | 186,727 (26.9) | 25,430 (41.7) | <0.0001 |
| Heart failure | 106,897 (14.2) | 94,311 (13.6) | 12,586 (20.6) | <0.0001 |
| Hypertension | 404,329 (53.5) | 361,589 (52.1) | 42,740 (70.0) | <0.0001 |
| Liver failure | 19,049 (2.5) | 17,254 (2.5) | 1795 (2.9) | 0.001 |
| Lymphoma | 11,149 (1.5) | 10,452 (1.5) | 697 (1.1) | <0.001 |
| Metastatic cancer | 65,512 (8.7) | 63,262 (9.1) | 2250 (3.7) | <0.0001 |
| Obesity | 120,971 (16.0) | 88,059 (12.7) | 32,912 (53.9) | <0.0001 |
| Peripheral vascular disease | 76,053 (10.1) | 68,667 (9.9) | 7386 (12.1) | <0.0001 |
| Renal failure | 78,876 (10.4) | 70,381 (10.1) | 8495 (13.9) | <0.0005 |
| Rheumatoid arthritis/collagen vascular diseases | 26,289 (3.5) | 23,896 (3.4) | 2393 (3.9) | 0.001 |
| Smoking | 176,290 (23.3) | 160,402 (23.1) | 15,888 (26.0) | <0.0001 |
| Solid tumors | 49,624 (6.6) | 46,950 (6.8) | 2674 (4.4) | <0.0001 |
| Substance abuse | 35,739 (4.7) | 33,187 (4.8) | 2552 (4.2) | 0.001 |
| Systemic thrombolysis | 8008 (1.1) | 7194 (1.0) | 814 (1.3) | <0.0001 |
| Thyroid disorders | 85,194 (11.3) | 76,583 (11.0) | 8611 (14.1) | <0.0001 |
| Type 2 diabetes | 157,591 (20.9) | 134,845 (19.4) | 22,746 (37.3) | <0.0001 |
| Valvular disease | 44,400 (5.9) | 40,686 (5.9) | 3714 (6.1) | 0.001 |
| Outcomes | ||||
| Inpatient mortality | 51952 (6.9) | 49,462 (7.1) | 2490 (4.1) | <0.0001 |
| Length of stay | 5 (3–9) | 5 (3–9) | 5 (3–8) | 0.05 |
| Total charge for hospitalization (×$1000) | 31.8 (17.3–64.0) | 31.5 (17.1–63.9) | 34.2 (19.5–65.0) | <0.0001 |
PE: pulmonary embolism; OSA: obstructive sleep apnea. Values are represented as mean ± SEM or median (IQR) for continuous variables and as n (%) for categorical variables. Continuous variables compared using Student t test or Mann–Whitney U test based on normality of variable. Categorical variable comparison by Pearson’s chi-squared test. Statistical significance considered at p < 0.001.
The prevalence of type 2 diabetes (37.3% vs. 19.4%, p < 0.0001), hypertension (70% vs. 52.1%, p < 0.0001), dyslipidemia (41.7% vs. 26.9%, p < 0.0001), coronary artery disease (26% vs. 20.3%, p < 0.0005), atrial fibrillation/flutter (15.9% vs. 13.8%, p < 0.0005), and congestive heart failure (20.6% vs. 13.6%, p < 0.0005) were all higher in the OSA+ group, whereas alcohol use (2.4% vs. 3.4%, p < 0.0005), iron deficiency anemia (18.7% vs. 20.6%, p < 0.0005), and chronic blood loss anemia (1.3% vs. 1.7%, p = 0.001) were lower. Although the total number of patients undergoing thrombolysis was small in both groups, use of systemic thrombolysis (1.3% vs. 1%, p < 0.0001) and catheter-directed thrombolysis (0.3% vs. 0.2%, p < 0.0001) was more common in the OSA+ group. Finally, despite these differences in comorbidity profiles, OSA+ patients had similar LOS (median, IQR: 5, 3–8 vs. 5, 3–9, p = 0.05) and lower in-hospital mortality (4.1% vs. 7.1%, p < 0.0001). However, they had a higher total hospitalization charge (in thousand dollars; median, IQR: 34.2, 19.5–65 vs. 31.5, 17.1–63.9; p < 0.0001).
Association between OSA and in-hospital PE mortality
Combining data from all years, we observed a lower in-hospital mortality in OSA+ PE patients compared to OSA– patients (odds ratio, 95% confidence interval: 0.556 0.53–0.58; p < 0.0001) (Table 2). Indeed, despite a higher incidence of traditional risk factors for inpatient mortality such as obesity, coronary artery disease, atrial arrhythmias, and diabetes mellitus, OSA was associated with lower risk of inpatient mortality, since the association between OSA and inpatient mortality was significant even after adjustment for demographics, primary insurance payer, median income quartile, Elixhauser and other comorbidities, non-invasive CPAP use, pulmonary hypertension, hypoxia, respiratory failure, and thrombolysis (0.55, 0.52–0.58; p < 0.0001). When assessed separately by CPAP utilization, the association between in-hospital mortality and OSA was stronger in those with CPAP use (0.35, 0.30–0.40; p < 0.0001) than those without (0.56, 0.53–0.59; p < 0.0001) in fully adjusted model (Supplementary Tables 5 and 6).
Table 2.
Association between presence of obstructive sleep apnea and in-hospital mortality in patients with pulmonary embolism.
| Model | Odds ratio | 95% CI | p |
|---|---|---|---|
| Unadjusted | 0.56 | 0.53–0.58 | <0.0001 |
| Adjusteda | 0.55 | 0.52–0.58 | <0.0001 |
CI: confidence interval.
aadjusted for age, sex, race, primary insurance payer, median income quartile by ZIP code, Elixhauser comorbidities, coronary artery disease, hyperlipidemia, atrial fibrillations/flutter, smoking, non-invasive continuous positive airway pressure ventilator use, thrombolysis, pulmonary hypertension, respiratory failure, and hypoxia.
Trends in prevalent OSA in PE patients
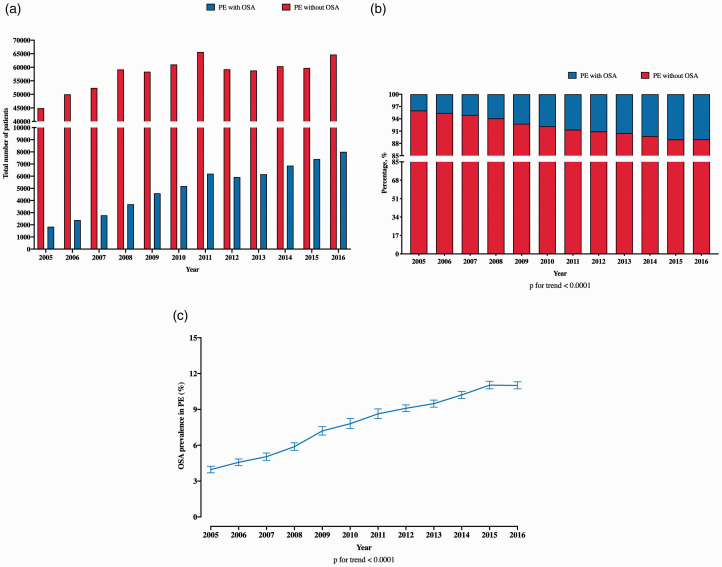
The total number of patients with PE in the NIS increased from 46,755 in 2005 to 72,706 in 2016 (Fig. 1a). Of total PE patients, the number of OSA+ patients also increased from 2005 to 2016 (Fig. 1a). The proportion of OSA+ PE patients included in the NIS increased over time from 4% in 2005 to 11% in 2016 (p for trend < 0.001) (Fig. 1b). The corresponding national trends for OSA+ PE patients demonstrated a similar trend (Fig. 1c).
Fig. 1.
Temporal trends in prevalence of OSA in in-hospital patients with PE. (a) Absolute number of PE patients with and without OSA; (b) proportion of PE patients with and without OSA; and (c) national trends in OSA prevalence in patients admitted with PE.
OSA: obstructive sleep apnea; PE: pulmonary embolism.
Trends in CPAP utilization and PE in-hospital outcomes
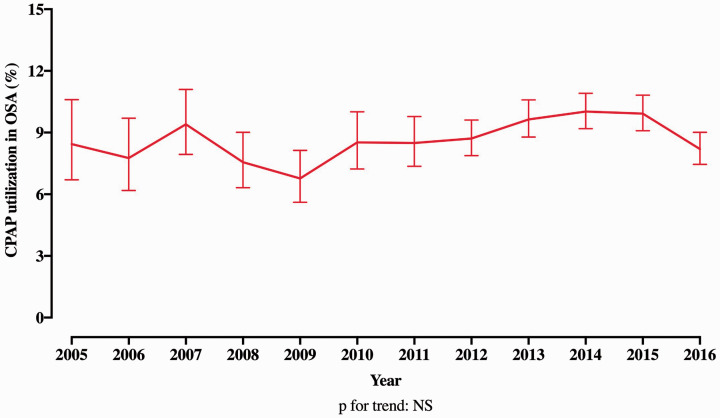
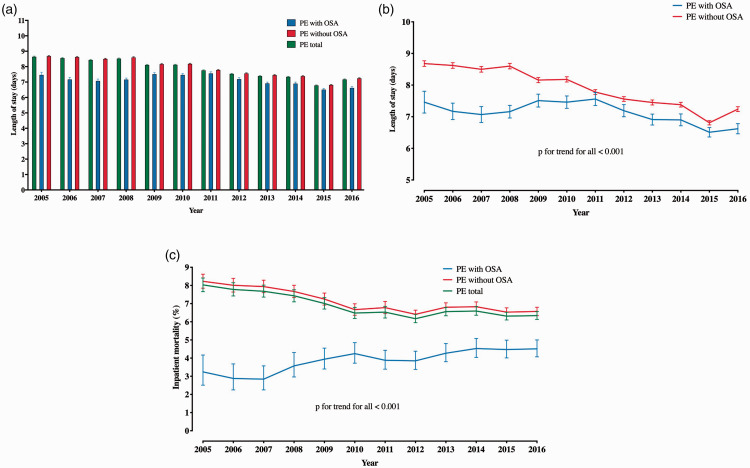
Analysis of trends in CPAP utilization revealed that 8.4% of OSA+ PE patients utilized CPAP in 2005, whereas this proportion was 9.9% in 2016. The trend in CPAP utilization was not statistically significant (Fig. 2). The difference in inpatient mortality between OSA+ and OSA– PE patients persisted in both groups of patients stratified by CPAP use in univariable as well as multivariable analyses (Supplementary Table 5 and 6). The LOS for entire PE population decreased from 2005 (median, IQR: 6, 4–10) to 2016 (4, 2–8) (p for trend <0.001) (Fig. 3a). While this LOS trend was similar in OSA– PE patients, OSA+ PE patients had an increasing LOS from 2005 to 2009, which later decreased and was similar to the OSA– PE patients in 2016 (Fig. 3b). Finally, the in-hospital mortality decreased for the overall PE population (p for trend < 0.001) over the time course of the study, which was primarily driven by decreased mortality in OSA– PE patients over time. There was a modest trend toward increasing mortality seen in OSA+ PE patients (Fig. 3c) over the study period. Despite these mortality trends, in-hospital mortality of OSA+ PE patients remained constantly lower than OSA– PE patients from 2005 to 2016.
Fig. 2.
Temporal trend in the utilization of non-invasive continuous positive airway pressure ventilation in patients with obstructive sleep apnea.
OSA: obstructive sleep apnea; CPAP: continuous positive airway pressure.
Fig. 3.
Temporal trends in outcome measures in patients with PE stratified by OSA. (a) Temporal trend of length of stay in all PE patients and in PE patients stratified by OSA; (b) national trends of length of stay in PE patients by OSA, and (c) national trends in in-hospital mortality among all PE patients and PE patients stratified by OSA.
PE: pulmonary embolism; OSA: obstructive sleep apnea.
Discussion
Using the Nationwide Inpatient Sample databases, we first demonstrated that prevalent OSA was associated with lower inpatient mortality in patients with PE despite a higher prevalence of traditional risk factors for cardiovascular mortality in a very large inpatient cohort. In addition, the prevalence of OSA increased in patients with PE over the study period. Overall inpatient mortality in PE patients decreased from 2005 to 2016, a trend predominantly driven by decrease in mortality among OSA– PE patients. Despite these decreases, the inpatient mortality in OSA+ PE patients remained consistently lower than the OSA– patients.
PE and OSA
Acute PE is a clinical syndrome initiated by the dislodgment of thrombus typically from lower extremity deep veins with embolization to the pulmonary vasculature, resulting in an abrupt increase in RV afterload, which may lead to acute hypoxemia, RV failure, and in some instances hemodynamic collapse. In the western world, the incidence of PE is about 1–2 cases per 1000 inhabitants per year,2 with an exponential rise in incidence with increasing age. PE contributes to a major burden of disease and is a leading cause of disability-adjusted life years lost.12 Known risk factors for PE include immobilization, trauma, surgery, cancer, obesity, hypercoagulable states, use of estrogen-based contraceptives, and autoimmune diseases.2,13–15 Although OSA is a known risk factor for cardiovascular diseases including hypertension, diabetes, heart failure, and cardiac arrhythmias, only recently, studies have recently suggested that OSA, especially severe OSA, may be associated with a procoagulant state.16 Contemporary clinical studies have further implied that OSA may be an independent risk factor for PE as well as recurrent venous thromboembolism.7,8 Another set of studies suggest that OSA is associated with increase in levels of tumor necrosis factor-alpha, fibrinogen, D-dimer as well as platelet activity and that CPAP use may alleviate some of these effects.17–20 However, a cross-over randomized trial evaluating the impact of CPAP in OSA and pulmonary hypertension did suggest that CPAP use decreased systolic pulmonary artery pressures in all patients.21 Despite our hypothesis that OSA-driven secondary pulmonary hypertension may lead to lower mortality in PE due to a gradual preconditioning of the RV to higher afterload, there is a relative lack of knowledge regarding this association, especially when OSA is also associated with an increased risk for PE.
OSA and inpatient outcomes in PE
We first demonstrated that OSA was associated with a consistently lower inpatient mortality in PE patients compared to those without OSA. In the initial years of the study from 2005 to 2010, trends revealed a modest increase in inpatient mortality in OSA+ PE patients, albeit still lower than OSA– patients, which in later years remained stable and lower than OSA– patients. The reason for this trend toward increasing inpatient mortality in OSA+ PE patients is uncertain. This may reflect coding practices for OSA rather than an actual increase in mortality since both the OSA prevalence and inpatient mortality in OSA+ patients remained stable after 2010. Alternatively, there may have been a higher outpatient utilization of CPAP therapy over this time frame, not captured in the inpatient coding database, which may have ameliorated some of the potentially protective effects in terms of RV preconditioning. Interestingly, we observed substantially higher inpatient mortality in both OSA+ and OSA– patients when they used CPAP during their hospitalization, although the difference in mortality was more striking for the OSA– patients. There are several potential explanations for this intriguing finding, including the possibility of confounding due to the potential for patients who required CPAP to have had higher PE severity or greater degrees of hypoxemia. Alternatively, the detrimental effects of positive airway pressure ventilation in a non-preconditioned right ventricle among OSA– PE patients could provide another potential physiologic explanation of the differences we observed. Though it is tempting to suggest caution with the use of CPAP in acute PE patients, given the inherent limitations of the ICD code-based database and lack of more detailed associated clinical variables such as PE severity and physiological measures suggestive of RV function post PE, the clinical implication of this finding should be viewed with caution before making recommendations regarding the use of CPAP in acute PE patients.
Despite the observation of trend of increasing mortality over the study period, our findings suggest that prevalent OSA is associated with lower inpatient mortality. OSA often contributes directly to development of both systolic and diastolic heart failure leading to secondary PH that may precondition the RV to generate higher PA pressures. Since these preconditioned RVs may be less prone to injury and hemodynamic collapse from acute PE, they may confer a protective effect and may, thus, be associated with a lower inpatient mortality. Another hypothesis is that OSA may lead to increased hemoglobin levels due to hypoxemia,22,23 which may be protective in acute PE preventing from worsening hypoxemia. We did observe a small difference (1.3%) in the prevalence of anemia—with predictably lower prevalence in OSA+ patients. While such a small difference in terms of prevalence of anemia may not be sufficient to explain the observed differences in PE mortality, this difference may illustrate the concept that OSA+ patients may have more nocturnal hypoxia, thus raising their hemoglobin and making them less likely to have anemia. Unfortunately, we did not have access to hemoglobin/hematocrit levels in the NIS databases. Finally, we observed a slightly higher proportion of patients receiving both systemic and catheter-directed thrombolysis in OSA+ group, but the absolute numbers were too small to explain the mortality difference between OSA+ and OSA– patients. Further, the association between lower inpatient mortality and OSA persisted beyond adjustment for both types of thrombolysis.
We also demonstrated that the overall prevalence of OSA significantly increased in PE patients during the study period as did the total number of inpatient hospitalizations for PE. The increase in overall OSA prevalence we observed could be attributed to increased awareness and coding practices for OSA, as well as the increased prevalence of obesity in the general population over the study period, which itself is strongly associated with OSA.24 One potential explanation for the slight overall increase in PE-related hospitalizations during the study period might be due to advances in imaging technology which have increased sensitivity to detect PE. Notably, the overall prevalence of venous thromboembolism over the last two decades has remained relatively stable, whereas the contribution of PE to this prevalence has increased.2,25,26 With the near ubiquitous adoption of high-resolution computed tomography (CT) angiography which can detect even small subsegmental PE that might previously have been incidental or asymptomatic, we hypothesize that some of the increase in PE hospitalizations we observed may reflect the increased sensitivity of this technology. The LOS decreased from a median of six days in 2005 to four days in 2016. While a similar trend was seen in OSA– PE patients, the LOS initially increased in OSA+ patients until 2009 but decreased in later years and in 2016 was similar to the OSA– PE patients. The LOS trends suggest that although patients were admitted for PE, they were stable for discharge sooner over the study period. The inpatient mortality decreased over the study period. The trends in LOS and inpatient mortality despite increasing numbers of PE admissions are consistent with the prior reports4,27,28 indicating that higher CT angiography use and sensitivity may have culminated in more PE diagnoses but apparent decreased mortality due to diagnosing low-risk patients in whom PE otherwise would go undiagnosed.
Strengths and limitations
Although we had a very large number of inpatient hospitalizations for PE, our study has certain strengths and limitations that warrant discussion. Use of the NIS allowed us to analyze trends in the outcomes of PE patients from hundreds of nationwide centers over a 12-year time frame, which are representative for the entire United States. However, our analyses are cross-sectional and observational, thus preclude any attempts at establishing causality. Being cross-sectional in nature, we did not have any temporal association between the diagnoses of OSA and PE and thus cannot exclude the possibility of OSA diagnosis post acute PE. Furthermore, as shown in the previous studies, OSA might be underdiagnosed in the inpatient clinical setting, and thus, prospective studies are needed to assess and confirm our findings. Additionally, they are subject to residual unmeasured confounding and hence should be treated as hypothesis generating. We did not have PE severity scores for patients studied; however, we attempted to overcome this by providing comprehensive comorbidity profiles for both the groups. Given administrative code-based nature of databases, OSA diagnoses were code based and not confirmed by sleep studies and thus were limited by lack of polysomnography results. Moreover, we did not have OSA severity measures that have prognostic value in PE as has been showed in the previous literature. Our CPAP utilization data are valid for inpatient admission only since outpatient utilization of CPAP may not be captured in NIS. Additionally, we did not have data on mandibular or dental surgeries or any dental appliances used during the CPAP treatment of OSA. Our mortality data are valid for only inpatient mortality and only for all-cause mortality. We had neither the 30-day or 1-year mortality data nor the PE-specific mortality data. We did not have data on hospital readmission since the NIS only included information from an index hospital admission, and we excluded patients who had a history of PE, ultimately limiting our ability to analyze any association between OSA and recurrent PE-related hospitalization. We did not have laboratory data such as D-dimer and N-terminal pro-B type natriuretic peptide or imaging data such as CT angiography or echocardiography, which may help prognosticate outcomes in PE and may provide details regarding clot burden as well as RV strain. Finally, there were no data available on medical treatments given to the patients during hospitalizations.
Future directions
Despite these limitations, we demonstrated a strong association between OSA and a lower inpatient mortality in PE patients. Though OSA has recently been showed to be a risk factor for PE, our results suggest that it may also have a protective effect. Hence, future research should confirm these results in prospective longitudinal studies of OSA patients. The Prognostic Significance of Obstructive Sleep Apnea in Patients With Acute Symptomatic Pulmonary Embolism (POPE) study plans to evaluate the prognostic impact of untreated OSA on cardiovascular outcomes in normotensive, stable outpatient with acute PE.29 This observational data will undoubtedly add to our findings, although given the much smaller sample size and inclusion of only hemodynamically stable outpatients, a similar difference in inpatient mortality may not be observed. Long-term mortality and mechanistic links behind apparent protective effect of OSA in PE patients should be investigated. As CPAP may be associated with decreased RV preconditioning—our proposed hypothesis behind the protective effect of OSA in PE patients—clinical studies should attempt to examine if CPAP use is associated with worse outcomes in OSA patients with PE.
Conclusions
We demonstrated that the inpatient mortality in OSA+ PE patients was consistently lower compared to OSA– patients throughout the study period, despite a numerical decrease in inpatient mortality among OSA– PE patients. Furthermore, the association between OSA and lower inpatient mortality in PE patients remained significant beyond adjustment for demographics and risk factors. We also showed that OSA prevalence increased in PE patients from 2005 to 2016 with a concomitant decrease in overall inpatient mortality among PE patients. Collectively, our results suggest a potential protective effect of OSA toward inpatient mortality in patients with PE; however, prospective studies need to confirm our findings.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894021996224 for Outcomes of patients hospitalized for acute pulmonary embolism by obstructive sleep apnea status by Aditya A. Joshi, Raef H. Hajjali, Avantee V. Gokhale, Triston Smith, Amit K. Dey, Garima Dahiya, Joseph B. Lerman, Aparna P. Sajja, Manreet Kanwar and Amresh Raina in Pulmonary Circulation
Acknowledgments
We would like to acknowledge Internal Medicine Residents and Fellows Research Committee, especially, Drs Tarun Sharma and Mario Castagnaro, for their help in making the data available for analyses.
Footnotes
Author contributions: The authors contributed to this work in the following ways: concept and design: AAJ, RHH, and AR; acquisition, analysis, and interpretation of data: AAJ, RHH, TS, AVG, JBL, APS, AKD, MK, and AR; drafting of the manuscript: AAJ, RHH, GD, and AR; critical revision for important intellectual content: AAJ, AVG, RHH, GD, JBL, APS, AKD, MK, and AR. All authors approved the final version of this article. Drs AAJ, RHH, and AR had full access to all data and take responsibility for the accuracy of data analyses.
Conflict of interest: Dr Raina is a speaker for Bayer, a consultant to Abbott and United Therapeutics, and reports clinical trial support from Actelion, United Therapeutics, Bellerophon, and Liquidia (none relevant to this paper). Dr Kanwar is a speaker for Bayer and Abiomed, a consultant to Bayer, and reports clinical trial support from AstraZeneca and CareDx (none relevant to this paper).
Primary data used in this study are available publicly under the Healthcare Cost and Utilization Project website, under subsection of National Inpatient Sample. Statistical codes can be requested from the corresponding author.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Guarantor: Amresh Raina.
ORCID iDs: Aditya A. Joshi https://orcid.org/0000-0003-4842-8666
Triston Smith https://orcid.org/0000-0002-4212-5448
Garima Dahiya https://orcid.org/0000-0003-1925-7722
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018; 137: e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primers 2018; 4: 18028. [DOI] [PubMed] [Google Scholar]
- 3.Arshad N, Isaksen T, Hansen JB, et al. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur J Epidemiol 2017; 32: 299–305. [DOI] [PubMed] [Google Scholar]
- 4.Smith SB, Geske JB, Kathuria P, et al. Analysis of national trends in admissions for pulmonary embolism. Chest 2016; 150: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok FA, Zondag W, van Kralingen KW, et al. Patient outcomes after acute pulmonary embolism. A pooled survival analysis of different adverse events. Am J Respir Crit Care Med 2010; 181: 501–506. [DOI] [PubMed] [Google Scholar]
- 6.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA 2003; 290: 1906–1914. [DOI] [PubMed] [Google Scholar]
- 7.Alonso-Fernandez A, de la Pena M, Romero D, et al. Association between obstructive sleep apnea and pulmonary embolism. Mayo Clin Proc 2013; 88: 579–587. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Fernandez A, Suquia AG, de la Pena M, et al. OSA is a risk factor for recurrent VTE. Chest 2016; 150: 1291–1301. [DOI] [PubMed] [Google Scholar]
- 9.Healthcare Cost and Utilization Project (HCUP). HCUP National Inpatient Sample (NIS). Rockville, MD: Agency for Healthcare Research and Quality, www.hcup-us.ahrq.gov/nisoverview.jsp (accessed 10 January 2019).
- 10.Healthcare Cost and Utilization Project (HCUP). Trend weights for HCUP NIS data, http://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp (accessed 10 January 2019).
- 11.Healthcare Cost and Utilization Project (HCUP). HCUP National Inpatient Sample (NIS). Rockville, MD: Agency for Healthcare Research and Quality, 2016, https://www.hcup-us.ahrq.gov/nisoverview.jsp (accessed 10 January 2018).
- 12.Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Semin Thromb Hemost 2014; 40: 724–735. [DOI] [PubMed] [Google Scholar]
- 13.Ahlehoff O, Wu JJ, Raunso J, et al. Cutaneous lupus erythematosus and the risk of deep venous thrombosis and pulmonary embolism: a Danish nationwide cohort study. Lupus 2017; 26: 1435–1439. [DOI] [PubMed] [Google Scholar]
- 14.Avina-Zubieta JA, Jansz M, Sayre EC, et al. The risk of deep venous thrombosis and pulmonary embolism in primary Sjogren syndrome: a general population-based study. J Rheumatol 2017; 44: 1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28: 370–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong SN, Lee SH. Hypercoagulability, obstructive sleep apnea, and pulmonary embolism-reply. JAMA Otolaryngol Head Neck Surg 2018; 144: 460. [DOI] [PubMed] [Google Scholar]
- 17.Alonso-Fernandez A, Toledo-Pons N, Garcia-Rio F. Obstructive sleep apnea and venous thromboembolism: overview of an emerging relationship. Sleep Med Rev 2020; 50: 101233. [DOI] [PubMed] [Google Scholar]
- 18.Arias MA, Garcia-Rio F, Alonso-Fernandez A, et al. CPAP decreases plasma levels of soluble tumour necrosis factor-alpha receptor 1 in obstructive sleep apnoea. Eur Respir J 2008; 32: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 19.Barcelo A, Morell-Garcia D, Sanchis P, et al. Prothrombotic state in children with obstructive sleep apnea. Sleep Med 2019; 53: 101–105. [DOI] [PubMed] [Google Scholar]
- 20.Garcia Suquia A, Alonso-Fernandez A, de la Pena M, et al. High D-dimer levels after stopping anticoagulants in pulmonary embolism with sleep apnoea. Eur Respir J 2015; 46: 1691–1700. [DOI] [PubMed] [Google Scholar]
- 21.Arias MA, Garcia-Rio F, Alonso-Fernandez A, et al. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J 2006; 27: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 22.Choi JB, Loredo JS, Norman D, et al. Does obstructive sleep apnea increase hematocrit? Sleep Breath 2006; 10: 155–160. [DOI] [PubMed] [Google Scholar]
- 23.Winnicki M, Shamsuzzaman A, Lanfranchi P, et al. Erythropoietin and obstructive sleep apnea. Am J Hypertens 2004; 17: 783–786. [DOI] [PubMed] [Google Scholar]
- 24.Jehan S, Myers AK, Zizi F, et al. Obesity, obstructive sleep apnea and type 2 diabetes mellitus: epidemiology and pathophysiologic insights. Sleep Med Disord 2018; 2: 52–58. [PMC free article] [PubMed] [Google Scholar]
- 25.Delluc A, Tromeur C, Le Ven F, et al. Current incidence of venous thromboembolism and comparison with 1998: a community-based study in Western France. Thromb Haemost 2016; 116: 967–974. [DOI] [PubMed] [Google Scholar]
- 26.Huang W, Goldberg RJ, Anderson FA, et al. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985-2009). Am J Med 2014; 127: 829–839. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheh SH, Bellin E, Freeman KD, et al. Pulmonary embolism diagnosis and mortality with pulmonary CT angiography versus ventilation-perfusion scintigraphy: evidence of overdiagnosis with CT? AJR Am J Roentgenol 2012; 198: 1340–1345. [DOI] [PubMed] [Google Scholar]
- 28.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med 2011; 171: 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manas E, Barbero E, Chiluiza D, et al. Impact of obstructive sleep apnea on cardiovascular outcomes in patients with acute symptomatic pulmonary embolism: rationale and methodology for the POPE study. Clin Cardiol 2017; 40: 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894021996224 for Outcomes of patients hospitalized for acute pulmonary embolism by obstructive sleep apnea status by Aditya A. Joshi, Raef H. Hajjali, Avantee V. Gokhale, Triston Smith, Amit K. Dey, Garima Dahiya, Joseph B. Lerman, Aparna P. Sajja, Manreet Kanwar and Amresh Raina in Pulmonary Circulation