Abstract
Acute respiratory distress syndrome is a common complication of severe viral pneumonia, such as influenza and COVID‐19, that requires critical care including ventilatory support, use of corticosteroids and other adjunctive therapies to arrest the attendant massive airways inflammation. Although recommended for the treatment of viral pneumonia, steroid therapy appears to be a double‐edged sword, predisposing patients to secondary bacterial and invasive fungal infections (IFIs) whereby impacting morbidity and mortality. Mucormycosis is a fungal emergency with a highly aggressive tendency for contiguous spread, associated with a poor prognosis if not promptly diagnosed and managed. Classically, uncontrolled diabetes mellitus (DM) and other immunosuppressive conditions including corticosteroid therapy are known risk factors for mucormycosis. Upon the background lung pathology, immune dysfunction and corticosteroid therapy, patients with severe viral pneumonia are likely to develop IFIs like aspergillosis and mucormycosis. Notably, the combination of steroid therapy and DM can augment immunosuppression and hyperglycaemia, increasing the risk of mucormycosis in a susceptible individual. Here, we report a case of sinonasal mucormycosis in a 44‐year‐old woman with hyperglycaemia secondary to poorly controlled diabetes following dexamethasone therapy on a background of influenza pneumonia and review 15 available literatures on reported cases of influenza and COVID‐19 associated mucormycosis.
Keywords: corticosteroid therapy, COVID‐19, influenza, mucormycosis, viral pneumonia
1. INTRODUCTION
Viral pneumonia is a worrisome health condition globally; it can be on a seasonal, sporadic, epidemic or even a pandemic scale with life‐threatening complications depending on the host's immune status and presence of co‐morbid conditions. 1 Notably, influenza and recently coronavirus disease 2019 (COVID‐19) are especially of specific concern because of the associated severe morbidity, high cost of care and unfavourable clinical outcome. 1 , 2 Globally, between 290,000 and 650,000 patients die annually from seasonal influenza 1 and the current COVID‐19 pandemic has claimed over 1.5 million lives within 10 months into the pandemic. 2 In patients with severe viral pneumonia, such as influenza and COVID‐19, acute respiratory distress syndrome (ARDS) is a common complication that requires intensive care unit (ICU) admission and mechanical ventilation (MV), use of corticosteroids and interleukin antagonists, for example tocilizumab to counter the massive inflammatory airways constriction and subsequent cytokine storm. 3 , 4 , 5
Importantly, acute viral pneumonia damages alveolar epithelial and endothelial tissues, dysregulates immune system and causes cellular immune dysfunction. 6 , 7 , 8 Upon the background lung damage, immune dysfunction and corticosteroid therapy in viral pneumonia, invasive fungal infections (IFIs) like aspergillosis and mucormycosis can spontaneously set in. 9 , 10 Cases of flu‐associated aspergillosis have been evidently highlighted in previous studies 9 , 11 , 12 ; however, mucormycosis in viral pneumonia cases have been clearly missed or under‐reported.
Mucormycosis—an acute and fatal fungal infection caused by ubiquitous fungal species belonging to Mucorales—is a fungal emergency with a highly aggressive tendency for contiguous spread, associated with a poor prognosis if not accurately and promptly diagnosed and managed. 13 Classically, uncontrolled diabetes mellitus (DM) and other immunosuppressive conditions such as neutropenia and corticosteroid therapy are known risk factors for mucormycosis. 14 The establishment of mucormycosis requires spores inhalation and/or seeding onto the airways or any vulnerable epithelium; germinating into angioinvasive hyphae—utilising host conditions such as hyperglycaemia, ketoacidosis, iron overload and neutropenia—causing endothelial damage, leading to local haemorrhage, thrombosis and necrosis; and eventual dissemination to involve multiple organs. 14 , 15
In this study, we present a case of sinonasal mucormycosis in an uncontrolled diabetic patient following dexamethasone therapy on a background influenza pneumonia and provide a review of current literature on influenza and COVID‐19 associated mucormycosis (CAM) cases.
2. CASE DESCRIPTION
A 44‐year‐old known diabetic woman with a history of poorly controlled diabetes presented to our centre at Shariati Hospital, Tehran, Iran, in February 2020 with a 5‐day history of fever, malaise, myalgia, dry cough and partial dyspnoea. She had no history of hypertension, asthma, tuberculosis, heart disease or contact with a confirmed COVID‐19 patient. She has been visiting a diabetic clinic, though irregularly, and has been sporadically taking insulin injections whenever she found her blood glucose raised. The dose of insulin injection could not be ascertained. Physical examination revealed a stable patient with mildly laboured breathing and nasal flaring. Her vital signs at presentation were temperature: 37.8°C; blood pressure: 130/80 mm Hg; pulse rate: 92 beats per minute; respiratory rate: 26 breaths per minute; and oxygen saturation: 94% (ambient air).
Her laboratory findings were blood glucose: 230 mg/dL; HbA1C: 8%; RT‐PCR of upper airways swab specimens (Qiagen) tested positive for influenza and negative for COVID‐19. HIV, hepatitis B and C serological tests were all negative. CBC revealed Hb: 14 mg/dL, WBC: 7 × 109/L with 60% neutrophil; CRP and ESR were both elevated. Moreover, her chest computed tomography (CT) demonstrated bilateral multifocal peripherally located patchy ground‐glass opacities (Figure 1). Therefore, acute influenza on basis of poorly controlled DM was diagnosed. She received 4 doses of intravenous (IV) dexamethasone (4 mg twice daily) on 2 consecutive days, and her symptoms subsided before she was discharged home on the fourth day of admission.
FIGURE 1.
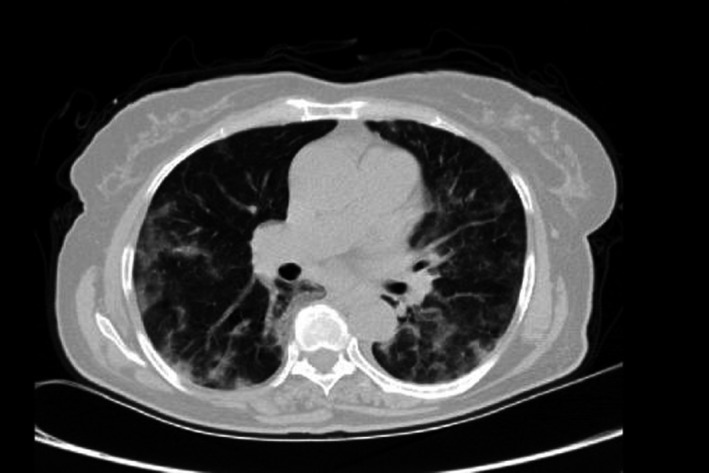
Axial view of chest computed tomography (CT) scan revealing peripheral bilateral ground‐glass opacities
Twenty days after her discharge, the patient complained of toothache and headache, which was followed by earache, nasal congestion and unilateral facial swelling. Subsequently, the patient visited by a dentist because she thought the facial swelling and pain were due to dental caries. The suspected tooth was evaluated for caries and managed. She was prescribed oral metronidazole, penicillin V and naproxen.
However, the symptoms did not improve. The patient was reassessed, and suspicion of mucormycosis was raised based on relevant clinical symptoms and associated risk factors. Therefore, she was admitted to the infectious disease ward, and empirical treatment with IV liposomal amphotericin B (Ambisome Gilead Co., 3 mg/kg daily, according to local guidelines 13 and experience in our centre) was commenced immediately. The hyperglycaemia was managed with subcutaneous insulin injections titrated against fasting blood glucose levels to maintain a blood sugar level of 150‐200 mg/dL. Accordingly, on the second day of admission, an otolaryngologist was consulted and she had functional endoscopic sinus surgery that revealed sinusitis with some partial necrosis in the right maxillary sinus; biopsy was made and the specimens were sent for mycological and pathological examination. CT of paranasal sinuses confirmed the evidence of mucosal thickening in the right maxillary sinus (Figure 2). However, neither palate necrosis nor ptosis and proptosis were noted.
FIGURE 2.
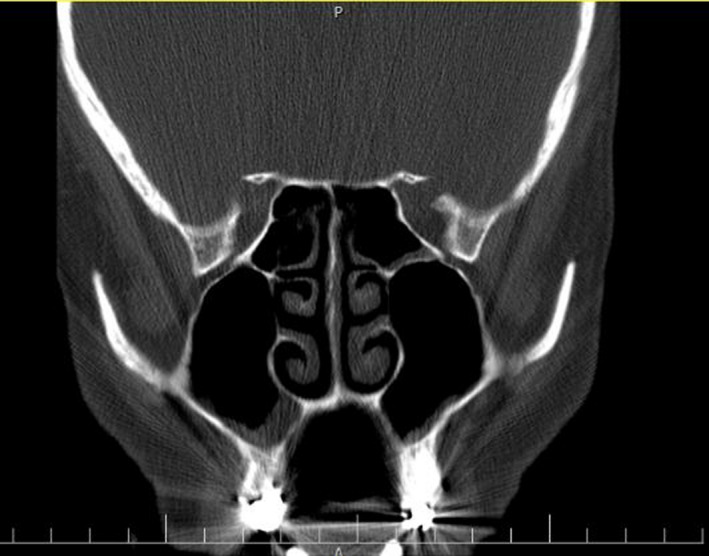
CT of paranasal sinuses illustrating maxillary sinus mucosal thickening
Direct and histopathological examinations using 10% potassium hydroxide and haematoxylin and eosin (H&E) staining both showed non‐septate, ribbon‐like, wide hyphae with right‐angle branching, suggestive of mucormycosis (Figure 3A,B). Although the culture remained negative probably due to pretreatment, the fresh specimen was subjected to manual DNA extraction, using phenol–chloroform–isoamyl extraction after tissue digestion with proteinase K and lysis buffer, 16 followed by semi‐nested PCR using Mucorales specific primers (ZM) as previously described by Bialek et al, 17 and the amplicon was sent for sequencing, which showed 99% similarity with the sequence of Rhizopus oryzae deposited in NCBI BLAST (https://www.blast.ncbi.nlm.nih.gov/Blast.cgi) and ISHAM barcoding (http://its.mycologylab.org) databases. The relevant sequence extracted from current study (R. oryzae) has been deposited in GenBank under accession number MW559073. Further, MRI did not show orbital or brain involvement.
FIGURE 3.
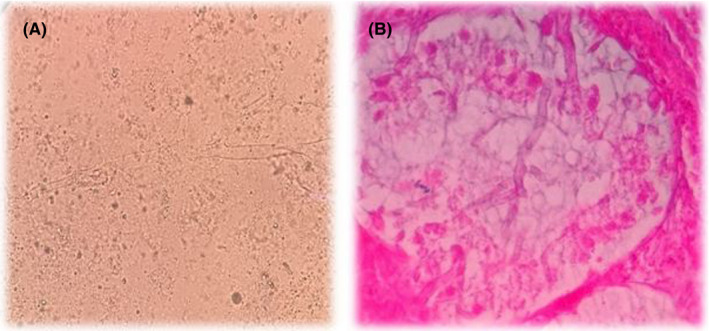
KOH examination (A) and haematoxylin and eosin (H&E) stain (B) showed abundant aseptate hyphae in the affected organs. Broad‐angled, aseptate hyphae were diagnostic of mucormycosis
Subsequently, the treatment with liposomal amphotericin B (Ambisome Gilead Co., 3 mg/kg per day) was continued for 2 weeks. When the patient's clinical conditions improved, liposomal amphotericin B was terminated at day 18, and the patient was eventually discharged on oral posaconazole (300 mg per day) on the 18th day. At the last follow‐up (8 months later), the patient showed no evidence of mucormycosis.
3. METHODS
We conducted a systematic review of the available literature to better characterise the extent of similar previous studies by searching electronic databases including PubMed, Scopus and Google Scholar for studies published in English. The search strategy was conducted using the term "mucormycosis" or "zygomycosis" combined with "influenza" OR "COVID‐19" OR "viral pneumonia".
Further, we manually searched references of relevant articles. The following data were extracted from selected studies and reviewed: demographic characteristics; underlying diseases; severity of viral pneumonia based on thoracic CT scan; a history of corticosteroid therapy; mucormycosis associated risk factors; evidence from histopathologic examinations; clinical manifestations, fungal aetiology of mucormycosis, forms and its extent; the time interval between diagnosis of viral pneumonia and mucormycosis; antifungal treatment and disease outcomes. Cases with molecular confirmation of influenza and COVID‐19 were solely included in our review.
4. RESULTS
The database search revealed a total of 17 articles: 10 on influenza‐associated mucormycosis (IAM) and 7 on CAM. Two articles on IAM were excluded based on missing data. The characteristics of the patients included in these studies are summarised in Table 1.
TABLE 1.
Characteristics of viral pneumonia patients with mucormycosis co‐infection
| S/N | Gender/age | Underlying diseases | Type of viral pneumonia | Severity of the disease/O2 supplementation with mechanical ventilation | Systemic corticosteroid therapy for viral pneumonia | Mucormycosis associated risk factor | Non‐septate hyphae on HE | Clinical manifestations of mucormycosis | Clinical form of mucormycosis & Etiologic agent | Time between diagnosis of viral pneumonia and Mucormycosis (days) | Surgical debridement done | Antifungal treatment | Outcome | Reference (year of publication) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/62 | CLL, SCT | Influenza | Severe/NA | No | Neutropenia, HM, Ibrutinib, Penbolizumab (immunosuppression) | No | Pulmonary infiltrates, necrotic nodular lesions | Pulmonary/Mucor sp | 59 | No | AMB, CSP, PSZ | Alive | Ajmal (2018) 10 |
| 2 | F/60 | Bipolar disorder, hypothyroidism, acute liver failure | Influenza | Severe/NA | Steroid for shock | Steroid | Yes | Pulmonary infiltration and necrosis, multiple necrotic skin nodules | Pulmonary, disseminated to skin/Rhizomucor pusillus | 6 | No | VRZ, AMB | Death | Huang (2019) 46 |
| 3 | M/74 | Autoinflammatory disease | Influenza | Severe/NA | Steroid for 12 years | Prolonged steroid therapy | Yes | Tracheal necrosis and dyspnoea | Tracheal/Lichtheimia sp | 7 | No | VRZ, AMB, PSZ | Alive | Leo (2018) 47 |
| 4 | M/48 | HCL | Influenza | Severe/NA | High‐dose steroid | Neutropenia, Steroid, HM, Rituximab (Immunosuppression) | Yes a,c | Erythromatous airways and dyspnoea |
Disseminated pulmonary mucormycosis/Apophysomyces Elegans |
15 | No | CSP. ISZ | Death | Kitmiridou (2018) 48 |
| 5 | M/59 | Diabetes, COPD, Hyperlipidaemia, hypertension | Influenza | Mild/No | Steroid for COPD | Uncontrolled diabetes, steroid | Yes | Necrotising skin lesions | Cutaneous/NA | 21 | Yes | AMB | Death | Person (2010) 49 |
| 6 | F/51 | Diabetes, hypothyroidism | Influenza | Severe/Yes | No | DKA | Yes | Respiratory failure, necrotic bronchial mucosa | Tracheal/NA | — | Yes | Yes; name not mentioned | Alive | Logan (2019) 50 |
| 7 | M/ 66 | Diabetes | Influenza | Mild/No | High‐dose steroid | Uncontrolled diabetes, steroid | Yes | Pleural effusion and respiratory failure | Pulmonary/Rhizopus spp. | 13 | Yes | VRZ, ISZ, AMB | Alive | Hoang (2020) |
| 8 | F/40 | Diabetes | Influenza | Severe/NA | No | Diabetes | Yes | Tracheal necrosis | Tracheal/NA | 30 | Yes | AMB | Alive | Mohindra (2014) 51 |
| 9 | M/60 | Diabetes | COVID‐19 | Severe/Yes b | Yes | Uncontrolled diabetes, steroid for COVID‐19 | Yes | Unilateral facial swelling, unilateral periorbital facial pain, eyelid oedema, ptosis, proptosis, right orbital cellulitis, acute vision loss | ROCM/NA | 12 | Yes | AMB | Death | Mehta (2020) 52 |
| 10 | F/33 | Diabetes, asthma, hypertension | COVID‐19 | Severe/NA | No | DKA | NA | Necrotic palate, necrotic nasal, left eye ptosis, altered mental status, ophthalmoplegia proptosis | ROCM/NA | 2 | Yes | AMB | Death | Werthman (2020) 53 |
| 11 | M/22 | Pancreatitis | COVID‐19 | Severe/Yes | Yes | Steroid for COVID‐19 | Yes a,c | NA | Disseminated (involving the hilar lymph nodes, heart, brain, and kidney)/NA | NA | No | No | Death | Hanley (2020) 54 |
| 12 | M/49 | COVID‐19 | Severe/Yes | Yes | Steroid for COVID‐19 | Yes | Right pneumothorax, bronchopulmonary fistula, necrotic empyema | Pulmonary/Rhizopus spp | 14 | Yes | AMB | Death | Placik (2020) 55 | |
| 13 | M/86 | Hypertension | COVID‐19 | Severe/Yes | Yes | Steroid for COVID‐19 | Yes | Gastric ulcers, acute diarrhoea, melena, severe anaemia, and fever | GIM/NA | 5 | No | No | Death | Monte (2020) 56 |
| 14 | M/60 | Diabetes, asthma, hypertension, hyperlipidaemia | COVID‐19 | Severe/Yes | Yes | Uncontrolled diabetes, steroid for COVID‐19 | Yes | Right globe proptosis, oedema of the eyelids and conjunctival chemosis. extensive opacification of right maxillary, ethmoid, and frontal sinuses | ROM/Rhizopus spp | 7 | Yes | AMB, CSP, PSZ | Death | Mekonnen (2020) 57 |
| 15 | M/66 | Hypertension | COVID‐19 | Severe/Yes | No | Lymphopenia | Yes | Pulmonary infiltrates with an increase of parenchymal thickening of the whole left lung, cavitary lesions in left lung and pleural effusion, opacification of the left maxillary sinus | SPM/Rhizopus spp | 21 | No | AMB, ISZ | Death | Pasero (2020) 58 |
Abbreviations: AMB, amphotericin B; CLL, chronic lymphocytic leukaemia; COPD, chronic obstructive airways disease; CSP, caspofungin; DKA, diabetes ketoacidosis; F, female; GIM, gastrointestinal mucormycosis; HCL, hairy cell leukaemia; HE, histopathological examination; HM, haematological malignancy; ISZ, isavuconazole; M, male; NA, not applicable (not mentioned in the article); PSZ, posaconazole; ROCM, rhino‐orbito‐cerebral mucormycosis; ROM, rhino‐orbital mucormycosis; SCT, stem cell transplant; SPM, sinopulmonary mucormycosis; VRZ, voriconazole.
Oxygen supplementation was mechanically provided and the patient intubated after mucormycosis detection.
Mucormycosis was diagnosed postmortem.
The mean age of patients with IAM was 57.5 years (range 40‐74) and that of the CAM patients was 53.7 years (range 22‐86); the mean age of all patients with viral pneumonia and mucormycosis co‐infection was 55.7 years. Males constituted 73.3% of the entire patients population and predominated in both CAM (85.7%) and IAM (62.5%) groups. Apart from the index of viral pneumonia, 93.3% of the patients had at least one underlying condition. In terms of severity, all patients with CAM (100%) presented with severe disease whereas severe form was present in 6/8 (75%) of IAM cases. The use of steroids was a prominent risk factor for the development of mucormycosis in 66.7% of patients with viral pneumonia. Specifically, 5/7 (71.4%) and 5/8 (62.5%) of patients with CAM and IAM, respectively, received steroids as an adjuvant treatment either for viral pneumonia or underlying medical conditions. Moreover, monoclonal antibodies such as rituximab were prescribed to 2/8 (25%) patients with IAM. Also, neutropenia was a documented risk factor in 25% of patients with IAM. Hyperglycaemia secondary to uncontrolled diabetes was a common risk factor for mucormycosis in 7/15 (46.7%) of patients with viral pneumonia. Among patients with IAM, 4/8 (50%) experienced hyperglycaemia and 43% of CAM patients presented with hyperglycaemia due to uncontrolled diabetes. Different clinical forms of mucormycosis were reported among the reviewed cases. Whereas pulmonary mucormycosis was the predominant clinical form (7/8 [87.5%]) in IAM, rhino‐orbito‐cerebral mucormycosis (ROCM) was the most common presentation (2/7 [28.6%]) in CAM. Although 7 (46.7%) articles have not provided the details of fungal agent causing mucormycosis, Rhizopus species was the most commonly encountered pathogen causing pneumonia‐associated mucormycosis (4/15, 26.7%). Surgical intervention was performed in 4/8 (50%) and 4/7 (57.1%) of IAM and CAM, respectively. Specific antifungal monotherapy was prescribed to 2/8 (25%) of IAM and 3/7 (43%) of CAM; in total, 5/15 (33.3%) of all cases received therapy with a single antifungal agent. Moreover, combined antifungal therapy with at least two different antifungal agents was reported in 5/8 (62.5%) of IAM and 2/7 (28.6%) of CAM. The average time between the diagnosis of viral pneumonia and mucormycosis in all cases was 16.3 days; specifically, it was 21.6 days and 10.1 days in IAM and CAM, respectively. The mortality rate among patients with viral pneumonia‐mucormycosis co‐infection was 66.7% (10/15, 3 out of 8 IAM cases compared to 100% of CAM cases). The outcomes of the disease differed with antifungal therapy. While the mortality rate was 50% among IAM patients who received antifungal monotherapy, 100% of CAM patients died despite 43% of them received antifungal monotherapy. The COVID‐19 patient with disseminated mucormycosis was diagnosed following postmortem examinations and neither received antifungal agent administration nor surgical debridement. Due to acute state and aggressive nature of the GI mucormycosis, 36 h after the oesophagogastroduodenoscopy and before the establishment of mucormycosis diagnosis, the patient (13th case) died and antifungal agents were not administered. Also, 3 out of 5 (60%) IAM patients who received combined antifungal therapy survived; however, only two patients out of all succumbed CAM patients received combined therapy (the mortality among them was 100%).
5. DISCUSSION
Certain conditions, notably, DM, hematologic malignancies (HMs), use of systemic steroids and cytokines antagonists, previous parenchymal lung damage and the attendant immune dysfunction following viral pneumonia, increase the individual's risk of secondary infections that further dwindle the quality of life and survival. 18 , 19 Previous studies have shown that patients with influenza and SARS‐CoV‐2 pneumonia are at increased risk for invasive pulmonary aspergillosis (IPA), invasive fusariosis or invasive candidiasis. 4 , 20 , 21 , 22 However, mucormycosis superinfections on viral pneumonia remain inconspicuous, probably due to challenges posed by the conventional diagnosis of Mucorales and the fact that many centres scaled‐down direct processing of at‐risk respiratory samples during the current COVID‐19 pandemic to minimise exposure to the virus. 23 , 24 Patients suffering from severe viral pneumonia are at greater risk for developing ARDS that necessitates ICU admission, external respiratory support and corticosteroid therapy on background lung damage, conditions that heighten the risk of IFIs and worsen the overall clinical outcome. In a study on 432 patients with severe influenza pneumonia admitted to ICU, IPA was reported in 83 (19%) within a median of 3 days after admission. Further, the study reported 3‐month mortality rates of 51% in patients with influenza/IPA co‐infection versus 28% in those without IPA. 22 Advances in diagnosis have impacted the increasing reports on invasive mucormycosis in susceptible patients like those with diabetes ketoacidosis secondary to uncontrolled diabetes, HMs, solid organ transplant (SOT), chronic respiratory diseases and corticosteroid therapy. 25 Since there are overlapping risk factors for developing IPA and pulmonary mucormycosis in patients with severe viral pneumonia, 26 it implies that pneumonia‐associated mucormycosis is presumably under‐diagnosed or under‐reported. The prominent risk factors for sinosal mucormycosis in our patient were hyperglycaemia, use of IV dexamethasone and underlying lung pathology as highlighted by other studies. 3 , 10 , 15
Although invasive aspergillosis leads the list of IFIs in the setting of viral pneumonia, reports of mucormycosis among viral influenza and recently COVID‐19 pneumonia are emerging. We found and analysed 15 cases of viral pneumonia/mucormycosis superinfection: eight cases of IAM and seven cases of CAM. The mean age of the patients was 55.7 years (range; 22‐86), with a predominance of male gender (73.3%). Although we report the case of a female patient with IAM, males predominated in other reports investigating pulmonary aspergillosis co‐infection in COVID‐19 and influenza cases. 20 , 22 Similarly, in our review, males constituted 85.7% and 62.5% of patients with CAM and IAM, respectively. However, the patients’ mean age (55.7 years) in our review was lower than 60 years, 22 63 years, 20 64 years 10 and 67 years 26 reported in other similar studies. This highlights the indifference to advanced age of secondary mucormycosis in severe viral pneumonia, especially with current COVID‐19 severe pneumonia.
Apparently, underlying diseases such as DM, HMs, SOT and use of corticosteroids are independent risk factors for IFIs. 13 , 29 , 30 , 31 In our reported case, hyperglycaemia secondary to poorly controlled diabetes and corticosteroid therapy (IV dexamethasone)(considering the fact that interval of 20 days between dexamethasone therapy and mucormycosis occurrence may be somewhat long to construct an association) were the noticeable risk factors for mucormycosis in the patient. Similarly, 14/15 (93.3%) of our reviewed cases had at least one underlying condition; DM is the most common underlying disease in 4/8 (50%) and 3/7 (43%) of IAM and CAM, respectively (Table 1). Exclusively, DM is a major risk factor for mucormycosis. 30 In addition, steroid therapy (such as dexamethasone) can tilt blood glucose levels to hyperglycaemia even in healthy individuals and lead to corticosteroid induced diabetes. More so, the combination of steroid therapy and DM can augment immunosuppression and hyperglycaemia, increasing the risk of infection. 31 Hyperglycaemia, acidosis and high‐dose corticosteroid treatment paralayse the ability and phagocytic functions of phagocytes, the principal host defence mechanism against mucormycosis, to immigrate to infected tissue and kill the organism. 32 Furthermore, considering the pathogenesis of viral pneumonia, the probability of mucormycosis in the background COVID‐19 and influenza may be hypothesised. As documented elsewhere, a marked acute cortisol stress response is mounted by COVID‐19 that can result in elevated level of serum cortisol and aggravate the control of blood sugar in both diabetic and non‐diabetic patients. 33 Meanwhile, it has been reported that ketosis or ketoacidosis, and induced diabetic ketoacidosis may be caused by COVID‐19 infection in those with diabetes. 34 All of the above mentioned justifications can make the COVID‐19 patients, both theoretically and practically, predisposed to mucormycosis development. 35 Also, the possible role of blood acidosis in viral pneumonia‐associated ARDS and elevated levels of serum ferritin cannot be ignored for mucormycosis susceptibility. 34 , 36 , 38 Thereby, acidic pH of the serum causes iron to be dissociated from sequestering proteins then serum iron availability increases which results in increased iron uptake by Mucorales species and consequently allows rapid fungal growth. 32 More so, intubation and MV have been described among the risk factors for IFIs, especially in severe viral pneumonia, 22 , 39 our reported case was not on MV. However, 7/15 (46.6%) of the our reviewed cases were on MV. Our reported case suffered from poorly controlled diabetes and steroid therapy on a background lung pathology—exposed to high risk of mucormycosis. Besides, 10 out of 14 (66.7%) cases in our review received systemic steroids to treat either the underlying disease or the primary viral pneumonia. Despite being recommended for the treatment of moderate to severe viral pneumonia, 3 , 40 steroid therapy appears to be a double‐edged sword, predisposing patients to secondary bacterial and IFIs whereby increasing morbidity and mortality. 31 A recent systematic review and meta‐analysis observed that corticosteroid therapy was associated with a higher mortality rate when compared with the placebo group, 39 mainly by causing further immunosuppression and prolonged viral shedding. 3
In terms of severity, on its own, severe pneumonia is a bad prognostic factor and secondary pulmonary IFI increases mortality rates. 10 Our reported case suffered mild/moderate pneumonia, and the secondary mucormycosis was timely diagnosed, promptly managed and successfully cured. Conversely, a single‐centre study in Germany observed 100% (3/3) mortality rate among patients with severe COVID‐19‐associated IPA. 40 Also, Schauwvlieghe et al 22 reported ICU mortality of 45% in critically ill `patients with IPA secondary to severe influenza pneumonia. These findings—higher fatality rates in severe COVID‐19 pneumonia‐associated IFIs compared to severe influenza‐associated IFIs—corroborate our observation in the 15 reviewed cases of viral pneumonia‐associated mucormycosis as we noted (7/7) 100% mortality in patients with severe disease of CAM, whereas among the six IAM patients with severe pneumonia only two died.
Depending on the underlying conditions and the risk factors, mucormycosis shows a specific predilection to particular anatomic sites. For example, ROCM is a typical presentation in diabetic patients; patients with profound neutropenia and graft‐versus‐host disease develop pulmonary mucormycosis. 41 Despite the apparent risk factors (DM, steroid therapy and lung pathology), our reported case ostensibly developed localised sinosal mucormycosis. Besides, in our reviewed cases, we observed different clinical forms of mucormycosis—pulmonary mucormycosis was the predominant clinical form 7/8 (87.5%) of IAM; ROCM was the most common presentation 2/7 (28.6%) of CAM; disseminated mucormycosis was reported in 2/8 (25%) of IAM and 1/7(14.3%) of CAM.
Mucormycosis is a fungal emergency with ROCM being the most fatal form. Despite the deep understanding of its pathogenicity, improved means of diagnosis and various therapeutic options, survival rates are poor (20‐60% depending on the underlying condition and site of infection). 15 , 25 A guideline on the management of mucormycosis issued by the European Confederation of Medical Mycology (ECMM) in concert with the Mycoses Study Group Education and Research Consortium affirms that survival can be improved via the early diagnosis, instituting prompt multidisciplinary care involving aggressive surgical therapy. 13 The guideline noted that lower mortality is achieved in localised sinus or skin infection, and surgical debridement may result in cure. We equally noted, in our review, that 4/8 (50%) and 4/7 (57.1%) of IAM and CAM cases, respectively, received surgical debridement. Interestingly, 3 of the 4 IAM cases who received surgical intervention survived the disease but all the surgically debrided cases of CAM died. This reveals that surgical intervention does not guarantee survival in severe cases of CAM.
Antifungal therapy is a hallmark life‐saving medical intervention in mucormycosis, and liposomal amphotericin B is the recommended first‐line drug. 13 Posaconazole and isavuconazole are the salvage drugs in case of intolerance or poor general condition. 13 The guidelines also recommend surgical debridement whenever feasible in parallel to antifungal treatment but note the doubtful benefit of antifungal combination therapy. However, combination therapy of mucormycosis showed remarkable outcomes in diabetic and leukaemic patients. 44 , 45 Although in our reviewed cases, only two patients with CAM received combination therapy, and 5/8 (62.5%) of IAM received combined drugs. Interestingly, 3 of 4 CAM patients who received liposomal AMB and surgical debridement were diabetic with ROCM/ROM, but they did not survive. Whereas the mortality rate was 37.5% among IAM patients, 100% of CAM patients died. This pronounces the high fatality of COVID‐19/mucormycosis superinfection despite aggressive management. Given the acute and aggressive nature of mucormycosis, timely diagnosis and prompt antifungal therapy is highly recommended in order to decrease the rate of mortality. 44 However, the lack of Mucorales specific circulating antigen, that is galactomannan test, the inefficiency of 1, 3 beta‐D glucan, negative results of blood cultures and absence of agents causing mucormycosis in cerebrospinal fluid during ROCM, usually deter the required speedy diagnosis and coupled with a paucity of standardised data to guide treatment decisions, the disease management is hampered by its aggressive course and ravaging complications. 44 The dependence on invasive procedures to take biological specimens from clinically involved tissues for the mucormycosis diagnosis and the fear of airborne transmission of SARS‐CoV‐2 during aerosol generating procedures in oral and maxillofacial surgery in COVID‐19 pandemic exacerbate the circumstance required for timely diagnosis of mucormycosis. 45
Our reported case of IAM survived due to a localised sinus involvement, early diagnosis, control of the blood sugar and aggressive treatment using combined antifungal therapy and surgical debridement.
6. CONCLUSION
Like other immunosuppressive conditions, severe viral pneumonia, accentuated by other risk factors, predisposes individuals to secondary IFIs such as IPA and mucormycosis. Literature review revealed that the most common presentation of IAM was pulmonary mucormycosis; other forms of mucormycosis such as pulmonary, gastrointestinal and disseminated mucormycosis were seen in CAM, but ROCM was the predominant presentation of CAM. A localised lesion, early diagnosis, regular control of the hyperglycaemia and aggressive treatment using combined antifungal therapy and surgical debridement improved the survival of our reported case of IAM. CAM appears to be more fatal than IAM, despite an aggressive treatment approach. Comprehensive research is needed to explore other bad prognostic factors in CAM and means of minimising their impact on morbidity and mortality.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Kazem Ahmadikia: Conceptualization (Lead); Formal analysis (Equal); Investigation (Equal); Methodology (Equal); Project administration (Equal); Writing—original draft (Lead); Writing—review and editing (Lead). Jamal Hashemi: Conceptualization (Equal); Methodology (Equal); Writing—original draft (Equal); Writing—review and editing (Equal). Sadegh Khodavaisy: Writing—original draft (Equal); Writing—review and editing (Equal). Muhammad Ibrahim Getso: Methodology (Equal); Writing—original draft (Equal); Writing—review and editing (Equal). Neda Alijani: Investigation (Equal); Methodology (Equal); Writing—review and editing (Equal). Hamid Badali: Methodology (Equal); Writing—review and editing (Equal). Hossein Mirhendi: Methodology (Equal); Writing—review and editing (Equal). Mohammadreza Salehi: Formal analysis (Equal); Writing—review and editing (Equal). Azin Tabari: Methodology (Equal); Writing—review and editing (Equal). Mojtaba Mohammadi Ardehali: Methodology (Equal); Writing—review and editing (Equal). Mohammad Kord: Methodology (Equal); Writing—original draft (Equal). Emmanuel Roilides: Conceptualization(Equal); Methodology (Equal); Writing—original draft (Equal); Writing—review and editing (Equal). Sassan Rezaie: Conceptualization (Equal); Methodology (Equal); Project administration (Equal); Supervision (Equal); Writing—original draft (Equal); Writing—review and editing (Equal).
ETHICAL STATEMENT
The study was approved by the ethical committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.SPH.REC.1397.298). To ensure anonymity, details that might disclose the identity of the subject under the study were not included. Written informed consent was obtained from the patient prior to being included in the study.
Ahmadikia K, Hashemi SJ, Khodavaisy S, et al. The double‐edged sword of systemic corticosteroid therapy in viral pneumonia: A case report and comparative review of influenza‐associated mucormycosis versus COVID‐19 associated mucormycosis. Mycoses. 2021;64:798–808. 10.1111/myc.13256
DATA AVAILABILITY STATEMENT
The data that support the finding of this study have been deposited in GenBank under accession number MW559073 and openly available.
REFERENCES
- 1. WHO , Influenza (seasonal) fact sheet. 2018, World Health Organisation Media Centre Geneva.
- 2. WHO . Coronavirus disease 2019 (COVID‐19). https://covid19.who.int/2020
- 3. Cao B, Gao H, Zhou B, et al. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med. 2016;44(6):e318‐e328. [DOI] [PubMed] [Google Scholar]
- 4. Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses. 2020;63(8):771‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID‐19). Int Care Med 2020;46(5):854‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klomp M, Ghosh S, Mohammed S, Nadeem Khan M. From virus to inflammation, how influenza promotes lung damage. J Leukoc Biol. 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herold S, Becker C, Ridge KM, Budinger GS. Influenza virus‐induced lung injury: pathogenesis and implications for treatment. Eur Respir J. 2015;45(5):1463‐1478. [DOI] [PubMed] [Google Scholar]
- 8. Rokni M, Ahmadikia K, Asghari S, Mashaei S, Hassanali F. Comparison of clinical, para‐clinical and laboratory findings in survived and deceased patients with COVID‐19: diagnostic role of inflammatory indications in determining the severity of illness. BMC Infect Dis. 2020;20(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 2018;31(6):471‐480. [DOI] [PubMed] [Google Scholar]
- 10. Ajmal S, Mahmood M, Abu Saleh O, Larson J, Sohail MR. Invasive fungal infections associated with prior respiratory viral infections in immunocompromised hosts. Infection. 2018;46(4):555‐558. [DOI] [PubMed] [Google Scholar]
- 11. Waldeck F, Boroli F, Suh N, et al. Influenza‐associated aspergillosis in critically‐ill patients—a retrospective bicentric cohort study. Eur J Clin Microbiol Infect Dis. 2020;39:1915–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van De Veerdonk FL, Kolwijck E, Lestrade PP, et al. Influenza‐associated aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2017;196(4):524‐527. [DOI] [PubMed] [Google Scholar]
- 13. Cornely OA, Alastruey‐Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405‐e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamilos G, Samonis G, Kontoyiannis DP Pulmonary mucormycosis . In: Baddley JW, Pappas PG, Seminars in respiratory and critical care medicine. © Thieme Medical Publishers; 2011;32(06):693–702. [DOI] [PubMed] [Google Scholar]
- 15. Petrikkos G, Tsioutis C. Recent advances in the pathogenesis of mucormycoses. Clin Ther. 2018;40(6):894‐902. [DOI] [PubMed] [Google Scholar]
- 16. Zaman K, Rudramurthy SM, Das A, et al. Molecular diagnosis of rhino‐orbito‐cerebral mucormycosis from fresh tissue samples. J Med Microbiol. 2017;66(8):1124‐1129. [DOI] [PubMed] [Google Scholar]
- 17. Bialek R, Konrad F, Kern J, et al. PCR based identification and discrimination of agents of mucormycosis and aspergillosis in paraffin wax embedded tissue. J Clin Pathol. 2005;58(11):1180‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manohar P, Loh B, Nachimuthu R, Hua X, Welburn SC, Leptihn S. Secondary bacterial infections in patients with viral pneumonia. Front Med. 2020;7:420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salehi M, Ahmadikia K, Badali H, Khodavaisy S. Opportunistic fungal infections in the epidemic area of COVID‐19: a clinical and diagnostic perspective from Iran. Mycopathologia. 2020;185(4):607‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID‐19. Lancet Respir Med. 2020;8(6):e48–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID‐19. Clin Microbiol Infect. 2020;26(11):1582‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schauwvlieghe AF, Rijnders BJ, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Resp Med. 2018;6(10):782‐792. [DOI] [PubMed] [Google Scholar]
- 23. Clancy CJ, Nguyen MH. COVID‐19, superinfections and antimicrobial development: What can we expect? Clin Infect Dis. 2020;71(10):2736–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pemán J, Ruiz‐Gaitán A, García‐Vidal C, et al. Fungal co‐infection in COVID‐19 patients: Should we be concerned? Rev Iberoam Micol. 2020;37(2):41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. J Fungi. 2019;5(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gangneux J‐P, Bougnoux M‐E, Dannaoui E, Cornet M, Ralph ZJ. Invasive fungal diseases during COVID‐19: we should be prepared. J Mycol Med. 2020;30(2):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoang K, Abdo T, Reinersman JM, Lu R, Higuita NIA. A case of invasive pulmonary mucormycosis resulting from short courses of corticosteroids in a well‐controlled diabetic patient. Medical Mycology Case Reports. 2020;29:22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia‐Vidal C, Sanjuan G, Moreno‐García E, et al. Incidence of co‐infections and superinfections in hospitalized patients with COVID‐19: a retrospective cohort study. Clin Microbiol Infect. 2020;27(1):83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bitar D, Lortholary O, Le Strat Y, et al. Population‐based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis. 2014;20(7):1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lansbury LE, Rodrigo C, Leonardi‐Bee J, Nguyen‐Van‐Tam J, Shen Lim W. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated cochrane systematic review and meta‐analysis. Crit Care Med. 2020;48(2):e98‐e106. [DOI] [PubMed] [Google Scholar]
- 31. Ardi P, Daie‐Ghazvini R, Hashemi SJ, et al. Study on invasive aspergillosis using galactomannan enzyme immunoassay and determining antifungal drug susceptibility among hospitalized patients with hematologic malignancies or candidates for organ transplantation. Microb Pathog. 2020;147:104382. [DOI] [PubMed] [Google Scholar]
- 32. Corzo‐León DE, Chora‐Hernández LD, Rodríguez‐Zulueta AP, Walsh TJ. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol. 2018;56(1):29‐43. [DOI] [PubMed] [Google Scholar]
- 33. Bonaventura A, Montecucco F. Steroid‐induced hyperglycemia: An underdiagnosed problem or clinical inertia? A narrative review. Diabetes Res Clin Pract. 2018;139:203‐220. [DOI] [PubMed] [Google Scholar]
- 34. Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID‐19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saegeman V, Maertens J, Ectors N, Meersseman W, Lagrou K. Epidemiology of mucormycosis: review of 18 cases in a tertiary care hospital. Med Mycol. 2010;48(2):245‐254. [DOI] [PubMed] [Google Scholar]
- 38. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verweij PE, Rijnders BJA, Brüggemann RJM, et al. Review of influenza‐associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46(8):1524‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Y, Jiang W, He Q, et al. Early, low‐dose and short‐term application of corticosteroid treatment in patients with severe COVID‐19 pneumonia: single‐center experience from Wuhan, China. medRxiv. 2020. [Google Scholar]
- 41. Vandroux D, Allyn J, Ferdynus C, et al. Mortality of critically ill patients with severe influenza starting four years after the 2009 pandemic. Infect Dis. 2019;51(11–12):831‐837. [DOI] [PubMed] [Google Scholar]
- 42. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Serris A, Danion F, Lanternier F. Disease entities in mucormycosis. J Fungi. 2019;5(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene‐caspofungin treatment of rhino‐orbital‐cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klimko NN, Khostelidi SN, Volkova AG, et al. Mucormycosis in haematological patients: case report and results of prospective study in Saint Petersburg, Russia. Mycoses. 2014;57(s3):91‐96. [DOI] [PubMed] [Google Scholar]
- 46. Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 2013. 98:492‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ahmad Z, Riaz N, Abid A, Shakir H, Mirza A, ul Haq E. Emergent aerosols generating procedures in oral & maxillofacial surgery in Covid‐19 pandemic. Ann King Edward Med Univ. 2020;26(2):330‐335. [Google Scholar]
- 48. Huang YQ, Tremblay J‐A, Chapdelaine H, Luong M‐L, Carrier FM. Pulmonary mucormycosis in a patient with acute liver failure: a case report and systematic review of the literature. J Crit Care. 2020;56:89‐93. [DOI] [PubMed] [Google Scholar]
- 49. Leo F, Zeh M, Prothmann A, Kurzai O, Kurz S, Grohé C. Tracheal, laryngeal and pulmonary mucormycosis followed by organizing pneumonia in a patient with adult onset still's disease. Med Mycol Case Rep. 2018;20:28‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kitmiridou D, Aung SN, Farmakiotis D. Disseminated Mucormycosis with Positive Aspergillus Galactomannan. Case Reports in Infectious Diseases. 2018;2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Person B, Bahouth H, Brauner E, Ben‐Ishay O, Bickel A, Kluger YS. Surgical emergencies confounded by H1N1 influenza infection‐a plea for concern. World J Emerg Surg. 2010;5(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Logan R, Coomes D, Adeyami O, Backous C. 592: rapidly progressive invasive pulmonary mucormycosis in the setting of severe diabetic ketoacidosis. Crit Care Med. 2019;47(1):276. [Google Scholar]
- 53. Mohindra S, Gupta B, Gupta K, Bal A. Tracheal mucormycosis pneumonia: a rare clinical presentation. Resp Care. 2014;59(11):e178‐e181. [DOI] [PubMed] [Google Scholar]
- 54. Mehta S, Pandey A. Rhino‐orbital mucormycosis associated with COVID‐19. Cureus. 2020;12(9):e10726–e10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Werthman‐Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID‐19. Am J Emerg Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID‐19: a post‐mortem study. Lancet Microbe. 2020;1(6):e245‐e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Placik DA, Taylor WL, Wnuk NM. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS‐CoV‐2 pneumonia. Radiol Case Rep. 2020;15(11):2378‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Monte Junior ESD, Santos MELD, Ribeiro IB et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID‐19 patient: a case report. Clin Endosc 2020;53(6):746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute invasive rhino‐orbital mucormycosis in a patient with COVID‐19‐associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pasero D, Sanna S, Liperi C, et al. A challenging complication following SARS‐CoV‐2 infection: a case of pulmonary mucormycosis. Infection. 2020;1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the finding of this study have been deposited in GenBank under accession number MW559073 and openly available.


