Abstract
Objective This study aimed to develop an institutional approach for defining data migration based on participatory design principles.
Methods We outline a collaborative approach to define data migration as part of an electronic health record (EHR) transition at an urban hospital with 20 ambulatory clinics, based on participatory design. We developed an institution-specific list of data for migration based on physician end-user feedback. In this paper, we review the project planning phases, multidisciplinary governance, and methods used.
Results Detailed data migration feedback was obtained from 90% of participants. Depending on the specialty, requests for historical laboratory values ranged from 2 to as many as 145 unique laboratory types. Lookback periods requested by physicians varied and were ultimately assigned to provide the most clinical data. This clinical information was then combined to synthesize an overall proposed data migration request on behalf of the institution.
Conclusion Institutions undergoing an EHR transition should actively involve physician end-users and key stakeholders. Physician feedback is vital for developing a clinically relevant EHR environment but is often difficult to obtain. Challenges include physician time constraints and overall knowledge about health information technology. This study demonstrates how a participatory design can serve to improve the clinical end-user's understanding of the technical aspects of an EHR implementation, as well as enhance the outcomes of such projects.
Keywords: participatory design, data migration, electronic health record vendor transition, end-user feedback, stakeholder feedback, physician engagement
Background and Significance
Medical institutions are increasingly faced with the challenge of transitioning to a new electronic health record (EHR) system as policies evolve, technologies advance, and health networks merge. 1 2 A complexity that often arises during this transition is data migration. 2 Data migration describes the transfer of EHR data from the current system to a new EHR. 3 This key step determines the clinical data content of the new EHR environment which may ultimately influence subsequent institutional and clinical outcomes. 4 5 6 There is currently a paucity of data on the best approach for defining institutional data migration based on the unique needs of clinical end-users. In this case study, end-user feedback was obtained to develop the display of historical clinical data.
The amount of clinical data that can be migrated may be limited due to resource constraints. 7 For example, data from a proprietary EHR may not be transferrable in a form that is compatible with the new EHR system. Conversions to ensure that data are fully accessible in a new EHR system can be complex, costly, and may take weeks or months to complete. 7 Scripted data transfer has been shown to be effective and efficient but depends on compatibility between systems, accurate master patient lists, and the ability to accurately assign appropriate codes to the clinical data being migrated. 8
Nonmigrated data is subsequently stored and is accessible via an archival legacy system. Failing to precisely define the scope of clinical data for an EHR migration jeopardizes the data integrity and clinical usefulness of the new environment. 9 Physicians may in turn spend additional time accessing the legacy archive to retrieve data, a process that vendors may strive to advertise as “only a few extra clicks,” but this may contribute to physician burnout. 10 11 Consequently, physicians may forgo archival data retrieval and simply elect to repeat medical tests leading to health care waste in the form of redundant testing and reduced patient safety. 12
To define data migration on behalf of 20 ambulatory clinics, we developed a framework based on participatory design (PD), a user-centered design approach that actively engages end-users and stakeholders to design technological interfaces and systems. 13 14 15 16 17 18 Specifically, our framework involved an institutional committee consisting of key stakeholders involved with the EHR transition. At this level, categories for data migration were reviewed, lookback periods were established, and end-users were identified to provide specialty-specific data migration feedback. PD is an approach that has been used in healthcare to address the interorganizational nature of healthcare informatics, 19 20 21 22 23 resulting in improved system quality, higher levels of user adoption during implementation, and decreased training needs. 24 25 26 Design based on end-user feedback has been shown to reduce end-user variations in EHR use that often exists within practice settings using a common EHR. 27 28 Although PD emphasizes end-user feedback to influence functionality and design outcomes, the goal of this study was to use PD to determine EHR content.
The aim of the data migration project was to elicit feedback from stakeholders to compile a detailed list of laboratory and documentation data to be included for data migration that reflected the unique needs of various specialties. 27 28 This consisted of individual laboratory types, documents, and other test results with corresponding lookback periods used to quantify the amount of data to be transferred. Other clinical domains, such as demographics, allergies, medications, past medical history, and imaging reports, were not subject to data limitations and thus were not included in end-user–focused provider feedback discussions. To our knowledge, there are no publications to date that presents an approach to defining data migration using PD in this setting. Here, we present an overview of our approach and recommend considerations for similar institutions facing this task.
Methods
Setting
The study was conducted at a 602-bed urban tertiary care hospital with 20 ambulatory clinics that are part of a larger health-network.
Committee and Key Stakeholders
After EHR vendor selection was established, a health-network governance committee consisting of representation from executive and medical leadership, health information technology, and vendor implementation specialists was appointed. This committee established the broad categories of clinical domains to be included for data migration and determined which would be subject to data limitations. Each hospital site then developed their own institutional governance committee that would submit a proposal for data migration and all requests would be combined to create a common EHR environment.
Our institution-specific EHR committee, which was overseen by the Chief Medical Information Officer (CMIO), consisted of 10 key stakeholders from health EHR vendor specialists, internal information technology (IT) specialists, executive management, physicians, and advanced care providers ( Fig. 1 ). Clinical department heads (chiefs of service) were also asked to participate in this collaborative effort. Data migration discussions began 8 months prior to the anticipated go-live date.
Fig. 1.
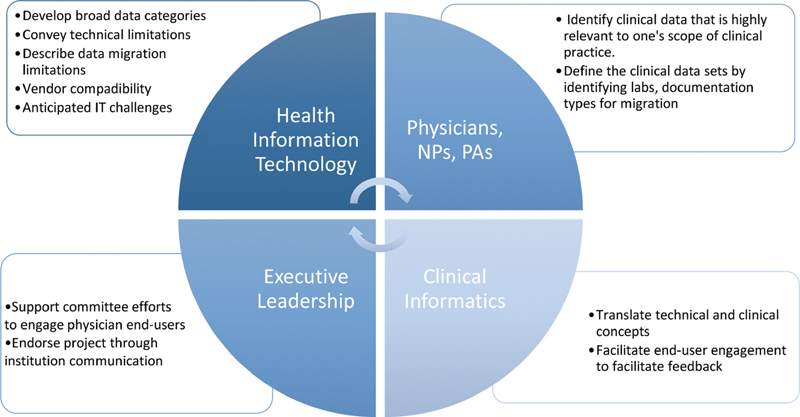
Key-stakeholder groups involved in developing a data migration project. IT, information and technology. NP, nurse practitioner; PA, physician assistant.
Defining the Scope of Data Migration
Specialty-specific feedback was elicited to define the scope of data migration for documentation note types, laboratory values, and diagnostic test/procedure reports ( Table 1 ). Physicians representing their respective ambulatory clinic were given the option to review all laboratory types ordered and document types used in the past year. They were then asked to indicate those suitable for data migration based on their perceived usefulness within their specialty. All radiology reports would be included for data migration. Radiographic images were not involved in data migration discussions, as they were available via the independent picture archiving and communication system (PACS).
Table 1. Inclusion and exclusion criteria of clinical data designated for data migration based on specialty specific feedback.
| Laboratory | Imaging | Direct care documents | Scanned documents | |
|---|---|---|---|---|
| Included | Any laboratory ordered at least once over the past year | All imaging reports | Physician documentation (phone calls, history and physical (H&P) notes, acute visits, etc.) Operative notes |
Advanced directives Consent to participate in research External facility results (laboratories, imaging reports, stress testing, mammography, etc.) |
| Excluded | Laboratory types not placed over the past one year | Radiology results a | Nonprovider documentation (i.e., nursing notes, pharmacy notes, social work, etc.) | Insurance forms Patient questionnaires Miscellaneous forms Procedural consent forms |
Radiology images were not included as these are accessible via a separate picture archiving and communication system (PACS) imaging system.
Classifying Laboratory Results
After committee review, three possible distinct “lookback” periods for lab values were designated: (1) “3-years,” (2) “10 years,” or (3) “lifetime.” This was done to ensure that the laboratory values migrated were relevant and contained enough data points to have adequate clinical utility. The threshold of 3 years was based on the clinical utility of trending commonly ordered values (i.e., prostate-specific antigen [PSA], international normalized ratio [INR], iron studies, lipid values, etc.). Physicians reported that this time frame likely provided enough historical data and additional data beyond 3 years was not perceived to be beneficial. If physician stakeholders indicated that a laboratory result should be included for data migration, they then assigned it to one of the three look-back periods to indicate the amount of historical data necessary ( Table 2 ).
Table 2. Lookback period classifications for labs assigned to data migration.
| Laboratory category | Corresponding lookback period | Examples |
|---|---|---|
| Trended | Trend all values on record from past 3 years | CBC, lipid panel, A1c, PSA |
| Last value | Last value recorded over the past 3 years | MMR and varicella titers, Helicobacter pylori stool antigen |
| Any value | Any value ever performed on record | Hemoglobin electrophoresis, ANA identification panel, interferon-gamma tuberculosis |
Abbreviations: A1c, glycated hemoglobin; ANA, antinuclear antibody; CBC, complete blood count; MMR,measles mumps and rubella; PSA, prostate specific antigen.
“Trended” laboratories were those that physicians usually compare with previous values when making diagnostic decisions. Therefore, laboratories in this category would include all values on file for patients over the past 3 years. Examples included but not limited to hemoglobin A1c, PSA, and lipids.
“Last value” laboratories would be a single discrete laboratory result for particular laboratory within the past 3 years. These refer to laboratories that are not generally repeated within a 3-year period. Examples included but not limited to immunization titers, Helicobacter pylori stool antigen, or heavy metal testing.
“Any value” are lifetime laboratories that are not routinely ordered with great frequency but carry a high clinical significance. Examples include, but are not limited to interferon-gamma tuberculosis (TB) testing, hemoglobin electrophoresis, hypercoagulable tests, and genetic studies. These laboratories had no limit to the lookback period performed.
Provider Feedback Forms
A master list of all the unique laboratories ordered over the past year ( n = 415), arranged by ordering frequency, was used as a template to obtain individual provider feedback. Providers were asked to review the top 100 most commonly ordered lab values (which accounted for 98% of all laboratories placed over the past year), with the option to review the remaining 315 less frequently ordered lab values. The aim of this approach was to maximize provider engagement by reducing the burden of laboratories for them to review. This would ensure that the laboratories with the largest amount of data to be migrated would be reviewed by all specialties interviewed.
Categorizing Documentation
Each clinic was asked to review their respective list of all the document types with the corresponding frequency of use over the past year and select which items were deemed necessary to be included for data migration.
Scanned Documents
Scanned documents posed a challenge for data migration and mapping as they contained nondiscrete data. There were no institution-wide guidelines for scanning, and subsequently each clinic had a unique approach to categorizing and labeling scanned documents. The master list of scanned document types was manually reviewed. Any documents that fell into one of the data-migration categories (i.e., laboratory values, procedures results, provider documentation, and end-of life care forms) were included in the final documentation list for data migration. Remaining scanned documents or “miscellaneous scanned documents,” would remain in the legacy data archive.
Standardized Technical Presentation Points
Discussions were held with the committee and clinic leaders to elicit feedback and address concerns. The following points were emphasized when presenting the concept of data migration to physician key stakeholders:
All existing results, notes, and historical data would be accessible after the EHR transition via an archival system.
The purpose of collecting their specialty-specific feedback was to identify clinically relevant data that would be commonly used to deliver patient care. Having this particular set of data migrated would ultimately save time and reduce the need for extra clicks to access historical data.
Simply migrating everything into the new system was not feasible due to the large amount of data which would require a lengthy and time-intensive conversion. This could also potentially lead to having less-pertinent historical data that would make a new user interface more challenging to adapt to.
Timeline
In phase 1 ( Fig. 2 ), organizational planning defined the scope of the project internally and determined how to best collaborate with physicians. Meetings were led by the CMIO and feedback was obtained about how to best classify lookback periods and elicit specialty-specific feedback. In phase 2, individual data review and feedback sessions were held with the physician leaders (chiefs of staff). In phase 3, this feedback from all specialties was analyzed and combined using an excel formula which summarized findings into a single master list ( Fig. 3 ). Once the master list of laboratories ( Supplementary Appendix A , available in the online version) and note types ( Supplementary Appendix B , available in the online version) were approved within the institution, a final copy ( Fig. 4 ) was submitted to the executive leadership committee, overseeing the network-wide EHR implementation project.
Fig. 2.

Organizational timeline for data migration project.
Fig. 3.
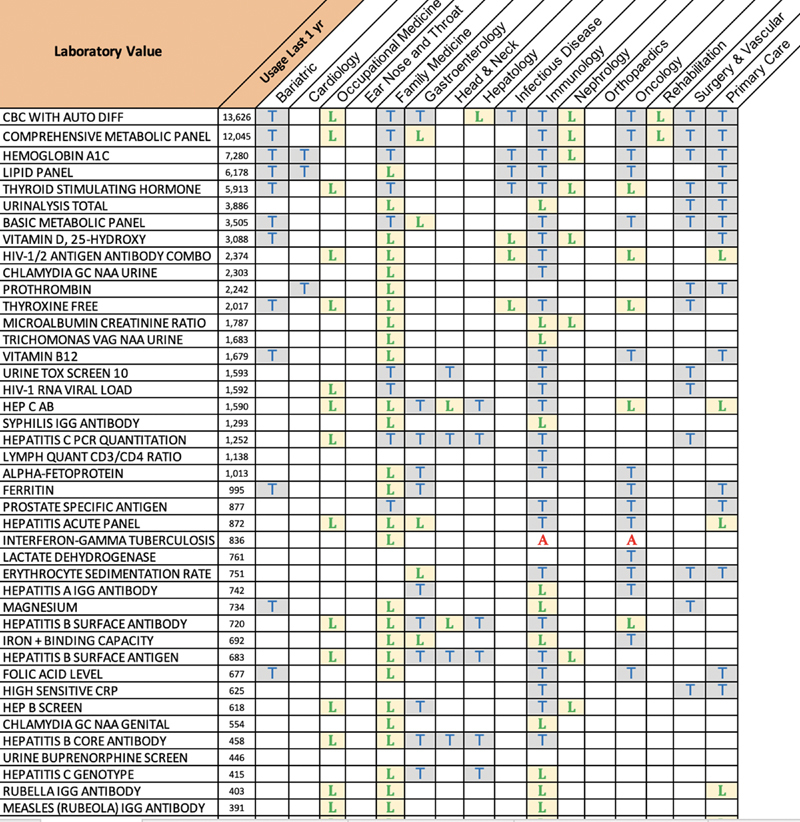
Sample of specialty-specific list of laboratories requested for data migration with lookback periods. “T” trended; all values on file over the past 3 years. “L” only the last value on file over the past 3 years to be migrated. “A” the last value on file, with no limit to the lookback period.
Fig. 4.
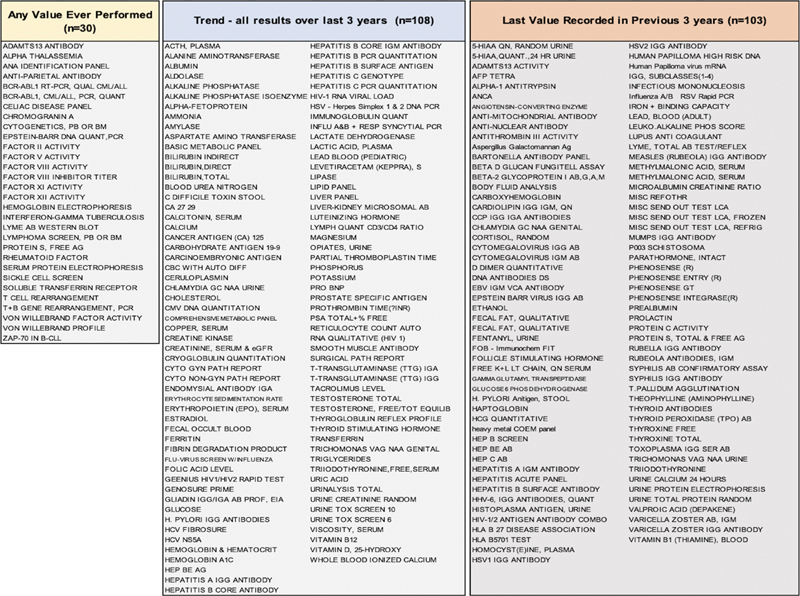
Master list of laboratory values compiled for data migration by look back period.
Results
Overall, 90% (17 of 19) of key-stakeholder physicians agreed to take part. The feedback compiled reflected a detailed list of clinically meaningful results that reflected the needs of each medical specialty. Requests for types of laboratory values and associated lookback periods varied based on specialty ( Fig. 5 ). Requests ranged from 2 laboratory types from rehabilitation medicine, to as many as 145 by immunology and infectious disease. Specialties that provided care on a long-term basis with more frequent patient visits (family medicine, immunology and infectious disease, and oncology) requested the most laboratory types ( n > 87). Rehabilitation medicine, orthopaedic surgery, and bariatric specialties requested fewer than 20 laboratory types. A total of 241 unique lab types were ultimately requested for migration ( Supplementary Appendix A , available in the online version). Interestingly, there was often a discrepancy regarding an ideal lookback period. When this occurred, the lookback period with the most data was chosen. Requests for historical provider documentation for a given clinic ranged from 3 note types to 43 with an average of 14 per clinic ( Supplementary Appendix B , available in the online version). Providers were generally enthusiastic about providing feedback and 41% of providers (7/17) elected to review and provide feedback for all lab values listed and not just the top 100. The most common questions received from providers involved the appearance and accessibility of the legacy data archive and the impact on their workflow if data was not migrated.
Fig. 5.
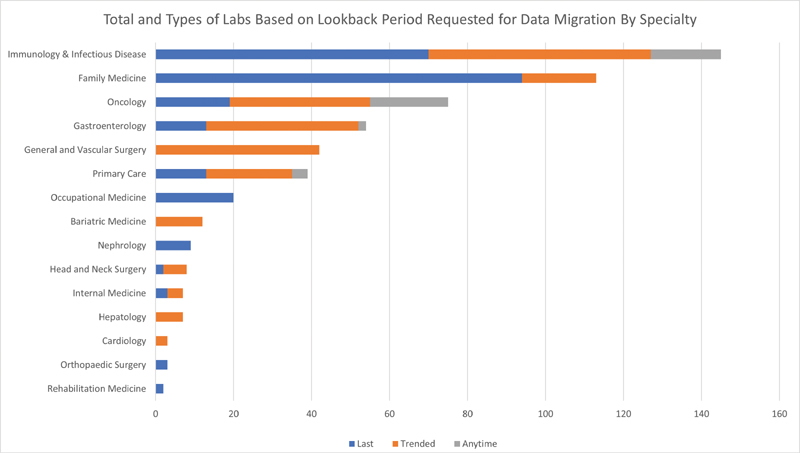
Laboratory orders requested for data migration based on specialty.
Discussion
This paper presents an approach to define the data migration needs of numerous ambulatory clinics using a PD-based method during the implementation of a new EHR system. By involving physician end-users, the clinical data designated for migration more accurately reflected their clinical needs. Physicians requested anywhere from 2 to 43 note types and from 2 to 145 unique laboratory types. These variations in feedback underscore the importance of tailoring data migration to end-users. This is in keeping with the well-observed phenomenon that even when controlling for EHR and practice setting, there exists a wide variation in individual physician practice patterns. 25 29
Feedback from all stakeholders including information technology and vendor representatives should be elicited early in the preimplementation planning phase. Data elements within the original EHR that are eligible for migration such as laboratory values and document types must be identified. The use of logical observation identifiers names and codes (LOINC) standards may facilitate this. Data types that are subject to data migration limitations should be defined based on end-user feedback to ensure clinically relevant data are included for migration. Initial strategies to define data migration were to take the top 100 most ordered laboratory types with 3-year lookback periods. While this would likely transfer a large volume of clinically useful data, it would have been suboptimal for certain specialties, such as oncology and infectious disease, who identified several rarely ordered lab types as being critical to transfer to a new EHR, such as genetic tests, electrophoresis, and, cytogenetics. Therefore, data migration for specialties may require protocols to include additional laboratory types.
Common barriers to implementing PD include time constraints, 30 poor organizational support, and lack of physician understanding of the technical aspects involved. 23 30 31 32 Our response rate (90%) for physician end-user participation which likely underscored the strong endorsement and support from executive leadership, as well as accommodating physicians' time and location for arranging meetings to obtain feedback. This project was orchestrated by the CMIO. A clinical informaticist plays a critical role within the health care setting by having the ability to understand and incorporate the unique needs of a diverse set of end-users for EHR optimization. By understanding the potential clinical implications of EHR functionality and design decisions, the clinical informaticist has the unique skill sets to engage end-users and advocate for a system that optimizes clinical outcomes.
Actively engaging end-users is only one part of PD which is based on several principles including mutual learning between designers and users, exploring alternative design visions, and simulation-based action. 33 34 Applying PD in the health care setting has been shown to enhance physician usability and satisfaction 6 21 24 35 36 and improve patient care. PD has great potential to revolutionize health care but it is still greatly underutilized. 37 Focusing on end-user feedback allowed our institution to successfully define its unique data-migration needs for an upcoming EHR transition.
Limitations
Our study has several limitations. Each specialty was represented by only one clinical end-user. Results would likely be more varied with a larger sample size within each specialty. Another limitation was that, at the time of this project, we were unable to show end-users what the legacy archive would look like. The most common question that providers had was regarding the appearance and accessibility of the legacy data archive. Gettinger and Csatari 2 described an unexpected and persistent reliance on legacy data to access administrative documentation consistently even 1-year post-EMR transition. Future research evaluating how accessing archival data impacts daily clinical practice patterns would be helpful.
Conclusion
PD describes a design approach based on feedback from all stakeholders. When transitioning to a new EHR system, it may not be feasible to migrate all clinical data. Eliciting physician end-user feedback to define data migration can be an effective approach for health care institutions faced with this task. Clinical informaticists are well suited to design and implement EHR optimization projects that integrate the needs of clinical, executive, and technical stakeholders.
Clinical Relevance Statement
When transitioning between electronic health record systems, clinical institutions may be faced with the task of defining the clinical data to be transferred. This paper outlines our approach in developing a detailed list of clinical data elements for data migration based on the unique needs of end-users. It may serve as a general framework for other institutions faced with the task of defining data migration.
Multiple Choice Questions
-
What is the benefit of using participatory design during an electronic health record transition?
Reduced costs.
Provides rigorous guidelines for governance.
Allows for design based on end-user feedback from stakeholders.
It requires fewer resources to implement.
Correct Answer: The correct answer is option c. Participatory design describes a process of design that attempts to actively engage end-users in the design process to ensure the final product meets their needs and is usable.
-
Why is it important to consider the needs of clinical end-users when discussing data migration for an electronic health record transition?
To ensure end-users have access to relevant clinical data.
To avoid relying on legacy databases to find clinical data on a routine basis.
To ensure that the new electronic health record environment is relevant to various clinical specialties.
All of the above.
Correct Answer: The correct answer is option d. Clinical end-users demonstrate variability in how they interact with an EHR system. To ensure that data migration results in an EHR environment that is useful and promotes efficiency, data migration should reflect the needs of clinical end-users.
Conflict of Interest None declared.
Protection of Human and Animal Subjects
Institutional Review Board approval was obtained. This work did not involve any interventions in human or animal subjects.
Supplementary Material
References
- 1.Manwell L B, Williams E S, Babbott S, Rabatin J S, Linzer M. Physician perspectives on quality and error in the outpatient setting. WMJ. 2009;108(03):139–144. [PubMed] [Google Scholar]
- 2.Gettinger A, Csatari A. Transitioning from a legacy EHR to a commercial, vendor-supplied, EHR: one academic health system's experience. Appl Clin Inform. 2012;3(04):367–376. doi: 10.4338/ACI-2012-04-R-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West S. Need versus cost: understanding EHR data migration options. J Med Pract Manage. 2013;29(03):181–183. [PubMed] [Google Scholar]
- 4.Babbott S, Manwell L B, Brown R.Electronic medical records and physician stress in primary care: results from the MEMO Study J Am Med Inform Assoc 201421(e1, E2):e100–e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gierl L, Feistle M, Müller H, Sliva K, Varnholt D, Villain S.Task-specific authoring functions for end-users in a hospital information system Comput Methods Programs Biomed 199548(1,2):145–150. [DOI] [PubMed] [Google Scholar]
- 6.Kroth P J, Morioka-Douglas N, Veres S. Association of electronic health record design and use factors with clinician stress and burnout. JAMA Netw Open. 2019;2(08):e199609. doi: 10.1001/jamanetworkopen.2019.9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paygude P, Devale P R. Automated data validation testing tool for data migration quality assurance. Int J Mod Eng Res. 2013;3(01):599–603. [Google Scholar]
- 8.Michel J J, Hsiao A, Fenick A. Using a scripted data entry process to transfer legacy immunization data while transitioning between electronic medical record systems. Appl Clin Inform. 2014;5(01):284–298. doi: 10.4338/ACI-2013-11-RA-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pageler N M, Grazier G'Sell M J, Chandler W, Mailes E, Yang C, Longhurst C A. A rational approach to legacy data validation when transitioning between electronic health record systems. J Am Med Informatics Assoc. 2016;23(05):991–994. doi: 10.1093/jamia/ocv173. [DOI] [PubMed] [Google Scholar]
- 10.Meigs S L, Solomon M.Electronic health record use a bitter pill for many physicians Perspect Heal Inf Manag 201613(Winter):1d. [PMC free article] [PubMed] [Google Scholar]
- 11.Sinsky C, Colligan L, Li L. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165(11):753–760. doi: 10.7326/M16-0961. [DOI] [PubMed] [Google Scholar]
- 12.Kwok J, Jones B. Unnecessary repeat requesting of tests: an audit in a government hospital immunology laboratory. J Clin Pathol. 2005;58(05):457–462. doi: 10.1136/jcp.2004.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irestig M, Hallberg N, Eriksson H, Timpka T. Peer-to-peer computing in health-promoting voluntary organizations: a system design analysis. J Med Syst. 2005;29(05):425–440. doi: 10.1007/s10916-005-6100-x. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Yu E. Designing information systems in social context: A goal and scenario modelling approach. Information Systems. 2004;29(02):187–203. [Google Scholar]
- 15.Sanders E B-N. New York, NY: Taylor and Francis; 2002. From user-centered to participatory design approaches; pp. 1–8. [Google Scholar]
- 16.Puri S K, Byrne E, Nhampossa J L, Quraishi Z B. Contextuality of participation in IS design: a developing country perspective. ACM. 2004;1:42–52. [Google Scholar]
- 17.Iglehart J K. Reform of the veterans affairs health care system. N Engl J Med. 1996;335(18):1407–1411. doi: 10.1056/NEJM199610313351821. [DOI] [PubMed] [Google Scholar]
- 18.Nøhr C, Kuziemsky C E, Elkin P L, Marcilly R, Pelayo S. Sustainable health informatics: health informaticians as alchemists. Stud Health Technol Inform. 2019;256:3–11. doi: 10.3233/SHTI190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scandurra I, Hägglund M, Koch S. From user needs to system specifications: multi-disciplinary thematic seminars as a collaborative design method for development of health information systems. J Biomed Inform. 2008;41(04):557–569. doi: 10.1016/j.jbi.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Tang T, Lim M E, Mansfield E, McLachlan A, Quan S D. Clinician user involvement in the real world: Designing an electronic tool to improve interprofessional communication and collaboration in a hospital setting. Int J Med Inform. 2018;110:90–97. doi: 10.1016/j.ijmedinf.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Clemensen J, Larsen S B, Kyng M, Kirkevold M. Participatory design in health sciences: using cooperative experimental methods in developing health services and computer technology. Qual Health Res. 2007;17(01):122–130. doi: 10.1177/1049732306293664. [DOI] [PubMed] [Google Scholar]
- 22.Bossen C. Evaluation of a computerized problem-oriented medical record in a hospital department: does it support daily clinical practice? Int J Med Inform. 2007;76(08):592–600. doi: 10.1016/j.ijmedinf.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Kanstrup A M, Madsen J, Nøhr C, Bygholm A, Bertelsen P. Developments in participatory design of health information technology - a review of PDC publications from 1990 - 2016. Stud Health Technol Inform. 2017;233:1–13. [PubMed] [Google Scholar]
- 24.Heponiemi T, Hyppönen H, Vehko T. Finnish physicians' stress related to information systems keeps increasing: a longitudinal three-wave survey study. BMC Medical Informatics and Decision Making. 2017;17(01) doi: 10.1186/s12911-017-0545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushniruk A W, Nøhr C. IOS Press; 2016. Participatory design, user involvement and health IT evaluation; pp. 139–151. [PubMed] [Google Scholar]
- 26.Tang T, Heidebrecht C, Coburn A. Using an electronic tool to improve teamwork and interprofessional communication to meet the needs of complex hospitalized patients: a mixed methods study. Int J Med Inform. 2019;127:35–42. doi: 10.1016/j.ijmedinf.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 27.HITEC Investigators . Ancker J S, Kern L M, Edwards A. How is the electronic health record being used? Use of EHR data to assess physician-level variability in technology use. J Am Med Inform Assoc. 2014;21(06):1001–1008. doi: 10.1136/amiajnl-2013-002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dewa C S, Loong D, Bonato S, Trojanowski L. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ Open. 2017;7(06):e015141. doi: 10.1136/bmjopen-2016-015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redd T K, Doberne J W, Lattin D. Variability in electronic health record usage and perceptions among specialty vs. primary care physicians. AMIA Annu Symp Proc. 2015;2015:2053–2062. [PMC free article] [PubMed] [Google Scholar]
- 30.Høstgaard A M, Bertelsen P, Nøhr C. Methods to identify, study and understand end-user participation in HIT development. BMC Med Inform Decis Mak. 2011;11:57. doi: 10.1186/1472-6947-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan B, Harris-Salamone K D. Health IT success and failure: recommendations from literature and an AMIA workshop. J Am Med Inform Assoc. 2009;16(03):291–299. doi: 10.1197/jamia.M2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratwani R M, Savage E, Will A. A usability and safety analysis of electronic health records: a multi-center study. J Am Med Informatics Assoc. 2018;25(09):1197–1201. doi: 10.1093/jamia/ocy088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kujala S. User involvement: a review of the benefits and challenges. Behav Inf Technol. 2003;22(01):1–16. [Google Scholar]
- 34.Van der Velden M, Mörtberg C. The Netherlands: Springer; 2015. Participatory design and design for values; pp. 41–66. [Google Scholar]
- 35.Ajami S, Bagheri-Tadi T. Barriers for adopting electronic health records (EHRs) by physicians. Acta Inform Med. 2013;21(02):129–134. doi: 10.5455/aim.2013.21.129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang M E.IT is from mars and physicians from venus: bridging the gap 20179(5S):S19–S25. [DOI] [PubMed] [Google Scholar]
- 37.Pollack A H, Miller A, Mishra S R, Pratt W. PD-atricians: Leveraging physicians and participatory design to develop novel clinical information tools. AMIA Annu Symp Proc. 2016;2016:1030–1039. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


