Key Points
Question
What are the oncologic outcomes and attributable costs associated with bacillus Calmette-Guérin (BCG) therapy for high-risk non–muscle-invasive bladder cancer (NMIBC)?
Findings
In this population-based cohort study within the Veterans Affairs Health System of 412 adults with high-risk NMIBC, patients with carcinoma in situ (Cis) had significantly increased risk of progression vs those with no Cis (85% vs 60%). Patients with progression had significantly increased costs as long as 5 years after diagnosis ($232 729 vs $94 879) compared with patients without progression.
Meaning
In this study, patients with Cis had an increased risk of progression that, regardless of Cis, was associated with significantly higher costs of care.
This cohort study describes real-world patient characteristics, long-term outcomes, and the economic burden in a population of US veterans with non–muscle-invasive bladder cancer.
Abstract
Importance
Management of high-risk non–muscle-invasive bladder cancer (NMIBC) represents a clinical challenge due to high failure rates despite prior bacillus Calmette-Guérin (BCG) therapy.
Objective
To describe real-world patient characteristics, long-term outcomes, and the economic burden in a population with high-risk NMIBC treated with BCG therapy.
Design, Setting, and Participants
This retrospective cohort study identified 412 patients with high-risk NMIBC from 63 139 patients diagnosed with bladder cancer who received at least 1 dose of BCG within Department of Veterans Affairs (VA) centers across the US from January 1, 2000, to December 31, 2015. Adequate induction BCG therapy was defined as at least 5 installations, and adequate maintenance BCG therapy was defined as at least 7 installations. Data were analyzed from January 2, 2020, to January 20, 2021.
Exposures
Intravesical BCG therapy, including adequate induction BCG therapy, was defined as at least 5 intravesical instillations of BCG within 70 days from BCG therapy start date. Adequate maintenance BCG therapy was defined as at least 7 installations of BCG within 274 days of the start (the first instillation) of adequate induction BCG therapy (ie, adequate induction BCG plus some form of additional BCG).
Main Outcomes and Measures
The Kaplan-Meier method was used to estimate outcomes, including event-free survival. All-cause expenditures were summarized as medians with corresponding interquartile ranges (IQRs) and adjusted to 2019 USD.
Results
Of the 412 patients who met inclusion criteria, 335 (81%) were male and 77 (19%) were female, with a median age of 67 (IQR, 61-74) years. Follow-up was 2694 person-years. A total of 392 patients (95%) received adequate induction BCG therapy, and 152 (37%) received adequate BCG therapy. For all patients with high-risk NMIBC, the 10-year progression-free survival rate and disease-specific death rate were 78% and 92%, respectively. Patients with carcinoma in situ (Cis) had worse disease-free survival than those without Cis (hazard ratio [HR], 1.85; 95% CI, 1.34-2.56). Total median costs at 1 year were $29 459 (IQR, $14 991-$52 060); at 2 years, $55 267 (IQR, $28 667-$99 846); and at 5 years, $117 361 (IQR, $59 680-$211 298). Patients with progressive disease had significantly higher median 5-year costs ($232 729 [IQR, $151 321-$341 195] vs $94 879 [IQR, $52 498-$172 631]; P < .001), with outpatient care, pharmacy, and surgery-related costs contributing.
Conclusions and Relevance
Despite adequate induction BCG therapy, only 37% of patients received adequate BCG therapy. Patients with Cis had increased risk of progression, and progression regardless of Cis was associated with significantly increased costs relative to patients without progression. Extrapolating cost figures, regardless of progression, resulted in nationwide costs at 1 year of $373 million for patients diagnosed with high-risk NMIBC in 2019.
Introduction
An estimated 81 400 new cases and 17 980 deaths due to bladder cancer occurred in the US in 2020.1 Although most patients are diagnosed with localized non–muscle-invasive bladder cancer (NMIBC), the risk of death is markedly increased once the disease invades the muscle.2 Non–muscle-invasive bladder cancer is a heterogeneous disease that can be stratified at the time of initial diagnosis based on findings from the transurethral resection of bladder tumor (TURBT) findings into low, intermediate, and high risk for progression to muscle invasion.3,4 Patients with high-risk NMIBC are at increased risk for progression and account for 25% of all newly diagnosed NMIBC.5 Identifying treatment patterns and response to these treatments among patients with NMIBC is paramount to improving the unchanged mortality due to this disease during the last 3 decades.6
Treatment patterns of NMIBC are heterogeneous with significant concerns regarding toxicity and lack of efficacy in treatments administered.7 Intravesical bacillus Calmette-Guérin (BCG) therapy after TURBT has been shown in multiple studies8,9,10 to reduce recurrence and progression in patients with high-risk NMIBC. However, despite the guideline recommendation, fewer than one-half of patients complete adequate maintenance BCG therapy, and fewer than 30% with high-risk NMIBC receive full induction courses of BCG therapy.9,11 Against this backdrop, bladder cancer also has one of the highest lifetime treatment costs of all cancers.12 Costs associated with bladder cancer care are substantial, with economic models estimating costs will be upward of $6 billion in 2020.13,14,15,16 Treatment patterns, outcomes, and costs have not been well studied among patients with high-risk NMIBC. In the present study, we determined treatment patterns, outcomes, and costs in patients with high-risk NMIBC in a real-world setting from the largest equal-access health care system in the US: the Department of Veterans Affairs (VA).
Methods
Data Source
Using inpatient and outpatient data and fee-basis claims (ie, care provided outside the VA system for which the VA paid), we queried data from the VA Informatics and Computing Infrastructure to identify patients with bladder cancer seen at any VA Health System site from January 1, 2000, to December 31, 2015. The study was approved by the institutional review board at the Durham VA Medical Center, Durham, North Carolina, which waived the need for informed consent for use of these retrospective data. This cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study Cohort
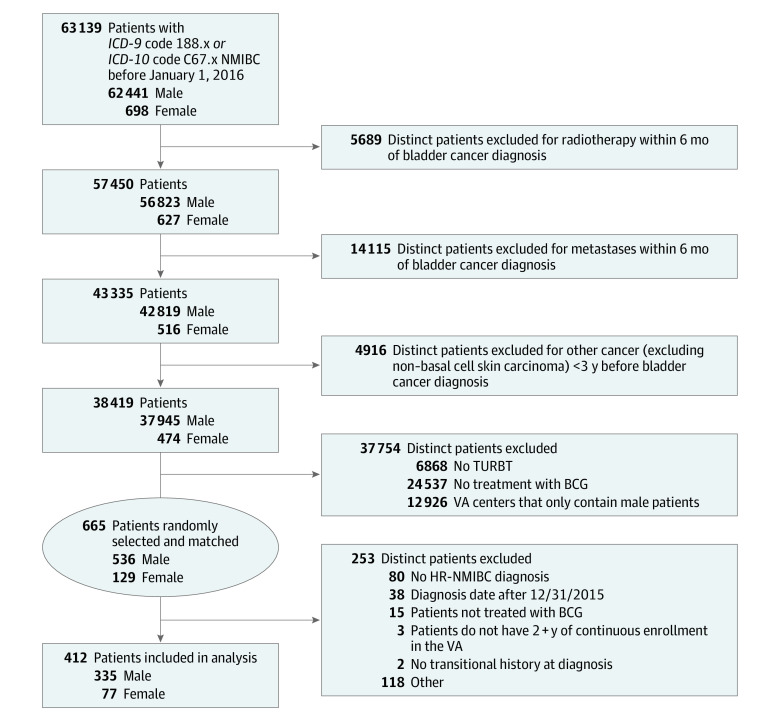
The study population consisted of patients with TURBT-confirmed NMIBC who were treated with BCG in the VA system. To identify a population of manageable size for screening and detailed review of medical records, we implemented a nationwide VA system query from January 1, 2000, to December 31, 2015 (Figure 1). Patients were required to have received care at the VA from January 1, 2000, to December 31, 2015; however, their bladder cancer could have been diagnosed any time before December 31, 2015. We identified 63 139 patients diagnosed with bladder cancer by using code 188.x from the International Classification of Diseases, Ninth Revision (ICD-9) and code C67.x from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10). Patients were excluded if they did not undergo TURBT or did not receive BCG in the VA. Patients were screened via review of medical records for all study inclusion and exclusion criteria. Patients may have had more than 1 exclusion factor warranting removal from the analysis. Complete inclusion and exclusion criteria are listed in Figure 1.
Figure 1. Cohort Selection.
Some excluded patients may be listed in more than 1 category. BCG indicates bacillus Calmette-Guérin; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; HR-NMIBC, high-risk non–muscle-invasive bladder cancer; TURBT, transurethral resection of bladder tumor; and VA, Department of Veterans Affairs.
After the above inclusion and exclusion query of the VA system, we then performed a detailed medical record review of 665 remaining patients with bladder cancer who were matched on sex within each VA center included (536 male and 129 female) to confirm patients were diagnosed with high-risk NMIBC before December 31, 2015, with at least 2 years of follow-up data available. We only selected patients who were treated with at least 1 installation of BCG therapy. We selected a male to female ratio of 4:1 to be representative of the general population of patients with NMIBC in the US.17 Female patients were selected randomly from all VA Medical Centers in the US. Male patients were matched by site, giving us a highly representative VA population across the entire country. After detailed medical record review with all inclusion and exclusion criteria followed by a 10% internal audit of all data, a total of 412 patients (335 male and 77 female) with high-risk NMIBC were included in the study.
Outcomes After BCG
Adequate induction BCG therapy was defined as at least 5 intravesical instillations of BCG within 70 days from the start date of BCG therapy.18 Adequate maintenance BCG therapy was defined as having received at least 7 installations of BCG within 274 days of start (the first instillation) of adequate induction BCG therapy (ie, adequate induction BCG plus some form of additional BCG therapy).18 We used response to BCG definitions and end points (persistence, recurrence, progression, and BCG unresponsiveness) as previously decribed.18,19 Disease progression was defined as (1) recurrent high-risk NMIBC, (2) pathological muscle-invasive disease (ie, T2 category or greater) upstaged from index TURBT findings, (3) development of nodal or visceral metastasis, (4) definitive treatment (cystectomy, systemic chemotherapy, or radiotherapy), or (5) bladder cancer–specific death. Disease-free survival was defined as time from index date (date of first BCG induction dose) to any TURBT finding of high-risk NMIBC, progression to MIBC or nodal or metastatic disease, or death due to bladder cancer. Overall survival was calculated from the index date to death due to any cause.
Study Covariates
Information on patient age, sex, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and non-Hispanic other races), and geographic region including the VA network were abstracted. Comorbidity was determined using the Charlson Comorbidity Index (CCI) by querying ICD-9 and ICD-10 codes.20 Codes were only available starting in the year 2000. Smoking status at bladder cancer diagnosis was defined as current, former, and never. For stage information, we used the TNM classification system according to the 8th edition of the American Joint Committee on Cancer Staging Manual.21 Clinical stages for patients with NMIBC were used to risk-stratify patients and categorize them as having high-risk vs low-risk NMIBC (which combined low- and intermediate-risk NMIBC).3,22 Clinical stage group for high-risk NMIBC was further subcategorized as presence vs absence of carcinoma in situ (Cis). The World Health Organization 1973 bladder cancer grading system (1, 2, and 3) was revised in 2004 (low and high).23,24,25 Thus, we used an accepted bladder cancer grade categorization as low (grade 1 and low) and high (grades 2 and 3 and high) grade.23,24,25 Patient tumor characteristics (stage and grade) at high-risk NMIBC diagnosis were abstracted.
Costs
Use of health care resources and costs were captured. To determine costs associated with NMIBC care, we summed all health care expenditures from inpatient, outpatient, and physician services, including laboratory, pharmacy, surgery, and radiology costs, starting from date of first BCG dose administered. Outpatient and inpatient expenditures were obtained from the outpatient and inpatient mean cost data of the VA Health Economics Resource Center and fee basis files.26,27 Costs were stratified by disease progression status. Primary analysis included costs within 2 years, and secondary analysis determined 1- and 5-year costs. All costs were inflated to 2019 USD using the medical component of the Consumer Price Index.28,29 Owing to the skewed distribution of cost data, we reported and compared median cost by progression status using the Wilcoxon rank sum test. Furthermore, we used a 2-part regression method to evaluate the association of costs and progression status while adjusting for age, sex, race/ethnicity, and year of bladder cancer diagnosis. To estimate the dollar amount of spending attributed to high-risk NMIBC, we extrapolated the adjusted median total costs associated with these patients to an analogous group of patients with bladder cancer in 2019.14,30,31 Specifically, we obtained the total number of estimated bladder cancer cases in 2019.17 We estimated that 90% of cases were transitional cell carcinoma. We then estimated that 70% were NMIBC and 25% of patients with NMIBC had high-risk disease.3,22,32 We then multiplied this number by $29 459 (1-year median all-cause expenditure per patient). To derive national costs for patients who received radical cystectomy, we multiplied the total number of patients with high-risk NMIBC diagnosed in 2019 by 9% (the rate of radical cystectomy in the present study) and $358 593 (median expenditure from BCG start to last follow-up or death per patient).
Statistical Analysis
Data were analyzed from January 2, 2020, to January 20, 2021. Patient demographic details and disease characteristics were summarized across all patients. The odds ratio of patient progression status (yes vs no) with any health care expenditures relative to no expenditures was estimated from logistic regression adjusting for age at bladder cancer diagnosis, sex, race/ethnicity, and year of bladder cancer diagnosis. For patients with health care expenditures, general linear modeling on the logarithm of the cost was performed by adjusting for the same covariates as ones in the logistic regression. We verified that general linear modeling assumptions were met. The start date of BCG induction therapy was treated as time zero for all analyses. Kaplan-Meier curves were created for time-to-event outcomes. Differences in time-to-event were stratified by presence or absence of Cis and tested using log-rank tests. A Cox proportional hazards regression model was used to examine variables associated with disease-free survival. No variable selection was performed. Candidate covariates were determined a priori and included age at first dose of BCG, sex (male vs female), smoking status (current vs former vs never), Cis at high-risk NMIBC diagnosis (yes vs no), and CCI at BCG therapy initiation (0-2 vs 3-4 vs ≥5). All statistical tests were 2 sided, and all analyses were performed using SAS, version 9.4 (SAS Institute Inc) and Stata, version 15.1 (StataCorp LLC). Statistical significance was defined as P < .05.
Results
Demographic details of patients with high-risk NMIBC are summarized in Table 1. Of the 412 patients who met inclusion criteria, 335 (81%) were male and 77 (19%) were female, with a median age of 67 (interquartile range [IQR], 61-74) years. Follow-up was 2694 person-years. Smoking status included never in 58 patients (14%), former in 205 (50%), and current in 149 (36%). At BCG therapy initiation, 383 patients (93%) had a CCI greater than 1, with 80 patients (19%) having a CCI of at least 6. Median follow-up was 70.5 (IQR, 42.5-105.3) months.
Table 1. Baseline Characteristics of Patients With High-Risk NMIBC.
| Characteristic | Patient data (n = 412)a |
|---|---|
| Age at BCG initiation, median (IQR), y | 67 (61-74) |
| Race | |
| Black | 50 (12) |
| White | 345 (84) |
| Other | 9 (2) |
| Unknown | 8 (2) |
| Ethnicity | |
| Hispanic/Latino | 15 (4) |
| Not Hispanic/Latino | 381 (92) |
| Unknown | 16 (4) |
| Sex | |
| Female | 77 (19) |
| Male | 335 (81) |
| VA Network | |
| New England | 21 (5) |
| Upstate New York | 54 (13) |
| Pennsylvania | 14 (3) |
| Washington, DC | 7 (2) |
| Mid-Atlantic | 14 (3) |
| Southeast | 28 (7) |
| Sunshine (Florida) | 52 (13) |
| Mid-South | 5 (1) |
| Ohio | 37 (9) |
| Great Lakes | 5 (1) |
| South Central | 36 (9) |
| Heart of Texas | 10 (2) |
| Southwest | 7 (2) |
| Rocky Mountain | 10 (2) |
| Northwest | 36 (9) |
| Sierra Pacific | 22 (5) |
| Desert Pacific | 44 (11) |
| Midwest | 10 (2) |
| Follow-up from BCG therapy, median (IQR), mo | 70.5 (42.5-105.3) |
| Smoking status | |
| Former | 205 (50) |
| Current | 149 (36) |
| Never | 58 (14) |
| CCI at BCG initiation | |
| 0 | 11 (3) |
| 1 | 8 (2) |
| 2 | 93 (23) |
| 3 | 97 (24) |
| 4 | 77 (19) |
| 5 | 36 (9) |
| ≥6 | 80 (19) |
| Missingb | 10 (2) |
| Clinical grade | |
| Unknown | 27 (6) |
| Low | 37 (9) |
| High | 348 (84) |
| Clinical stage group | |
| Unknown | 2 (1) |
| Cis with or without T1 or TaHG | 69 (16) |
| TaHG or T1 without Cis | 341 (83) |
Abbreviations: BCG, bacillus Calmette-Guérin; CCI, Charlson Comorbidity Index; Cis, carcinoma in situ; IQR, interquartile range; NMIBC, non–muscle-invasive bladder cancer; TaHG, Ta high-grade; VA, Department of Veterans Affairs.
Unless otherwise specified, data are expressed as number (percentage) of patients.
Patients with BCG initiation before 2000 did not have CCI available.
A total of 392 patients (95%) received adequate induction BCG therapy, and 152 (37%) received adequate maintenance BCG therapy (Table 2). Median length of BCG induction was 42 (IQR, 35-288) days. A total of 71 patients (17%) experienced progression, of whom patients with Cis had significantly worse progression-free survival vs patients without Cis as detailed below (Figure 2). Among patients who received adequate induction BCG therapy and had a complete response (196 [48%]), 61 (31%) subsequently had recurrent high-risk NMIBC. Thirty-three patients with Cis received adequate induction BCG therapy and had a complete response. Of these, 13 patients (39%) had recurrent high-risk NMIBC. In the entire cohort, 30 patients (7%) underwent systemic chemotherapy; 12 (3%), radiotherapy; and 39 (10%), radical cystectomy. Among patients undergoing radical cystectomy, median time to surgery was 23 (IQR, 8-42) months from BCG therapy initiation.
Table 2. Treatments and Outcomes After BCG.
| Characteristic or outcome | Patient data (n = 412)a |
|---|---|
| Year of BCG initiation, median (range) | 2010 (2007-2013) |
| Adequate induction therapy | 392 (95) |
| Adequate maintenance BCG therapy | 152 (37) |
| Length of BCG induction, median (IQR), d | 42 (35-288) |
| Cystectomy | 39 (9) |
| Clinical stage group, No./total No. (%) | |
| Cis with or without T1 or TaHG | 13/69 (19) |
| TaHG or T1 without Cis | 26/341 (8) |
| Progression | |
| Unknown | 2 (1) |
| No | 339 (83) |
| Yes | 71 (17) |
| Distant metastasisb | |
| Unknown | 2 (1) |
| No | 385 (94) |
| Yes | 25 (6) |
| Persistent high-risk NMIBC | |
| NA | 20 (5) |
| No | 91 (23) |
| Yes | 57 (15) |
| Unknownc | 244 (62) |
| BCG unresponsive | |
| NAd | 20 (5) |
| No | 0 |
| Yes | 26 (7) |
| Unknowne | 366 (93) |
| Complete response | |
| No | 216 (52) |
| Yes | 196 (48) |
| Recurrent high-risk NMIBC after complete response | |
| No complete response or nonadequate induction | 223 (54) |
| No | 47 (11) |
| Yes | 61 (15) |
| Unknownf | 81 (20) |
| Dead | 166 (40) |
| Death due to BC | 27 (7) |
Abbreviations: BC, bladder cancer; BCG, bacillus Calmette-Guérin; Cis, carcinoma in situ; IQR, interquartile range; NA, not available; NMIBC, non–muscle-invasive bladder cancer; TaHG, Ta high-grade.
Unless otherwise indicated, data are expressed as number (percentage) of patients. Percentages are rounded and may not equal 100.
This category represents the number with distant metastasis ever during follow-up after high-risk NMIBC.
These patients did undergo a transurethral resection of bladder tumor (TURBT) in the required time frame to determine whether the patient had persistent disease.
Twenty patients did not have the required BCG treatment to determine BCG-unresponsive disease.
These patients did undergo a TURBT in all of the required time frames to determine BCG unresponsiveness.
These patients did undergo a TURBT within 786 days of last BCG exposure to determine recurrent status.
Figure 2. Kaplan-Meier Curves for Time to Progression, Overall and Stratified by Clinical Stage Group.
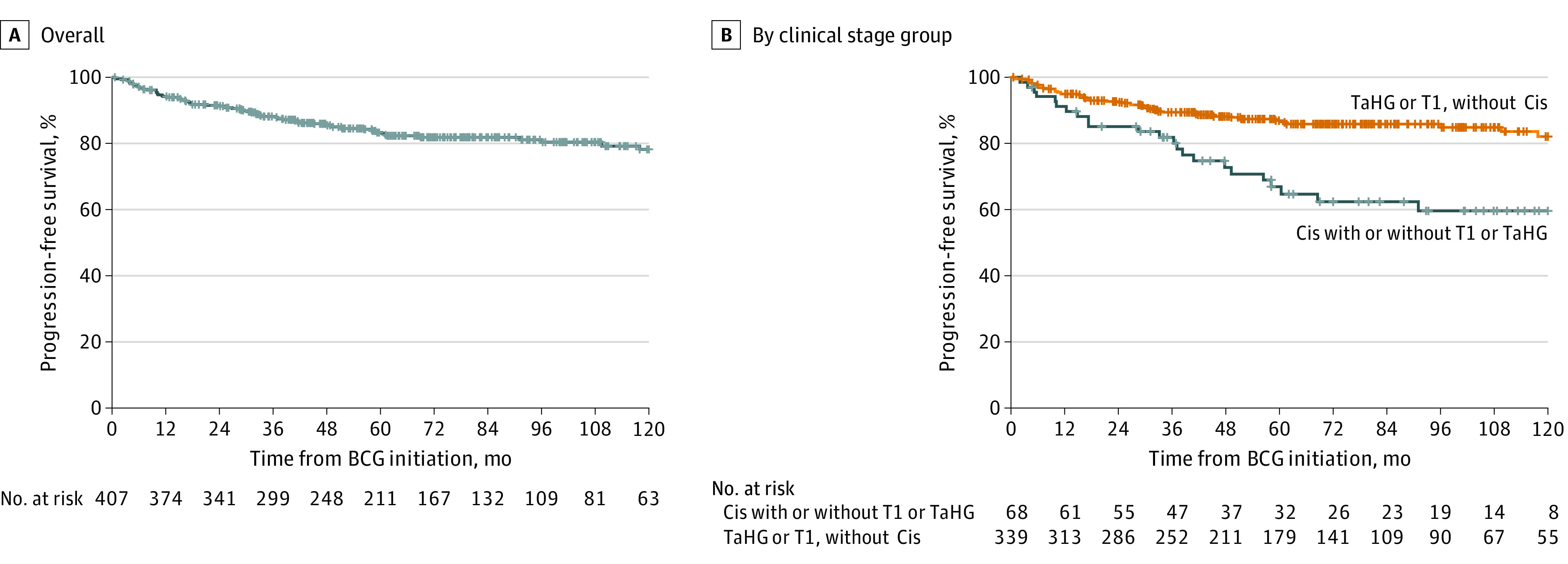
Plus sign indicates censored. BCG indicates bacillus Calmette-Guérin; Cis, carcinoma in situ; and TaHG, Ta high-grade.
A total of 166 patients (40%) died, 27 (7%) with bladder cancer–specific causes. Although there was no difference in all-cause mortality, patients with Cis had significantly worse disease-free survival vs patients without Cis as detailed below (eFigures 1 and 2 in the Supplement). Patient characteristics independently associated with worse disease-specific survival included Cis at high-risk NMIBC diagnosis (hazard ratio, 1.99; 95% CI, 1.44-2.75) (eTable 1 in the Supplement). Three-, 5-, 10-, and 15-year Kaplan-Meier event-free survival estimates for time to progression, cystectomy, death, and bladder cancer death are shown in eTable 2 in the Supplement. Specifically, at 10 years, patients with Cis had worse progression-free survival (60% vs 82%; P < .001), cystectomy-free survival (76% vs 91%; P = .004), bladder cancer–specific survival (87% vs 94%; P < .001), and overall survival (49% vs 55%; P = .32) compared with patients without Cis. For all patients with high-risk NMIBC, the 10-year progression-free survival rate and disease-specific death rate were 78% and 92%, respectively. Patients with Cis had worse disease-free survival than those without Cis (hazard ratio [HR], 1.85; 95% CI, 1.34-2.56).
Patient characteristics as well as cost data available for patients diagnosed from 2010 to the present (401 [97%]) are summarized in Table 3. Total median costs at 1 year from the start of BCG induction therapy were $29 459 (IQR, $14 991-$52 060); 2 years, $55 267 (IQR, $28 667-$99 846); and 5 years, $117 361 (IQR, $59 680-$211 298). Patients with disease progression had significantly higher median 5-year costs ($232 729 [IQR, $151 321-$341 195] vs $94 879 [IQR, $52 498-$172 631]; P < .001), with outpatient care, pharmacy, and surgery-related costs contributing largely to the higher costs associated with disease progression. A 2-part regression method was used to evaluate the association of costs and progression status, noting significantly higher costs with progression (eTables 3 and 4 in the Supplement). Of 39 patients who received a cystectomy, 33 (85%) received their cystectomy within the VA system and were included in the costs analysis (eTable 5 in the Supplement). Among patients who underwent radical cystectomy from initial BCG dose to end of follow-up, the median all-cause expenditure per patient was $366 857 (IQR, $278 462-$668 378). Extrapolating these figures to the total US population results in $465 million in total costs for patients with high-risk NMIBC who underwent radical cystectomy for patients diagnosed in 2019. In comparison, extrapolating cost figures resulted in nationwide costs at 1 year of $373 million for patients diagnosed with high-risk NMIBC in 2019.
Table 3. Health Care Cost Data, Overall and Stratified by Progression Status From Time of BCG Induction Therapy Start Date to 1, 2, and 5 Years of Follow-upa.
| Variable | Progression status | P value | ||
|---|---|---|---|---|
| No progression (n = 330) | Progression (n = 71) | All (n = 401) | ||
| Age at BC diagnosis, median (IQR), y | 66 (59-74) | 65 (60-73) | 66 (59-74) | .87b |
| Race, No. (%) | ||||
| Black | 42 (13) | 7 (10) | 49 (12) | .50c |
| White | 273 (83) | 62 (87) | 335 (84) | |
| Other | 7 (2) | 2 (3) | 9 (2) | |
| Unknown | 8 (2) | 0 (0) | 8 (2) | |
| Ethnicity, No. (%) | ||||
| Hispanic/Latino | 13 (4) | 2 (3) | 15 (4) | .15c |
| Not Hispanic/Latino | 301 (91) | 69 (97) | 370 (92) | |
| Unknown | 16 (5) | 0 (0) | 16 (4) | |
| Sex, No. (%) | ||||
| Female | 58 (18) | 15 (21) | 73 (18) | .48c |
| Male | 272 (82) | 56 (79) | 328 (82) | |
| Year of BC diagnosis, median (range) | 2010 (2006-2013) | 2008 (2005-2011) | 2010 (2006-2013) | .049b |
| Costs, median (IQR), USDd | ||||
| Total | ||||
| 1 y | $27 056 ($13 205-$48 193) | $35 179 ($18 128-$95 247) | $29 459 ($14 991-$52 060) | .001b |
| 2 y | $51 355 ($25 562-$82 467) | $102 718 ($46 691-$182 997) | $55 267 ($28 667-$99 846) | <.001b |
| 5 y | $94 879 ($52 498-$172 631) | $232 729 ($151 321-$341 195) | $117 361 ($59 680-$211 298) | <.001b |
| Inpatient | ||||
| 1 y | $0 ($0-$0) | $0 ($0-$15 016) | $0 ($0-$0) | <.001b |
| 2 y | $0 ($0-$0) | $12 051 ($0-$76 069) | $0 ($0-$6263) | <.001b |
| 5 y | $0 ($0-$23 418) | $67 517 ($15 187-$108 524) | $2282 ($0-$46 101) | <.001b |
| Outpatient | ||||
| 1 y | $26 445 ($12 945-$44 159) | $34 227 ($17 861-$68 418) | $27 070 ($13 832-$47 681) | .003b |
| 2 y | $46 923 ($25 218-$76 282) | $72 014 ($46 691-$123 257) | $52 030 ($27 751-$83 454) | <.001b |
| 5 y | $88 874 ($49 405-$141 366) | $163 656 ($107 993-$203 412) | $97 877 ($53 859-$163 656) | <.001b |
| Laboratory | ||||
| 1 y | $833 ($146-$1798) | $1315 ($329-$3917) | $861 ($162-$2029) | .007b |
| 2 y | $1586 ($451-$3153) | $3374 ($1600-$8349) | $1773 ($616-$3597) | <.001b |
| 5 y | $3147 ($1177-$5974) | $9851 ($4307-$16 364) | $3693 ($1536-$7879) | <.001b |
| Pharmacy | ||||
| 1 y | $2014 ($897-$4211) | $3341 ($1279-$6903) | $2064 ($973-$4823) | .008b |
| 2 y | $3691 ($1567-$7835) | $7536 ($2667-$23 308) | $4068 ($1640-$9251) | <.001b |
| 5 y | $7217 ($3653-$17 949) | $18 306 ($10 210-$39 045) | $9065 ($4018-$21 945) | <.001b |
| Surgery | ||||
| 1 y | $1371 ($0-$6627) | $4141 ($0-$16 062) | $1696 ($0-$7515) | .008b |
| 2 y | $3966 ($0-$12 105) | $13 945 ($3652-$34 165) | $5185 ($0-$13 561) | <.001b |
| 5 y | $9726 ($1342-$23 221) | $33 383 ($17 495-$66 406) | $12 159 ($2936-$30 905) | <.001b |
| Radiology | ||||
| 1 y | $489 ($0-$1263) | $843 ($136-$2730) | $521 ($0-$1416) | .013b |
| 2 y | $1108 ($330-$2416) | $2508 ($889-$10 787) | $1238 ($383-$2988) | <.001b |
| 5 y | $2778 ($1132-$5710) | $10 965 ($4648-$19 851) | $3326 ($1348-$8241) | <.001b |
Abbreviations: BC, bladder cancer; BCG, bacillus Calmette-Guérin; IQR, interquartile range.
Cost data are only available from 2010 to present, so only patients treated with BCG starting in 2000 are included in this table.
Calculated using Wilcoxon rank sum test.
Calculated using χ2 test.
All costs are adjusted to 2019 USD using the medical component of the Consumer Price Index.
Discussion
Most cases of newly diagnosed bladder cancer are NMIBC; however, once bladder cancer invades the muscle, the 5-year risk of death due to bladder cancer is approximately 50%.32 High-risk NMIBC accounts for approximately 25% of all newly diagnosed NMIBC, with prior reports of 33% progressing to muscle invasion in 10 years.33 Treatments are limited, with cases unresponsive to BCG needing more definitive treatments, including radical cystectomy and/or systemic therapies. In this study, we found that although almost all patients completed adequate induction BCG therapy, only 37% of patients completed adequate maintenance BCG therapy. Patients with Cis had significantly increased risk of progression when compared with patients with no Cis. Moreover, we observed significantly increased costs among patients with vs without progression of disease.
Our study has several important findings. First, to our knowledge, this is one of the largest cohorts with high-risk NMIBC described with individual patient data from an equal-access setting. We included patients with NMIBC who received BCG and had at least 2 years of follow-up within the national VA Health System. Recognizing the male predominance within the VA population, our cohort included a male to female ratio of 4:1 to be representative of the general population of patients with NMIBC in the US. Although other population-based data sets have been developed, high-risk NMIBC was not defined according to the NMIBC risk definition noted in guidelines3,22 and lacked the detail to control for potential confounders such as smoking, which has been previously associated with adverse bladder cancer outcomes (ie, disease progression and survival).7,33 The present study overcomes these limitations in other data sets but also has the clinical and pathological detail in accordance with current high-risk NMIBC clinical trials to provide real-world comparisons.
Second, we describe treatment patterns of high-risk NMIBC that include intravesical therapy but also subsequent definitive treatments. We found that although almost all patients (95%) completed adequate induction BCG therapy, only 37% of patients completed adequate maintenance BCG therapy. Prior studies have characterized how the number of endoscopic resections of bladder tumors affects use of BCG, showing that 40% of patients with high-risk NMIBC receive any BCG after a single TURBT, with more than 60% of those who do receive BCG having an incomplete induction course.7,34 Our study, although restrictive and excluding large numbers of patients and thus limiting generalizability, suggests strong adherence to guidelines for adequate induction BCG therapy within the VA Health System consistent with other studies, suggesting higher quality of cancer care in patients treated in the VA than elsewhere.35 However, as noted in other studies, opportunity remains to improve use of adequate maintenance BCG therapy3,4 given that maintenance BCG therapy reduced progression risk.9,18,36 In our study, 30 patients (7%) underwent systemic chemotherapy, 12 (3%) underwent radiotherapy, and 39 (10%) underwent radical cystectomy. These findings are in accordance with prior studies37 noting that only a small fraction of patients undergo definitive therapy with cystectomy, radiotherapy, or systemic chemotherapy.
Third, we found that patients with Cis had significantly increased risk of progression and worse disease-free survival than patients with no Cis. These findings support prior studies3,4 noting increased risk of progression associated with Cis and current clinical trial designs that stratify patients by NMIBC tumor histology (Cis vs no Cis) at randomization. Research efforts are underway to elucidate the underlying biological aggression of high-risk NMIBC, including Cis.19 This effort is balanced by health policy considerations regarding appropriate allocation of BCG to reduce progression among patients with high-risk NMIBC and, in particular, those with Cis given recent worldwide BCG shortages.38
Fourth, we observed high costs associated with high-risk NMIBC and especially among patients with progression. Patients with progression had significantly higher 5-year costs ($232 729 vs $94 879; P < .001), with outpatient care, pharmacy, and surgery-related costs contributing largely to the higher costs associated with disease progression. We also described the additional cost burden of radical cystectomy in this cohort. Although prior studies have determined costs associated with NMIBC, these studies either focused on surveillance or dosing of BCG and/or used Markov modeling, including all patients with NMIBC (low, intermediate, and high risk) to ascertain costs and cost-effectiveness research.39,40 We provide the first study, to our knowledge, to show detailed costs associated with the management of high-risk NMIBC and substantial costs associated with radical cystectomy.41,42 These costs associated with progression are important given the recent advances and costs associated with novel immune and gene therapy treatment options in patients with BCG-refractory high-risk NMIBC.43,44 As we move toward approval of these newer agents by the US Food and Drug Administration, there should be further interrogation of global costs of care in addition to oncologic efficacy.
Limitations
Our findings need to be interpreted within the context of the study design. First, this is a retrospective cohort study with inherent selection bias and cannot derive causation. Second, although we controlled for possible confounders not included in other studies (ie, smoking, detailed pathological findings, and access to care), we recognize the inability to adjust for all potential known and unknown confounders that may affect our findings. This limitation is exemplified by increased numbers of BCG installations associated with worse disease-free survival observed in the present study when there is evidence that induction and maintenance therapy result in improved disease-free survival.18 Unmeasured confounders including tumor size, lymphovascular invasion, and lack of central pathological findings may affect our results.3,22,32 Third, the study cohort was performed within the largest equal-access health care system in the US, and we recognize the inability to capture all possible treatments (and associated costs) received outside of the VA. Last, our cost estimates were based on expenditures from the VA, which have differing payment systems than other insurance programs. Moreover, our cost extrapolation was likely an overestimate because it assumed similarly high adherence to the population included in this study. The Congressional Budget Office has concluded that it could not properly compare absolute health care costs across the VA and the private sector.45 Previous studies of the cost of care provided by VA hospitals have not shown evidence that VA hospitals were significantly more or less efficient than nonfederal hospitals and that it was not more (or less) costly to provide care in VA facilities than in non-VA facilities.46,47 Thus, although absolute cost comparisons across systems with dissimilar cost estimation practices are a challenge, costs observed within the VA exhibit similar patterns of costs observed in other health systems and insurance programs (ie, Medicare).
Conclusions
In the present report, we describe treatment patterns, outcomes, and costs in patients with high-risk NMIBC within the VA Health System. We found that 95% of patients completed adequate induction BCG, but only 37% of patients completed adequate maintenance BCG therapy. Patients with Cis had a significantly increased risk of progression compared with patients with no Cis. We observed significantly increased costs associated with progression. Extrapolating cost figures, regardless of progression, resulted in nationwide costs at 1 year of $373 million for patients diagnosed with high-risk NMIBC in 2019. Our findings demonstrate the need for additional therapies to reduce the risk of progression and further improve outcomes and decrease costs.
eFigure 1. Kaplan-Meier Curves for Disease-Free Survival, Overall and Stratified by Clinical Stage Group
eFigure 2. Kaplan-Meier Curves for Time to All-Cause Mortality, Overall and Stratified by Clinical Stage Group
eTable 1. Univariable and Multivariable Association Between Demographic and Disease Characteristics and Disease-Free Survival (n = 401)
eTable 2. Kaplan-Meier Event-Free Survival Estimates From Time of BCG Initiation
eTable 3. Comparisons of Number of Patients With Any Cost vs No Cost Stratified by Progression Status
eTable 4. Association Between Progression Status and Costs (if Any)
eTable 5. Health Care Cost Data From Time of BCG Induction Start Date and 30 and 90 Days After Cystectomy (n = 33)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Scher H, Bahnson R, Cohen S, et al. ; National Comprehensive Cancer Network . NCCN urothelial cancer practice guidelines. Oncology (Williston Park). 1998;12(7A):225-271. [PubMed] [Google Scholar]
- 3.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non–muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):1021-1029. doi: 10.1016/j.juro.2016.06.049 [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Burger M, Zigeuner R, et al. ; European Association of Urology . EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639-653. doi: 10.1016/j.eururo.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 5.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178(6):2314-2330. doi: 10.1016/j.juro.2007.09.003 [DOI] [PubMed] [Google Scholar]
- 6.Zehnder P, Studer UE, Skinner EC, et al. Unaltered oncological outcomes of radical cystectomy with extended lymphadenectomy over three decades. BJU Int. 2013;112(2):E51-E58. doi: 10.1111/bju.12215 [DOI] [PubMed] [Google Scholar]
- 7.Lenis AT, Donin NM, Litwin MS, et al. ; Urologic Diseases in America Project . Association between number of endoscopic resections and utilization of bacillus Calmette-Guérin therapy for patients with high-grade, non–muscle-invasive bladder cancer. Clin Genitourin Cancer. 2017;15(1):e25-e31. doi: 10.1016/j.clgc.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? a meta-analysis of randomized trials. Urology. 2006;67(6):1216-1223. doi: 10.1016/j.urology.2005.12.014 [DOI] [PubMed] [Google Scholar]
- 9.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guérin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163(4):1124-1129. doi: 10.1016/S0022-5347(05)67707-5 [DOI] [PubMed] [Google Scholar]
- 10.Sylvester RJ, van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guérin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168(5):1964-1970. doi: 10.1016/S0022-5347(05)64273-5 [DOI] [PubMed] [Google Scholar]
- 11.Chamie K, Saigal CS, Lai J, et al. ; Urologic Diseases in America Project . Compliance with guidelines for patients with bladder cancer: variation in the delivery of care. Cancer. 2011;117(23):5392-5401. doi: 10.1002/cncr.26198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Burden of Disease Health Financing Collaborator Network . Evolution and patterns of global health financing 1995-2014: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet. 2017;389(10083):1981-2004. doi: 10.1016/S0140-6736(17)30874-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. doi: 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29(12):1517-1524. doi: 10.1200/JCO.2010.31.1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Accordino MK, Wright JD, Vasan S, et al. Use and costs of disease monitoring in women with metastatic breast cancer. J Clin Oncol. 2016;34(24):2820-2826. doi: 10.1200/JCO.2016.66.6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leow JJ, Cole AP, Seisen T, et al. Variations in the costs of radical cystectomy for bladder cancer in the USA. Eur Urol. 2018;73(3):374-382. doi: 10.1016/j.eururo.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 17.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 18.Kamat AM, Sylvester RJ, Böhle A, et al. Definitions, end points, and clinical trial designs for non–muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol. 2016;34(16):1935-1944. doi: 10.1200/JCO.2015.64.4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamat AM, Colombel M, Sundi D, et al. BCG-unresponsive non–muscle-invasive bladder cancer: recommendations from the IBCG. Nat Rev Urol. 2017;14(4):244-255. doi: 10.1038/nrurol.2017.16 [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 21.Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93-99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 22.Babjuk M, Burger M, Compérat EM, et al. European Association of Urology guidelines on non–muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur Urol. 2019;76(5):639-657. doi: 10.1016/j.eururo.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 23.Compérat E, Varinot J, Moroch J, Eymerit-Morin C, Brimo F. A practical guide to bladder cancer pathology. Nat Rev Urol. 2018;15(3):143-154. doi: 10.1038/nrurol.2018.2 [DOI] [PubMed] [Google Scholar]
- 24.Amin MB, McKenney JK, Paner GP, et al. ; International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder Cancer 2012 . ICUD-EAU International Consultation on Bladder Cancer 2012: pathology. Eur Urol. 2013;63(1):16-35. doi: 10.1016/j.eururo.2012.09.063 [DOI] [PubMed] [Google Scholar]
- 25.Amin MB, Trpkov K, Lopez-Beltran A, Grignon D; Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group . Best practices recommendations in the application of immunohistochemistry in the bladder lesions: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38(8):e20-e34. doi: 10.1097/PAS.0000000000000240 [DOI] [PubMed] [Google Scholar]
- 26.Wagner TH, Chen S, Barnett PG. Using average cost methods to estimate encounter-level costs for medical-surgical stays in the VA. Med Care Res Rev. 2003;60(3)(suppl):15S-36S. doi: 10.1177/1077558703256485 [DOI] [PubMed] [Google Scholar]
- 27.Smith VA, Arterburn DE, Berkowitz TSZ, et al. Association between bariatric surgery and long-term health care expenditures among veterans with severe obesity. JAMA Surg. 2019;154(12):e193732. doi: 10.1001/jamasurg.2019.3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu JC, Chughtai B, O’Malley P, et al. Perioperative outcomes, health care costs, and survival after robotic-assisted versus open radical cystectomy: a national comparative effectiveness study. Eur Urol. 2016;70(1):195-202. doi: 10.1016/j.eururo.2016.03.028 [DOI] [PubMed] [Google Scholar]
- 29.Cipriano LE, Romanus D, Earle CC, et al. Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value Health. 2011;14(1):41-52. doi: 10.1016/j.jval.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 31.Noone AM, Howlander N, Krapcho M, et al. SEER cancer statistics review, 1975-2015. Updated September 10, 2018. Accessed June 1, 2020. https://seer.cancer.gov/archive/csr/1975_2015/
- 32.Clark PE, Agarwal N, Biagioli MC, et al. ; National Comprehensive Cancer Network (NCCN) . Bladder cancer. J Natl Compr Canc Netw. 2013;11(4):446-475. doi: 10.6004/jnccn.2013.0059 [DOI] [PubMed] [Google Scholar]
- 33.Chamie K, Litwin MS, Bassett JC, et al. ; Urologic Diseases in America Project . Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119(17):3219-3227. doi: 10.1002/cncr.28147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer BA, McBride RB, Hershman DL, et al. Adjuvant intravesical bacillus Calmette-Guérin therapy and survival among elderly patients with non–muscle-invasive bladder cancer. J Oncol Pract. 2013;9(2):92-98. doi: 10.1200/JOP.2011.000480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gidwani-Marszowski R, Faricy-Anderson K, Asch SM, Illarmo S, Ananth L, Patel MI. Potentially avoidable hospitalizations after chemotherapy: differences across Medicare and the Veterans Health Administration. Cancer. 2020;126(14):3297-3302. doi: 10.1002/cncr.32896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU Cancers Group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63(3):462-472. doi: 10.1016/j.eururo.2012.10.039 [DOI] [PubMed] [Google Scholar]
- 37.Chamie K, Ballon-Landa E, Daskivich TJ, et al. ; Urologic Diseases in America Project . Treatment and survival in patients with recurrent high-risk non–muscle-invasive bladder cancer. Urol Oncol. 2015;33(1):20.e9-20.e17. doi: 10.1016/j.urolonc.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma V, Wymer KM, Borah BJ, et al. Cost-effectiveness of maintenance bacillus Calmette-Guérin for intermediate and high risk nonmuscle invasive bladder cancer. J Urol. 2020;204(3):442-449. doi: 10.1097/JU.0000000000001023 [DOI] [PubMed] [Google Scholar]
- 39.Mossanen M, Wang Y, Szymaniak J, et al. Evaluating the cost of surveillance for non–muscle-invasive bladder cancer: an analysis based on risk categories. World J Urol. 2019;37(10):2059-2065. doi: 10.1007/s00345-018-2550-x [DOI] [PubMed] [Google Scholar]
- 40.Heijnsdijk EAM, Nieboer D, Garg T, Lansdorp-Vogelaar I, de Koning HJ, Nielsen ME. Cost-effectiveness of surveillance schedules in older adults with non–muscle-invasive bladder cancer. BJU Int. 2019;123(2):307-312. doi: 10.1111/bju.14502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams SB, Shan Y, Jazzar U, et al. Comparing survival outcomes and costs associated with radical cystectomy and trimodal therapy for older adults with muscle-invasive bladder cancer. JAMA Surg. 2018;153(10):881-889. doi: 10.1001/jamasurg.2018.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams SB, Shan Y, Ray-Zack MD, et al. Comparison of costs of radical cystectomy vs trimodal therapy for patients with localized muscle-invasive bladder cancer. JAMA Surg. 2019;154(8):e191629. doi: 10.1001/jamasurg.2019.1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamat AM, Shore N, Hahn N, et al. KEYNOTE-676: phase III study of BCG and pembrolizumab for persistent/recurrent high-risk NMIBC. Future Oncol. 2020;16(10):507-516. doi: 10.2217/fon-2019-0817 [DOI] [PubMed] [Google Scholar]
- 44.Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non–muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021;22(1):107-117. doi: 10.1016/S1470-2045(20)30540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gidwani R, Asch SM, Needleman J, et al. End-of-life cost trajectories in cancer patients treated by Medicare versus the Veterans Health Administration. J Am Geriatr Soc. Published online December 24, 2020. doi: 10.1111/jgs.16941 [DOI] [PubMed] [Google Scholar]
- 46.Stroupe KT, Smith B, Weaver FM, et al. Healthcare utilization and costs for patients with Parkinson’s disease after deep brain stimulation. Mov Disord Clin Pract. 2019;6(5):369-378. doi: 10.1002/mdc3.12765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hendricks AM, Remler DK, Prashker MJ. More or less? Methods to compare VA and non-VA health care costs. Med Care. 1999;37(4)(suppl Va):AS54-AS62. doi: 10.1097/00005650-199904002-00008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Kaplan-Meier Curves for Disease-Free Survival, Overall and Stratified by Clinical Stage Group
eFigure 2. Kaplan-Meier Curves for Time to All-Cause Mortality, Overall and Stratified by Clinical Stage Group
eTable 1. Univariable and Multivariable Association Between Demographic and Disease Characteristics and Disease-Free Survival (n = 401)
eTable 2. Kaplan-Meier Event-Free Survival Estimates From Time of BCG Initiation
eTable 3. Comparisons of Number of Patients With Any Cost vs No Cost Stratified by Progression Status
eTable 4. Association Between Progression Status and Costs (if Any)
eTable 5. Health Care Cost Data From Time of BCG Induction Start Date and 30 and 90 Days After Cystectomy (n = 33)