Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), emerged in China in December 2019 and rapidly spread worldwide. Epidemiological studies in the general population 1 , 2 , 3 identified older age, diabetes, obesity, hypertension, cardiovascular diseases, chronic lung diseases, end‐stage renal and liver diseases, cancer and haematological malignancies, as risk factors for severity and mortality. Allogeneic haematopoietic stem cell transplantation (alloHSCT) recipients were also considered at risk of severe COVID‐19, as respiratory viral infections in alloHSCT recipients are associated with significant morbidity and mortality. 4
During the first European peak, the Société Francophone de Greffe de Moelle et de Thérapie cellulaire (SFGM‐TC) and European Society for Blood and Marrow Transplantation (EBMT) issued recommendations 5 to postpone alloHSCT whenever feasible. However, many alloHSCT could not be delayed, and all previously transplanted patients were considered at risk of severe COVID‐19. Although alloHSCT recipients were advised to limit social interactions, several were still infected. The SFGM‐TC conducted a multicentre study of COVID‐19 alloHSCT recipients, aiming to identify risk factors for severity and mortality.
All SFGM‐TC centres were invited to report alloHSCT recipients diagnosed with COVID‐19 in France, Belgium and Switzerland from February 2020. Only patients with a laboratory‐confirmed SARS‐CoV‐2 infection (reverse transcriptase‐polymerase chain reaction performed on nasopharyngeal swab) were included. Data were retrospectively collected from the patients’ charts and the ProMISe (Project Manager Internet Server) database. Biological data were collected at COVID‐19 diagnosis. Most chest computed tomography (CT) scans were collected and reviewed by two researchers (H.S., M.L.C.C.), combining visual and automated analysis of the extension of the COVID‐19‐related opacities thanks to a prototype software (Siemens Healthineers®, Erlangen, Germany). Radiological patterns were classified as previously published. 6 The conditioning regimen was considered myeloablative or reduced‐intensity/non‐myeloablative as previously published. 7 Graft‐versus‐host disease (GVHD) was considered acute before day 100 and chronic after. Pneumonia was defined according to the Fourth European Conference on Infections in Leukaemia (ECIL‐4) guidelines for the definition of pneumonia attributable to community‐acquired respiratory viruses (clinical symptoms and signs of a low respiratory tract infection, virus detected in nasopharyngeal swab). Pneumonia was considered probable in cases of infiltrates on imaging or hypoxaemia and possible in their absence. 8 Patients transferred to an intensive care unit (ICU) or deceased due to COVID‐19 were classified as severe, and non‐severe in all other cases. 9 All patients gave their written consent for data collection. The study followed the Declaration of Helsinki.
Continuous variables are expressed as median (range) or mean (±SD) and compared using Student’s t‐test or Mann–Whitney test for normally and abnormally distributed data respectively. Categorical variables are expressed as frequencies (percentages) and compared using chi‐squared or Fisher’s tests as appropriate. Risk factors for severe COVID‐19 were assessed using both uni‐ and multivariate logistic regressions. All analyses were performed using the Statistical Analysis System (SAS), version 9.4.6 (SAS Institute Inc., Cary, NC, USA). A two‐tailed significance level P < 0·05 was used.
A total of 54 alloHSCT recipients were diagnosed with COVID‐19 between March and May 2020. The incidence was 0·40% in all 25 participating centres and 0·64% for the 14 centres with patients. Centres reported a median (range) of 2·5 (1–9) patients. Cases were mostly diagnosed in Ile‐de‐France (44·4%) and in the East of France (31·5%). The incidence varied among centres (0·14–1·50%), even within the same region.
Patient characteristics are described in Table S1: 21 (38·8%) had severe COVID‐19, including 13 transferred to ICU and eight deceased from COVID‐19 outside of ICU (Figure S1). Overall survival is shown in Fig 1. The breakdown of severe and non‐severe cases according to time from alloHSCT to COVID‐19, and in patients receiving immunosuppressive treatment or not are shown in Figure S2A,B. Biological data at COVID‐19 diagnosis, available for a subset of patients, are shown in Table S1. Radiological findings are described in Data S1.
Fig 1.
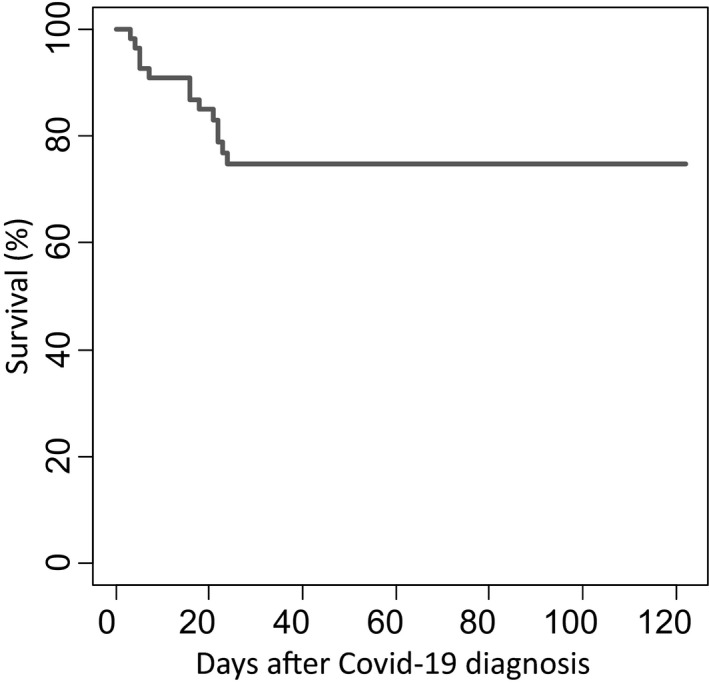
Survival curve after COVID‐19 diagnosis.
Haematological characteristics did not vary between patients with severe or non‐severe forms of COVID‐19 (Table S1). Uni‐ and multivariable analyses of risk factors for severe COVID‐19 are shown in Table I: probable pneumonia, symptoms other than respiratory and immunosuppressive treatment were associated with severity in multivariable models. Risk factors for death from COVID‐19 (Table II) were older age at alloHSCT, shorter time from alloHSCT to COVID‐19 diagnosis, probable pneumonia, co‐infection during the course of COVID‐19 and lower platelet count.
Table I.
Uni‐ and multivariable logistic regression analyses for association with a severe form of COVID‐19.
| Characteristic | Univariable* | Multivariable*, † | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Time between alloHSCT and COVID‐19 diagnosis* | |||||
| Quartile 1 (11–211 days) | Ref | Ref | |||
| Quartile 2 (212 days–1.1 years) | 0·49 (0·09–2·57) | 0·40 | 2·33 (0·15–36·81) | 0·55 | |
| Quartile 3 (1.4–3.7 years) | 0·11 (0·02–0·70) | 0·02 | 0·14 (0·01–3·44) | 0·23 | |
| Quartile 4 (4.5–18.8 years) | 0·10 (0·01–0·69) | 0·02 | 0·13 (0·01–3·16) | 0·21 | |
| Probable pneumonia | 14·18 (1·63–123·20) | 0·02 | 45·20 (1·33 −999·99) | 0·03 | |
| Possible and probable pneumonia | 20·78 (2·42–178·42) | 0·006 | |||
| Other symptoms ‡ | 3·40 (1·01–11·52) | 0·05 | 20·89 (1·41–309·62) | 0·03 | |
| Any immunosuppressive treatment | 5·11 (1·32–19·77) | 0·02 | 16·37 (1·25–215·00) | 0·03 | |
| Any comorbidity | 3·24 (0·86–12·24) | 0·08 | 0·05 (0·01–2·11) | 0·12 | |
| Co‐infection | 6·34 (1·71–23·51) | 0·006 | 14·80 (0·70–311·44) | 0·08 | |
| Platelet count (×109/l ) | |||||
| Tertile 1 (11–79) | 13·33 (1·78–100·13) | 0·01 | |||
| Tertile 2 (95–189) | 3·12 (0·47–20·58) | 0·24 | |||
| Tertile 3 (221–781) | Ref | ||||
Excluding patients aged <15 years from analysis did not modify the results. Due to the low number of patients, multivariable analysis was performed considering only clinical factors (excluding platelet count). alloHSCT, allogeneic haematopoietic stem cell transplantation; COVID‐19, coronavirus disease 2019.
Adjusted for age at COVID‐19 and sex.
Due to the low number of patients, multivariate analysis was performed considering only clinical factors (excluding platelet count).
Other symptoms: asthenia (n = 5), neurological symptoms (n = 4), myalgia (n = 2), dysgeusia (n = 1), skin lesions (n = 1), arthralgia (n = 1), other (n = 3).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table II.
Univariable logistic regression analysis for association with death from COVID‐19 (dead = 13, alive = 33).
| Characteristic | Univariable* | |
|---|---|---|
| OR (95% CI) | P | |
| Time between alloHSCT and COVID‐19 diagnosis* | ||
| Quartile 1 (54–216 days) | Ref | 0·02 |
| Quartile 2 (234 days–1.4 years) | 0·61 (0·09–4·07) | 0·61 |
| Quartile 3 (1.4–3.7 years) | 0·16 (0·02–1·35) | 0·09 |
| Quartile 4 (4.5–18.8 years) | 0·05 (0·01–0·73) | 0·03 |
| Probable pneumonia | 9·97 (1·09–91·57) | 0·04 |
| Possible and probable pneumonia | 6·62 (0·70–62·36) | 0·10 |
| Other symptoms † | 2·35 (0·56–9·86) | 0·24 |
| Any immunosuppressive treatment | 2·93 (0·69–12·50) | 0·15 |
| Any comorbidity | 3·27 (0·58–18·34) | 0·18 |
| Co‐infection | 12·04 (1·84–78·87) | 0·01 |
| Platelet count (×109/l) | ||
| Tertile 1 (11–79) | 21·37 (1·71–267·11) | 0·01 |
| Tertile 2 (95–189) | 2·97 (0·23–39·30) | 0·41 |
| Tertile 3 (221–781) | Ref | |
| Age at alloHSCT (years) ‡ | ||
| Quartile 1 (2·4–35·0) | Ref | |
| Quartile 2 (37·4–54·7) | 0·80 (0·04–15·35) | 0·89 |
| Quartile 3 (55·6–63·4) | 4·21 (0·38–46·11) | 0·24 |
| Quartile 4 (63·7–74·1) | 12·81 (1·19–137·34) | 0·04 |
Excluding patients aged <15 years from analysis did not modify the results. The effective included in the analysis does not allow to perform multivariate analysis. alloHSCT, allogeneic haematopoietic stem cell transplantation; COVID‐19, coronavirus disease 2019.
Adjusted for age at COVID‐19 and sex.
Other symptoms: asthenia (n = 5), neurological symptoms (n = 4), myalgia (n = 2), dysgeusia (n = 1), skin lesions (n = 1), arthralgia (n = 1), other (n = 3).
Adjusted for sex.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
This international study is the first to describe risk factors for severe infection and death from COVID‐19 in alloHSCT recipients. AlloHSCT recipients are at high risk of COVID‐19 severity and mortality, with a fatality rate of 25%. Previous studies of patients with haematological malignancy with COVID‐19 reported a fatality rate of 39–61%. 10 , 11 , 12 The lower mortality rate in our present study might be related to the young age of our patients, as well as the inclusion of non‐hospitalised patients with milder forms of COVID‐19. In our present study, as previously seen in haematology patients, 2 , 12 the risks of severity and death were not associated with the factors seen in the general population (male sex, diabetes and obesity). Probable pneumonia was the only factor associated with severity and death. Immunosuppressive treatment was a risk factor for severity but not for death: the impact of immunosuppression on the course of COVID‐19 in alloHSCT recipients is difficult to determine. Recent studies reported the efficacy of steroids and/or tocilizumab in the treatment of severe COVID‐19. 13 , 14 Immunosuppression may be an aggravating factor (decreasing the ability to control viral replication), but may also be a protective factor (decreasing the inflammatory phase). Unfortunately, we were not able to perform longitudinal cytokines and immunological analyses over the course of COVID‐19. Concerning biological data, only a low platelet count, but not a low lymphocyte count, was associated with poorer outcomes in our present population. However, the lymphocyte count was low in the entire population, as expected in alloHSCT recipients.
The central review of CT scans showed that atypical lesions were not rare, so COVID‐19 should not be ruled out on the sole basis of radiological findings in alloHSCT recipients. Bronchiectasis within opacities may alert us to the risk of further onset of fibrotic lesions. Indeed, respiratory viruses infections can be a trigger for pulmonary chronic GVHD 15 and alloHSCT recipients who survive COVID‐19 should be closely monitored and evaluated for the development of pulmonary GVHD.
The total number of COVID‐19 cases among all centres of the SFGM‐TC was lower than could have been feared, probably related to the education of alloHSCT recipients: alloHSCT recipients probably applied the instructions of social distancing, mask wearing and handwashing better than the general population. Some nosocomial clusters have been reported, 10 but none found in the participating centres.
Our present study has limitations: due to the retrospective data collection, not all biological and clinical data could be fully exhaustive. Patient collection was prospective, so no case should have been missed in the participating centres, but we cannot exclude an underestimation of the true incidence of COVID‐19 among alloHSCT recipients if pauci‐symptomatic patients did not report to their transplant centre.
To date, no vaccine against SARS‐CoV‐2 is yet commercially available and vaccination in alloHSCT recipients has limited efficacy. As SARS‐CoV‐2 continues to spread internationally, alloHSCT recipients should maintain a high level of awareness to avoid contamination.
Conflict of Interest
The authors declare that they have no competing interests.
Supporting information
Table SI. Characteristics of the 54 patients.
Fig S1. Flow chart.
Fig S2. (A) Proportion of severe and non‐severe cases, according to time from alloHSCT to COVID‐19 diagnosis. (B) Breakdown of cases of COVID‐19 by severity and immunosuppressive treatment.
Data S1. Radiological findings.
Acknowledgements
Alienor Xhaard, Constance Xhaard, Maud D’Aveni, Helene Salvator, Jacques‐Olivier Bay, Marie Robin, Stephanie N’Guyen‐Quoc and Marie‐Therese Rubio participated in research design, data analysis and writing the article. Marie‐Laure Chabi, Tereza Coman, Yves Beguin, Yves Chalandon, Xavier Poiré, Michaël Loschi, Catherine Paillard, Benedicte Bruno, Patrice Ceballos, Jean‐Hugues Dalle, Karin Bilger participated in the performance of the research. All authors critically revised the manuscript. The authors would like to thank Prof. Gérard Socié for providing a careful and insightful review of the manuscript. The authors would like to thank Dr Corinne Balleyguier, Dr Madleen Chassang, Prof. Eric Delabrousse, Prof. Eric de Kerviler, Prof. Boris Guiu, Prof. Valérie Laurent, Prof. Olivier Lucidarme, Dr Constance de Margerie‐Mellon, Dr Paul Meunier, Dr Perriguey and Prof. Francis Veillon for providing CT scans.
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meng Y, Lu W, Guo E, Liu J, Yang B, Wu P, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID‐19 patients: a propensity score‐matched analysis. J Hematol Oncol. 2020;13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020;383:1757–66. [DOI] [PubMed] [Google Scholar]
- 4. Wolfromm A, Porcher R, Legoff J, Peffault de Latour R, Xhaard A, de Fontbrune FS, et al. Viral respiratory infections diagnosed by multiplex PCR after allogeneic hematopoietic stem cell transplantation: long‐term incidence and outcome. Biol Blood Marrow Transplant. 2014;20:1238–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S, et al. The challenge of COVID‐19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T‐cell therapy. Bone Marrow Transplant. 2020;55:2071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hani C, Trieu NH, Saab I, Dangeard S, Bennani S, Chassagnon G, et al. COVID‐19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging. 2020;101:263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL‐4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. 2013;56:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. [DOI] [PubMed] [Google Scholar]
- 10. He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID‐19 in persons with haematological cancers. Leukemia. 2020;34:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malard F, Genthon A, Brissot E, van de Wyngaert Z, Marjanovic Z, Ikhlef S, et al. COVID‐19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55:2180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah V, Ko Ko T, Zuckerman M, Vidler J, Sharif S, Mehra V, et al. Poor outcome and prolonged persistence of SARS‐CoV‐2 RNA in COVID‐19 patients with haematological malignancies; King’s College Hospital experience. Br J Haematol. 2020;190:e279–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramiro S, Mostard RL, Magro‐Checa C, van Dongen CM, Dormans T, Buijs J, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID‐19‐associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79. 2020‐218479. DOI: 10.1136/annrheumdis‐2020‐218479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. RECOVERY Collaborative Group , Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. 2020. [Online ahead of print]. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 15. Sheshadri A, Chemaly RF, Alousi AM, Shah PK, Rondon G, Bashoura L, et al. Pulmonary impairment after respiratory viral infections is associated with high mortality in allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2019;25:800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Characteristics of the 54 patients.
Fig S1. Flow chart.
Fig S2. (A) Proportion of severe and non‐severe cases, according to time from alloHSCT to COVID‐19 diagnosis. (B) Breakdown of cases of COVID‐19 by severity and immunosuppressive treatment.
Data S1. Radiological findings.


