Abstract
Recently, transplantation of cryopreserved ovarian tissue is the method for fertility preservation for oncologic and nononcologic reasons. The main challenge of ovarian cryopreservation followed by transplantation is that ischemia reperfusion injury (IRI) induced the loss of follicles. The aim of this study was to evaluate the effects of glutathione (GSH), ulinastatin (UTI) or both (GSH+UTI) on preventing ischemia reperfusion-induced follicles depletion in ovarian grafts.
Ovarian fragments were collected from 20 women aged 29±6 years. Frozen-thawed human ovarian tissue was xenografted into SCID mice, at the same time GSH, UTI and GSH+UTI was administrated respectively. The ovarian grafts were collected at the 1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after xenotransplantation. Follicle survival rate was measured by H&E staining and Live/Dead staining. Angiogenic activity and macrophage recruitment was evidenced by immunohistochemical staining. The oxidative stress and inflammatory cytokines in human ovarian xenografts were measured by real-time PCR. The results indicated that after the treatments of GSH, UTI and GSH+UTI in the hosts, follicular survival in ovarian grafts were improved. The level of VEGF, CD31, and antioxidant enzymes superoxide dismutase 1 and superoxide dismutase 2 in ovarian grafts were increased. Accumulation of macrophages, level of IL6 and TNF-α, as well as malondialdehyde was decreased in ovarian grafts from treated groups. In conclusion, administration of GSH, UTI and GSH+UTI decreased the depletion of follicles in human grafts post-transplantation by inhibiting IRI-induced antiangiogenesis, oxidative stress and inflammation.
Keywords: Xenotransplantation, ischemia reperfusion, oxidative stress, inflammation, macrophages
Introduction
Plentiful cancers have been not viewed as incurable diseases, but treatments of cancers induce premature ovarian insufficiency. Therefore, fertility preservation of young women patients now needs to be addressed. Cryopreservation of ovarian tissue is the best choice for young patients, because it can conserve fertility function1 and can even conserve endocrine function of ovary2. The ultimate objective of cryopreservation of ovarian tissue is to implant them into the pelvic cavity (orthotopic site) or in a heterotopic site. In 2004, the study reported the first pregnancy by orthotopic transplantation of ovarian tissue3. The number of live births has climbed to more than 130 until 20174. Nevertheless, the loss of ovarian follicles following transplantation is a huge issue and it is hampering the success rates of transplantation. Lots of literatures demonstrated that ischemia reperfusion (IR) after transplantation induced the accumulation of inflammatory cytokines and free radical release in the ovarian grafts, therefore, resulted in the abundant follicle loss5–7. Hence, the inhibition of oxidative stress and inflammatory cytokines during the initial post-transplantation period plays a significant role on success of ovarian tissue transplantation8–10. Actually, many studies have been conducted with antiinflammation factors or antioxidants agents to enhance follicular survival rate11–13. Some other studies showed that ischemia–reperfusion injury (IRI) could be decreased by using substances to stimulate revascularization of the graft2,14. In our study, we used GSH, UTI, GSH+UTI to minimize IRI in ovarian grafts by inducing angiogenesis and suppressing both inflammation and oxidative stress.
Glutathione (GSH) is a tripeptide of γ-L-glutamyl-L-cysteinyl-glycine, and it is a significant antioxidant by taking part in cellular redox reactions and thioether formation. Ulinastatin (UTI) is an acidic glycoprotein extracted from human urine, which can both inhibit pancreas-derived proteases and neutrophil elastase to finally suppress inflammatory response15. According to the literatures, GSH and UTI have abilities to resist IRI in many organs because of their antioxidative and antiinflammatory effects15,16. However, seldom studies have discussed the possible effects of GSH and UTI on the prevention of IRI in ovarian grafts.
Taking the results of previous research into account, our objective in this study was to investigate the protective effects of GSH and UTI on the transplanted ovarian grafts against IRI. Furthermore, because of the different mechanism and targets of GSH and UTI, we also evaluate the synergistic effect of both on ovarian grafts.
Materials and Methods
Collection of Ovarian Tissue
The protocol of this study was reviewed and approved by Ethics Committee at The First Affiliated Hospital, Sun Yat-sen University (Approval number: 2014-7), and was carried out following the Code of Ethics of the World Medical Association (Declaration of Helsinki). Informed consent was obtained for experimentation with human subjects, and the privacy rights of human subjects must always be observed. Human ovarian tissue was collected from 20 women aged 29 ± 6, receiving laparoscopic surgery for benign gynecologic disease. All patients admitted in the Reproductive Center, The First Affiliated Hospital, Sun Yat-Sen University have been subjected to donation of ovarian tissues samples for research purposes with their written informed consent. Ovarian biopsies were immediately transported to the laboratory in Leibovitz L-15 medium (Gibco, Waltham, MA, USA).
Freezing and Thawing Procedure
The human ovarian tissue was frozen according to slow-freezing protocols. Freezing of ovarian strips was performed as previously described17, with some modifications. Ovarian tissues were sliced into small fragments (the size of 1.0–1.5 mm cubes) and were suspended in cryoprotectant (CPA) solution consisting of MEM-Glutamax supplemented with 4 mg/ml HSA and 10% DMSO at 4°C, and then transferred to 2 ml cryovials (Simport, Bernard-Pilon Beloeil, Canada) containing 0.8 ml of the CPA solution. The cryovials were cooled in a programmable freezer (Freezer Control CL-8800i; Cryologic, Victoria, Australia) using the following program: (1) cooled from 0 to −8°C at −2°C/min, (2) seeded manually, (3) cooled to −40 at −0.3°C/min, and (4) cooled to −140 at −30°C/min and transferred to LN for storage. Thawing steps were indicated as fellow: The tissue fragments were air warmed for 1 minute then immersed in a 37°C warm water bath, and then washed in a serial process of L-15 medium supplemented with 10% FBS and 0.25 M, 0.1 M, and 0 M sucrose for 5 minutes each to release from the protective cryopreservation medium.
Transplantation to SCID Mice
Eighty-four SCID mice (SPF level, 5-6 weeks old) purchased from Animal Center of Sun Yat-sen University North Campus were used for the study. All animal experiments should comply with the ARRIVE guidelines and should be carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). In our study, the sex of mice is female. The mice were housed and bred as previously described18. The mice randomly assigned to four groups (21 mice/group). Surgery was performed with the mice anesthetized by ketamine (75 mg/kg body weight (bwt); Anesketin, Eurovet, Heusden-Zolder, Belgium) and medetomidine (1 mg/kg bwt; Domitor, Cambridge, MA, USA), and buprenorphine (0.1 mg/kg bwt; Temgesic, Kenilworth, NJ, USA) for analgesia. The mice were kept on a warming plate and the incision site was disinfected with pure alcohol and covered with a sterile towel. Dorsum skin incision (2∼3 mm) was made. Six ovarian grafts were stitched under the dorsal subcutaneous. The dorsum skin incision was then closed with absorbable 7/0 Vicryl (Ethicon, Irvine, CA, USA). After surgery, gentamicin was intravenously (i.v.) injected as a single dose (5 mg/kg bwt; MCE, Monmouth, NJ, USA). At the day of the xenotransplantation, GSH (200 mg/kg bwt; Sigma, Burlington, MA, USA)16, UTI (2500 U/kg bwt, Techpool, Guangdong, China)19, and GSH+UTI (GSH 100 mg/kg bwt and UTI 1250 U/kg bwt) was i.v. injected into respective group once per day for seven consecutive days. Control group with cryopreserved-thawed was daily i.v. injections of physiological saline per day for seven consecutive days. Three mice in each group at each time point (1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after transplantation) were sacrificed to take the grafts (18 grafts/group/time point). The details about the ovarian grafts in each experiment were showed in supplemental Tables S1 and 2.
Isolation of Follicles and Viability Assessment with Fluorescence Dye
Isolation
We used 2 ml Collagenase I (3 mg/ml) to incubate ovarian grafts in a water bath at 37°C with gentle agitation until the tissue was completely digested, assessed by visualization of a homogeneous suspension. During that time, the ovarian digest was mechanically disrupted by pipetting up and down every 15 min. We added an equal volume of PBS supplemented with 10% HSA (Sigma, Burlington, MA, USA) to stop digestion. After enzymatic digestion, the follicles were picked up using a polycarbonate micropipette and stored in PBS with 10% HSA at room temperature, until the process of follicular retrieval was completed.
Viability Assessment
Follicle viability was assessed by double fluorescent labeling with calcein AM and ethidiumhomodimer stains (Live/Dead Viability Assay kit; Invitrogen, Waltham, MA, USA) as described by Maltaris et al.20. The steps were followed as below: isolated follicles were incubated in 1 ml PBS containing 1% HSA, 1μM calcein AM and 2μM ethidiumhomodimer for 45 min at 37°C in the dark. Nonfluorescent cell-permeant calcein AM enters the cell and is cleaved by esterase in living cells giving an intense uniform green fluorescence. Ethidiumhomodimer-I enters cells with damaged membranes binding to DNA with high affinity, resulting in a bright red fluorescence. After exposure to fluorescent dyes, the isolated follicles were washed in PBS containing 1% HSA and observed under an inverted fluorescence microscope (TH4-200; Olympus, Tokyo, Japan). Green fluorescence was visualized in vital cells and red fluorescence in dead cells. Dead follicles have been defined as follicles with both the oocyte and all follicular cells dead21, and others have been viewed as live follicles. Follicle viability (%) was calculated follow the formula:
Histology and Follicle Counting
Histological analysis was performed on frozen-thawed ovarian tissue before and after transplantation. Ovarian grafts were collected from SCID mice at the 1st day (the same day of xenograft), 3rd day, 7th day, 14th day, 28th day, 56th day, and 85th day after transplantation. In our study, hematoxylin-eosin staining (H&E staining) were used to observe follicular morphology. First, half of the grafts were fixed in 4% paraformaldehyde. After fixation, the ovarian fragments were dehydrated, embedded in paraffin and serially sectioned (5μm). Every the fifth section was stained with hematoxylin-eosin (Sigma, Burlington, MA, USA) and examined by three independent observers under a light-microscope (Olympus, Tokyo, Japan). Follicles were counted only in the section in which the oocyte nucleus was visible, to avoid overcounting. Follicles were defined as healthy (morphologic integrity) or dead (abnormal morphology) following the guideline of GOOK et al.22. Primordial was defined as oocyte partially or completely encapsulated by squamous pregranulosa cells23.
The rate of morphologically integrated follicles was calculated follow the formula:
Morphologically integrated follicles = [integrated follicles / (integrated follicles + abnormal follicles)] %;
Immunohistochemical Studies
Immunohistochemical staining was performed according to our previous study24. The slides were prepared as described in H&E staining, immersed in citrate buffer, and heated in a microwave oven for 15 min. Endogenous peroxidase was blocked with 3% H2O2 solution in methanol for 20 min. After three times washing with TBS, to determine the VEGF expression of graft in different group, slides were reacted with anti-VEGF primary antibody (rabbit monoclonal, dilution 1:100; Abcam, Cambridge, Britain) for human, and then were reacted with anti-rabbit secondary antibody at 37°C for 30mins followed by three washes with PBS. To evaluate microvessels density (MVD) of murine microvessels in the graft, slides were reacted with anti-CD31 primary antibody (rabbit monoclonal, dilution 1:200; Abcam, Cambridge, Britain) for mouse. Immunohistochemistry for CD68, a macrophage and activated microglia marker, was also performed on slides using anti-CD68 primary antibody for human (rabbit monoclonal, dilution 1:200; Abcam, Cambridge, Britain). Pretransplantation group were included using rat and rabbit IgG (Sigma, Burlington, MA, USA) diluted to the same concentrations as the primary antibodies. Diaminobenzidine (DAB) Kit (Beijing Zhongshan Biotechnology Co., Beijing, China) was used to visualize immunoreactive proteins. The protein levels were detected using Polink-2 Plus IHC Detection System (Beijing Zhongshan Biotechnology Co., Beijing, China). DAB was added to the slides for 3 min as a chromogen. Followed by rinsing in running tap water, slides were counterstained with hematoxylin (blue). Finally, slides were dehydrated in absolute ethanol and mounted.
All of the stained slides were observed under the light microscope (Olympus, Tokyo, Japan) at ×200 and ×400 magnifications. Histochemical Score was used to evaluate the VEGF expression level as Andrea Castro described25. Three independent observers selected different optical fields in a random manner to assess the positive staining in 1,000 cells for each sample. The intensity grade (i) of the staining was evaluated as strong (grade 3), moderate (grade 2), or weak (grade 1), and integrated in a semiquantitative parameter named the histochemical score (H SCORE = [% of positive cells × (i + 1)] / 100).
Microvessel density was measured by light microscopy in areas of graft containing the largest number of microvessels (Hot Spot). Endothelial cell or endothelial cell cluster with positive staining for CD31 was considered as a single and countable microvessel. If vessels with a lumen diameter greater than 8 red blood cells and thick muscle layers, it is not counted. Image J was used to measure the lumen diameter and count the microvessels. The number of microvessels in the five areas at Hot Spot in the low-power field of view for each slice was count. The area of the microvessels in the ×200 magnification field equal to 0.7386 mm2 per field. The density was calculated by the equation: Density = Number / Area.
For macrophage quantification, twenty high power field (HPF) areas per group were investigated. HPF was defined as the microscopically visible area at ×400 magnification. Image J program was used for positive cells counting. Quantification procedures were applied to whole ovarian slides.
Measurement of Lipid Peroxidation
Lipid peroxidation is a significant factor to evaluate oxidative stress. The concentration of malonyldialdehyde (MDA) was considered as a marker of lipid peroxidation, which can reveal the oxidative damage. The MDA level in human ovarian grafts was measured according to previous study24 by Lipid Peroxidation Assay Kit (Abcam, Cambridge, Britain). Grafts were removed from mice under ethylether anesthesia and diluted homogenates to 5 mg tissue with 303µl lysis buffer by TubeMill control (IKA, Deutschland, Germany). Homogenates were centrifuged at 4°C, 13000 × g for 10 min. The supernatant was pipetted into a new 1.5 ml microtube and added 600 µl TBA solution to 200 µl supernatant. All the samples were incubated at 95°C for 60 min and cooled to room temperature in an ice bath for 10 min. After that, we pipetted 200 µl from each mixture into a 96-well plate and measured the plate immediately at OD532 nm for colorimetric assay.
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from human ovarian grafts using the homogenizer with 1 ml trizol and the RNeasy Mini Kit (Qiagen, Germantown, Germany). Prime Script RT reagent Kit (Takara Bio, Kyoto, Japan) was used to prepare complementary DNA. Quantitative polymerase chain reaction (qPCR) was performed using the StepOnePlus Real-Time PCR System (Life Technologies, Waltham, MA, USA). Primer sequences were showed in Table 1. PCR was initiated at 95°C for 2 min, followed by 40 cycles at 95°C for 10 s, 58°C for 30 s and 72°C for 30 s. Target gene expression was calculated by the ratio to the housekeeping gene.
Table 1.
List of Primers Generated for qPCR.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| IL-6 | 5’-ACCAGAGGAAATTTTCAATAGGC-3’ | 5’-TGATGCACTTGCAGAAAACA-3’ |
| TNF-α | 5’-AGGGTCTGGGCCATAGAACT-3’ | 5’-CCACCACGCTCTTCTGTCTAC-3’ |
| SOD1 | 5’-GTGATTGGGATTGCGCAGTA-3’ | 5-’TGGTTTGAGGGTAGCAGATGAGT-3’ |
| SOD2 | 5’-GCTTGATAGCCTCCAGCAAC-3’ | 5’-ACTGAAGTTCAATGGTGGGG-3’ |
| Actin | 5’-TGAGAGGGAAATCGTGCGT-3’ | 5’-TCATGGATGCCACAGGATTCC-3’ |
IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
Statisics Analysis
All data are presented as mean and standard deviation (mean ± SD). Statistical significance was calculated using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). One-Way analysis of variance (ANOVA) was applied for multiple comparisons. P<0.05 was considered statistically significant.
Results
Collection of Ovarian Tissue Grafts from SCID Mice
There are 20 patients attended in our experiments, each patient offered 26 pieces of ovarian tissues in the size of 1.0–1.5 mm cubes. The total 520 pieces of ovarian tissues were used to cryopreservation, and 504 pieces were transplanted into mice after thawing, the rest frozen-thawed tissues were used to calculate the follicular survival rate before transplantation, and perform q-PCR and measure MDA as the pretransplantation group (Supplemental Tables S1 and 2).
There were 84 SCID mice in this study, and randomly assigned to four groups (21 mice/group). Ovarian grafts were stitched under the dorsal subcutaneous of each SCID mice (6 grafts/mouse). Three mice in each group were sacrificed to take the grafts (18 grafts/group/time point) at the 1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after transplantation respectively. The details about the ovarian grafts in each experiment were showed in Supplemental Tables S1 and 2. H&E staining was used to evaluate the condition of frozen-thawed human ovary tissue (Table 2.). The results indicated that the follicular survival rate of frozen-thawed human ovary tissue was 84.4 , and it of fresh human ovary tissue is 97.6 . As shown in the Table 2, the survival rate of primordial, primary, and secondary were 79.3%, 17.9%, and 2.8% respectively. Therefore, the quality and fertility preservation of frozen-thawed human ovary tissue is available for our transplantation experiments.
Table 2.
Average Number of Follicles Survival Rate of Follicles in Frozen-Thawed Human Ovary Tissue Before Transplantation.
| Total Follicles (%) | Classification of follicle (% of survival) | |||
|---|---|---|---|---|
| Primordial | Primary | Secondary | Antral | |
| 84.4% | 20±10.2 (79.3%) | 4.5±2.1 (17.9%) | 0.7±0.3 (2.8%) | 0 |
The results are shown as the mean ± SD. Total 84 tissue specimen of frozen-thawed human ovary were measured. The number of follicles in a view field of 1 mm2, having the highest number of follicles in each section, was counted (in a total of 6 fields of view). Total follicles (%) were calculated as the number of survival follicles/the number of all follicles (survival + death). The survival rates (% of survival) of certain follicles were calculated as number of the stage certain of follicles /the total number of follicles in pretransplant ovarian tissue samples.
Follicular Morphology Integrity and Viability Rate
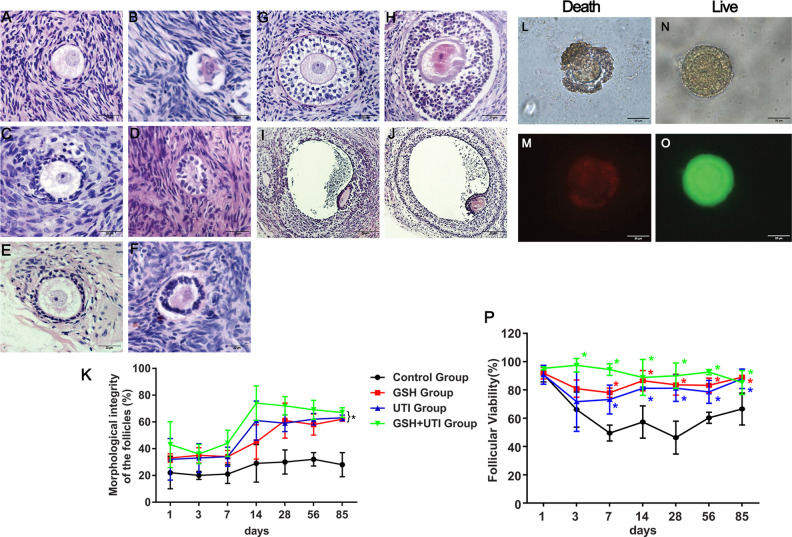
In order to evaluate the protective effects of GSH, UTI and GSH + UTI on follicular integrity and viability, H&E staining and Live/Dead staining was performed. The morphology of healthy and abnormal primordial follicle, primary follicle, secondary follicle, preantral follicle and antral follicle in human ovarian grafts post-xenotransplantation were showed (Fig. 1A–F). The imaging of live and dead follicles in bright field were showed in Fig. 1L and P, while the imaging of red and green staining for dead (Fig. 1M) and live (Fig. 1O) follicles were also showed. Follicle integrity/morphology and viability at the 1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after xenotransplantation are shown in Fig. 1K and P, respectively. The rate of follicle integrity and viability showed a decreased trend at the first week, which suggested that ovarian grafts suffered from IRI during this time. GSH, UTI, and GSH + UTI groups showed a higher follicular integrity rate and viability rate than control group at the 1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after xenotransplantation, and GSH + UTI group presented with the highest follicular integrity and viability. These results demonstrated that follicle integrity (percentage of healthy follicle) and viability were better preserved in the group of GSH, UTI, and GSH + UTI compared to control.
Figure 1.
Survival rate of follicles in each group at the 1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after xenotransplantation. Healthy (A) and abnormal (B) primordial follicle in human ovarian grafts at the 3rd day post-xenotransplantation; healthy (C) and abnormal (D) primary follicle in human ovarian grafts at the 14th day post-xenotransplantation; healthy (E) and abnormal (F) secondary follicle in human ovarian grafts at the 14th day post-xenotransplantation; healthy (G) and abnormal (H) preantral follicle in human ovarian grafts at the 14th day post-xenotransplantation; healthy (I) and abnormal (J) antral follicle in human ovarian grafts at the 14th day post-xenotransplantation. Curve graph (K) indicated the survival rate of follicles in each group at the 1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after xenotransplantation by H&E staining. Live/Dead assay was used to calculate the survival rate of follicles (L-P). The red and green staining has meaning for dead (M) and live (O) follicles. The dead and live follicles of bright field imaging were showed in (L) and (N). Curve graph (P) showed survival rate of follicles in each group at the 1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after xenotransplantation by Live/dead assay. *Indication of statistical significance (P < 0.05) when compare to control group.
The Expression of VEGF in the Graft
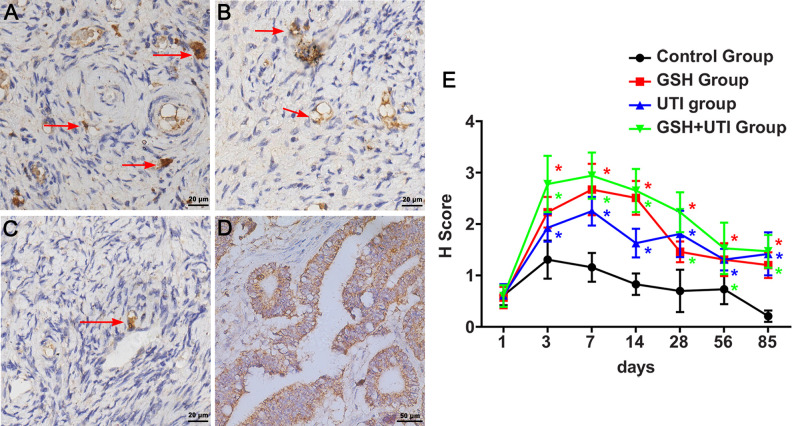
VEGF is a signal protein secreted by various cells to induce angiogenesis. It relates to a mechanism that renews the oxygen supply to tissues when ischemia reperfusion occurred. Therefore, we used immunohistochemical staining to evaluate the VEGF expression of ovarian graft in different groups. The intensity grade of the VEGF staining was showed as strong (grade 3, Fig. 2A), moderate (grade 2, Fig. 2B), or weak (grade 1, Fig. 2C). The H score of VEGF in the ovarian grafts markedly increased and reached the peak at the first week after transplantation, while in GSH group, UTI group and GSH+UTI group it was higher than control group (Fig. 2E, *P < 0.05). These data suggested that ovarian grafts expressed an increasing number of VEGF to induce angiogenesis at the first week after xenotransplantation. Furthermore, host with the treatment of GSH, UTI and GSH+UTI improved angiogenesis in ovarian grafts by expressing more VEGF.
Figure 2.
Immunostaining of VEGF in human ovarian grafts in control group, GSH group, UTI group and GSH+UTI group. Strong intensity grade of VEGF expression (grade 3, A), moderate intensity grade of VEGF expression (grade 2, B), and weak intensity grade of VEGF expression (grade 1, C) were showed (VEGF positive cells were indicated by arrow). The positive control of VEGF in epityphlon were showed in (D). Histochemical score of VEGF levels after xenotransplantation were shown as curve graphs in (E). *Indication of statistical significance (P < 0.05) when compare to control group.
MVD of Murine Microvessels in the Human Ovarian Graft
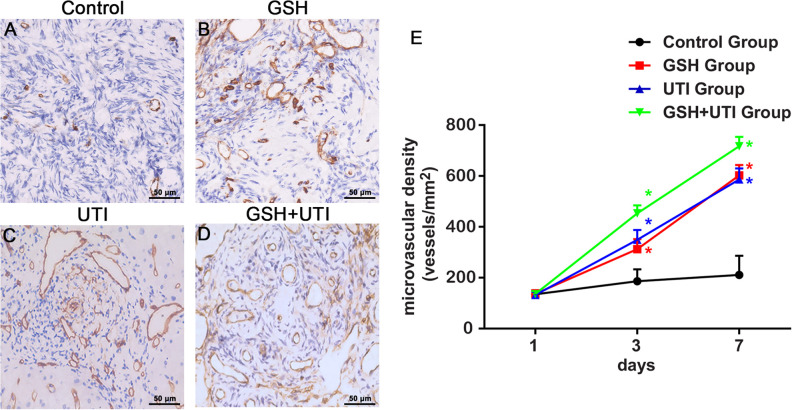
It has been well-known that during the IR period, microvessels reconstruction is an important step to re-supply nutrition and oxygen for transplanted ovarian grafts26. In order to calculate the MVD of murine microvessels (mMVD), CD31 was used to label microvessels. Fig. 3A–D showed mMVD in the human ovarian grafts from control group, GSH group, UTI group and GSH+UTI group at the 7th day after transplantation. On the 1st day post-transplantation, the mMVD levels were low, and there were no significant differences between control group and other treated groups. On the 3rd and 7th day post-transplantation, mMVD in GSH group, UTI group and GSH+UTI group showed an increasing tendency, but mMVD in control group was still at a low level. These results indicated that the first signs of host angiogenesis were occurred on the 3rd day post-transplantation, and the treatment with GSH, UTI and GSH+UTI potentiated the formation of host microvessels (Fig. 3E).
Figure 3.
Analysis of the murine microvessel in the human ovarian grafts. Immunohistochemical staining of murine microvessel in the human ovarian grafts from control group (A), GSH group (B), UTI group (C) and GSH+UTI group (D) at the 7th day after xenotransplantation. Curve graph (E) showed mMVD in human ovarian grafts from each group form at the 1st, 3rd and 7th day after xenotransplantation *Indication of statistical significance (P < 0.05) when compare to control group.
Effects of UTI, GSH and GSH+UTI on Suppressing the Infiltration of Macrophage and Inflammation in the Ovarian Grafts
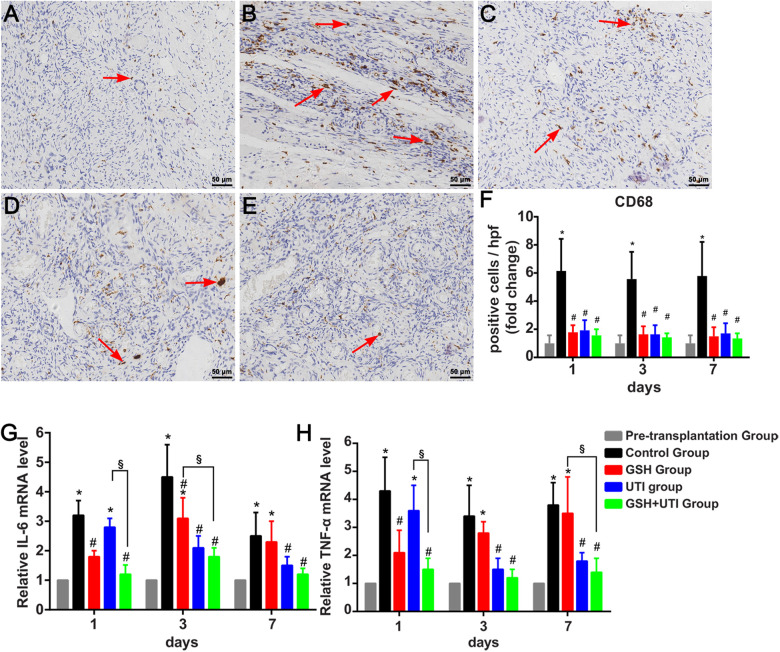
IR-induced inflammatory cytokines have been proved to induce the loss of follicles in ovarian grafts. Inflammatory cytokines such as interlukin-6 (IL6) and Tumor Necrosis Factor-alpha (TNF-α), secreted by macrophages27. The antiinflammatory effects of GSH, UTI, or GSH+UTI on ovarian grafts were evaluated during the IR period. Pretransplanted frozen-thawed human ovarian tissues were labeled as pretransplantation group, and transplanted frozen-thawed human ovarian tissues with saline treatment were labeled as control group. The results indicated that substantial macrophages labeled by CD68 were recruited into ovarian xenografts in control group, the numbers of them were decreased in ovarian grafts by the administration of GSH, UTI, and GSH+UTI on the host (Fig. 4A–F). Therefore, the adding of GSH, UTI, and GSH+UTI played an antiinflammation role on the ovarian grafts. Other results further supported this concept that the administration of GSH, UTI, and GSH+UTI could attenuate the increasing expression of inflammatory cytokines (IL6 and TNF-α) in the ovarian grafts (Fig. 4G, H). Of note, GSH+UTI group showed the least macrophages and lowest level of IL6 and TNF-α, compared with GSH group and UTI group.
Figure 4.
GSH, UTI, and GSH+UTI inhibit the infiltration of macrophages and inflammatory cytokines in human ovarian grafts. Immunohistochemical staining of CD68+ cells in the frozen-thawed ovarian tissues from pretransplantation group (A), control group (B), GSH group (C), UTI group (D) and UTI+GSH group (E). The red arrows indicated the CD68+ cells in each group. The results of CD68+ cells/hpf in the 1st, 3rd, and 7th day after transplantation were normalized to pretransplantation group (F). The mRNA level of inflammatory cytokines IL6 (G) and TNF-α (H) in the transplanted ovarian grafts was assessed by Real-time PCR. Data were normalized to pretransplantation group. *Indication of statistical significance (P < 0.05), when compare to pretransplantation group. #Indication of statistical significance (P < 0.05), when compare to control group. §Indication of statistical significance (P < 0.05), when compare to GSH+UTI group.
Treatment with UTI, GSH and GSH+UTI Decreased Oxidative Stress in Ovarian Grafts during IR Period
Previous study indicated that IR induced amount of oxidative stress in ovarian grafts. To assess the antioxidative effects of GSH, UTI and GSH+UTI on ovarian grafts, we measured the level of MDA, superoxide dismutase 1 (SOD1) and SOD2 in grafts (Fig. 5). Normalize to pretransplantation group, MDA level was increased in control group, which would be attenuated by the administration of GSH, UTI, GSH+UTI. Simultaneously, the expression levels of SOD1 and SOD2 were decreased in grafts in control group, and they were increased followed by the administration of GSH, UTI, GSH+UTI on the host. These results showed that GSH, UTI, GSH+UTI could inhibit oxidative stress in ovarian grafts by decreasing the expression of MDA and improving the level of SOD1 and SOD2. GSH+UTI showed the best antioxidative effective than GSH alone and UTI alone.
Figure 5.
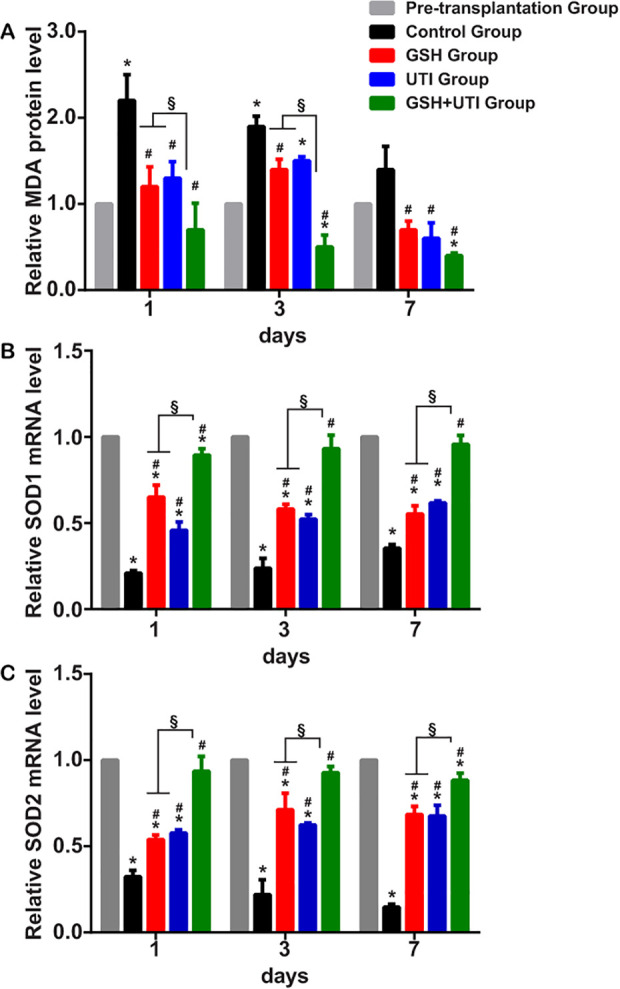
GSH, UTI and GSH+UTI decreased the oxidative stress in human ovarian grafts. Lipid peroxidation was assessed by the measurement of malondialdehyde (MDA) in pretransplantation tissue and transplanted ovarian grafts (A). Mean ± SD are shown (n = 3 mice/group). Antioxidant enzymes (superoxide dismutase 1 [SOD1] and SOD2) in pretransplantation tissue and transplanted ovarian grafts were measured at the 1st, 3rd, and 7th day after transplantation (B&C). Mean ± SD are shown (n = 3 mice/group). *Indication of statistical significance (P < 0.05), when compare to pretransplantation group #Indication of statistical significance (P < 0.05), when compare to control group. §Indication of statistical significance (P < 0.05), when compare to GSH+UTI group.
Discussion
IRI in the ovarian grafts related to insufficient perfusion and limitation of the blood flow, increasing the accumulation of oxidative stress and inflammatory cytokine which can lead to huge follicular loss in the ovarian grafts. GSH and UTI have been proved to protect many organs against IRI, such as kidney, liver, heart and brain28–30. However, the anti-IRI effects of GSH, UTI and GSH+UTI on transplanted ovarian grafts and their mechanism have not been discussed so far. Previous studies indicated that IR occurred at the first week after ovarian grafts transplantation, being regarded as the most critical period to improve the follicle survival rate as well as preserve the ovarian functions of the grafts31,32. Therefore, our study daily administrated GSH, UTI, GSH+UTI into the host at the first week after transplantation and observed their protective effects on the ovarian grafts. The result indicated the follicle integrity and viability were better preserved in the group of GSH, UTI, and GSH+UTI at the 1st, 3rd, 7th, 14th, 28th, 56th, and 85th day after transplantation. Further, the protective mechanisms of them were discussed: (1) Improved the process of angiogenesis in grafts after transplantation. (2) Decreased IL6 and TNF-α, as well as inhibit the infiltration of macrophages to suppress IR-induced inflammation. (3) Decreased the MDA and increased the SOD1/SOD2 to suppress IR-induced oxidative stress.
As reported, unlike other organs’ transplantation where blood reperfusion can be achieved immediately by reanastomosis, small-grafted ovarian fragments can only obtain blood support by the newly growth of blood vessels33, which possibly begin within 48 hours after transplantation34. In the transplanted ovarian grafts, the production of VEGF was increased at the beginning because of the stimulation of ischemia and hypoxia; but with the accumulation of reactive oxygen species (ROS) and inflammatory cytokines, the cells membrane proteins were injured, therefore they could not continue to produce enough VEGF to compensate ischemia and hypoxia. Followed by that, the revascularization progress was impeded which induced the loss of follicles in grafted ovarian tissues35. Our results first time indicated that the administration of GSH, UTI and GSH+UTI could effectively increase the follicular integrity and viability in human ovarian grafts because revascularization processes were promoted by inducing the production of VEGF. As we know, VEGF, one of important signaling proteins involved in angiogenesis, can stimulate the formation of blood vessel in either physiological or pathological condition. Henry et.al. had indicated VEGF is able to reduce the hypoxic period and improve follicular preservation by promoting newly-growth vessel maturation after ovarian transplantation36. Our results showed that in the group of GSH, UTI and GSH+UTI, the expression of VEGF increased rapidly at the first week after transplantation (Fig. 2), and higher murine microvessel density were showed (the CD31; Fig. 3).
Lots of literatures demonstrated that follicle loss after transplantation was attributed to IRI. The insufficient blood and oxygen induced the increase of ROS, leading to oxidative stress. Oxidative stress leads to overproduction of free radicals and DNA damage, causing a negative effect on oocyte quality such as follicular loss and apoptosis37–39. The increase of ROS in ovarian tissue also led to various pro-inflammatory cytokines releasing, such as TNF-α and IL6. This cytokines could activate the death reporter pathway and cause proteolysis, which then induces apoptosis40. Importantly, TNF-α and IL6 could recruit more macrophage to the ovarian grafts and aggravated the inflammation responses by releasing ROS and inflammatory cytokines41,42. In short, the interactions among oxidative stress, TNF-α/IL6, and macrophage exacerbated the inflammatory response in the ovarian grafts, which brought about follicles loss.
Although GSH and UTI have been used to resist IR-induced oxidative stress and inflammations, their mechanisms are different. Eken MK et al. indicated that treatment with etanercept restored GSH levels in ovarian from IR rats and increase SOD levels and decrease MDA levels, implicating GSH attenuated oxidative ovarian reserve against IRI in a rat model43. Similarly, UTI has been proven to alleviate IRI in the most organs, including heart, lung, and liver by regulating the levels of TNF-α and IL628. Our study had the consistent results that administration of GSH and UTI improve the follicular integrity and viability in the ovarian grafts by regulating antioxidative and antiinflammatory parameters. Novelty, our study evaluated the synergistic effect of the combination of GSH and UTI on the ovarian grafts against IRI, because of the different mechanisms and targets of GSH and UTI. Predominate mechanism of GSH in antioxidant defense is that of reducing H2O2 into H2O and removing hypochlorous acid (HOCl). In addition, GSH can conjugate with human neutrophil elastase (HNE) to eliminate them, and restored another nonenzymatic antioxidant. These reactions can appear in any organ44. On the other hand, the main anti-IRI mechanism of UTI was due to inhibit various inflammatory proteases such as chymotrypsin and neutrophil elastase. Expectedly, our study for the first time proved the synergistic effect of GSH and UTI on ovarian grafts against IRI. The combination of GSH and UTI had more powerful effects on attenuating IR-induced oxidative stress and inflammation in the ovarian grafts, thereby showing better preservation of follicles than GSH or UTI alone.
TNF-α and IL6 are two significant mediators of IRI, as they are produced from macrophages as early inflammatory responses. Especially, the levels of TNF-α reached the peak in 2 h after inflammation, recruiting other inflammatory cytokines and inflammatory cells to tissues45. Our results found that during IR period, GSH and UTI decreased the levels of TNF-α and IL6, as well as suppressed the infiltration of macrophage in ovarian grafts. In addition, MDA, a marker of lipid peroxidation, has been viewed as an indicator of oxidative stress levels. Superoxide dismutase also plays an essential role in converting ROS to innocuous substance46. Our study also pointed out that UTI and GSH could decrease MDA and increase SOD in ovarian grafts.
The limitation of our study was that of unrevealing the interaction between inflammation/oxidative stress and revascularization progress in transplanted ovarian grafts. In the future, we will pay more attention on clearly describing their mechanism and signal pathway.
Conclusion
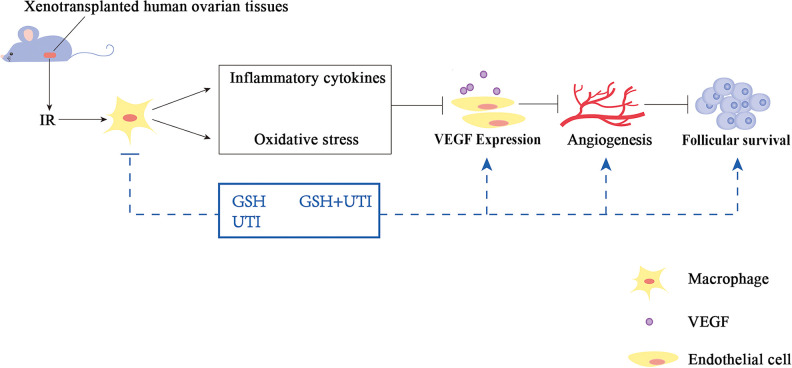
The host with the treatment of GSH, UTI and GSH+UTI improved the follicle integrity and viability in the transplanted ovarian grafts, because the IR-induced oxidative stress and inflammation were suppressed, and the angiogenesis progression was improved (Schema). Especially, the combination of GSH and UTI exerted the best protective effects, compared with the treatment of GSH alone or UTI alone. Therefore, therapeutic strategies employing GSH, UTI or GSH+UTI have become promising methods to be effective for the prevention of follicular loss in frozen-thawed ovary after transplantation.
Schema.
The treatment of GSH, UTI, GSH+UTI in the mice could decrease the IRI-induced oxidative stress and inflammation, and improved angiogenesis in ovarian grafts, which result in the increase of follicular survival rate in human ovarian grafts.
Supplemental Material
Supplemental Material, sj-pdf-1-cll-10.1177_0963689721997151 for Protective Effects of Reduced Glutathione and Ulinastatin on Xeno-transplanted Human Ovarian Tissue Against Ischemia and Reperfusion Injury by Yubin Li, Yue Hu, Shunye Zhu, Ying Tuo, Bin Cai, Tengfei Long, Wen Zhao, Xiaoxin Ye, XiaoFang Lu and Lingli Long in Cell Transplantation
Acknowledgments
The authors thank Weiping Yu (University of Texas at Austin, USA) for her comments on the paper.
Author Contributions: Yubin Li and Yue Hu contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by Ethics Committee at The First Affiliated Hospital, Sun Yat-sen University (Approval number: 2014-7).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from Natural Science Foundation of Guangdong Province (2015A030313036).
ORCID iD: Lingli Long  https://orcid.org/0000-0002-4022-2076
https://orcid.org/0000-0002-4022-2076
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Ethics Committee at The First Affiliated Hospital, Sun Yat-sen University (Approval number: 2014-7), and was carried out following the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Statement of Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Meirow D. Fertility preservation in hematology cancer patients. Aust Nz J Obstet Gyn. 2007;47:A21. [Google Scholar]
- 2. Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;9(12):735–749. [DOI] [PubMed] [Google Scholar]
- 3. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410. [DOI] [PubMed] [Google Scholar]
- 4. Donnez J, Dolmans MM. Fertility Preservation in Women, N Engl J Med 2017;377(17):1657–1665. [DOI] [PubMed] [Google Scholar]
- 5. Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, Dolmans MM. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model, Fertil Steril. 2010;93(5):1676–1685. [DOI] [PubMed] [Google Scholar]
- 6. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolusari A, Okyay AG, Kockaya EA. The Effect y. Reprod Sci. 2018;25(3):406–413. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki H, Ishijima T, Maruyama S, Yanagimoto Ueta Y, Abe Y, Saitoh H. Beneficial effect of desialylated erythropoietin administration on the frozen-thawed canine ovarian xenotransplantation. J Assist Reprod Genet. 2008;25(11-12):571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang H, Lee HH, Lee HC, Ko DS, Kim SS. Assessment of vascular endothelial growth factor expression and apoptosis in the ovarian graft: can exogenous gonadotropin promote angiogenesis after ovarian transplantation? Fertil Steril. 2008;90(4 Suppl):1550–1558. [DOI] [PubMed] [Google Scholar]
- 10. Mahmoodi M, Soleimani Mehranjani M, Shariatzadeh SM, Eimani H, Shahverdi A. N-acetylcysteine improves function and follicular survival in mice ovarian grafts through inhibition of oxidative stress. Reprod Biomed Online. 2015;30(1):101–110. [DOI] [PubMed] [Google Scholar]
- 11. Martinez-Madrid B, Donnez J, Van Eyck AS, Veiga-Lopez A, Dolmans MM, Van Langendonckt A. Chick embryo chorioallantoic membrane (CAM) model: a useful tool to study short-term transplantation of cryopreserved human ovarian tissue. Fertil Steril . 2009;91(1):285–292. [DOI] [PubMed] [Google Scholar]
- 12. Aksak Karamese S, Toktay E, Unal D, Selli J, Karamese M, Malkoc I. The protective effects of beta-carotene against ischemia/reperfusion injury in rat ovarian tissue. Acta Histochem. 2015;117(8):790–797. [DOI] [PubMed] [Google Scholar]
- 13. Campbell BK, Hernandez-Medrano J, Onions V, Pincott-Allen C, Aljaser F, Fisher J, Mcneilly AS, Webb R, Picton HM. Restoration of ovarian function and natural fertility following the cryopreservation and autotransplantation of whole adult sheep ovaries. Hum Reprod. 2014;29(8):1749–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donnez J, Dolmans MM. Transplantation of ovarian tissue. Best Pract Res Clin Obstet Gynaecol. 2014;28(8):1188–1197. [DOI] [PubMed] [Google Scholar]
- 15. Liao XL, Danzeng QZ, Zhang W, Hou CS, Xu BB, Yang J, Kang Y. Role of using two-route ulinastatin injection to alleviate intestinal injury in septic rats. Chin J Traumatol. 2018;21(6):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suyavaran A, Ramamurthy C, Mareeswaran R, Subastri A, Rao PL, Thirunavukkarasu C. TNF-alpha suppression by glutathione preconditioning attenuates hepatic ischemia reperfusion injury in young and aged rats. Inflamm Res. 2015;64(1):71–81. [DOI] [PubMed] [Google Scholar]
- 17. Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langedonckt A, Donnez JA. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92(1):374–381. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Q, Wang SM, Yao PB, Zhang L, Zhang YJ, Chen RX, Fu Y, Zhang JM. Effects of L-carnitine on follicular survival and graft function following autotransplantation of cryopreserved-thawed ovarian tissues. Cryobiology. 2015;71(1):135–140. [DOI] [PubMed] [Google Scholar]
- 19. Che H, Lv YF, Liu YG, Hou YX, Zhao LY. Effect of ulinastatin on myocardial ischemia reperfusion injury through ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(10):4458–4464. [DOI] [PubMed] [Google Scholar]
- 20. Maltaris T, Koelbl H, Fischl F, Seufert R, Schmidt M, Kohl J, Beckmann MW, Binder H, Hoffmann I, Mueller A, Dittrich R. Xenotransplantation of human ovarian tissue pieces in gonadotropin-stimulated SCID mice: the effect of ovariectomy. Anticancer Res 2006;26(6B):4171–4176. [PubMed] [Google Scholar]
- 21. Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology 2013;67(1):64–69. [DOI] [PubMed] [Google Scholar]
- 22. Gook DA, Edgar DH, Stern C. Effect of cooling rate and dehydration regimen on the histological appearance of human ovarian cortex following cryopreservation in 1, 2-propanediol. Hum Reprod. 1999;14(8):2061–2068. [DOI] [PubMed] [Google Scholar]
- 23. Grosbois J, Demeestere I. Dynamics of PI3 K and Hippo signaling pathways during in vitro human follicle activation. Hum Reprod. 2018;33(9):1705–1714. [DOI] [PubMed] [Google Scholar]
- 24. Long L, Wang J, Lu X, Xu Y, Zheng S, Luo C, Li Y. Protective effects of scutellarin on type II diabetes mellitus-induced testicular damages related to reactive oxygen species/Bcl-2/Bax and reactive oxygen species/microcirculation/staving pathway in diabetic rat. J Diabetes Res. 2015;2015:252530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castro A, Johnson MC, Anido M, Cortinez A, Gabler F, Vega M. Role of nitric oxide and bcl-2 family genes in the regulation of human endometrial apoptosis. Fertil Steril. 2002;78(3):587–595. [DOI] [PubMed] [Google Scholar]
- 26. Rahimi G, Isachenko V, Kreienberg R, Sauer H, Todorov P, Tawadros S, Mallmann P, Nawroth F, Isachenko E. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Eur J Obstet Gynecol Reprod Biol 2010;149(1):63–67. [DOI] [PubMed] [Google Scholar]
- 27. Hiatt LA, McKenzie JR, Deravi LF, Harry RS, Wright DW, Cliffel DE. A printed superoxide dismutase coated electrode for the study of macrophage oxidative burst. Biosens Bioelectron. 2012;33(1):128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Peng C, Zhang Z, Shi J, Lin Y, Gu L, Ma X, Li H. Intravenous infusion of ulinastatin attenuates acute kidney injury after cold ischemia/reperfusion. Int Urol Nephrol 2019;51(10):1873–1881. [DOI] [PubMed] [Google Scholar]
- 29. Li X, Su L, Zhang X, Zhang C, Wang L, Li Y, Zhang Y, He T, Zhu X, Cui L. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurol Res. 2017;39(4):367–373. [DOI] [PubMed] [Google Scholar]
- 30. Han P, Qin Z, Tang J, Xu Z, Li R, Jiang X, Yang C, Xing Q, Qi X, Tang M, Zhang J, et al. RTA-408 Protects Kidney from Ischemia-Reperfusion Injury in Mice via Activating Nrf2 and Downstream GSH Biosynthesis Gene. Oxid Med Cell Longev. 2017;2017:7612182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schubert B, Canis M, Darcha C, Artonne C, Smitz J, Grizard G. Follicular growth and estradiol follow-up after subcutaneous xenografting of fresh and cryopreserved human ovarian tissue. Fertil Steril. 2008;89(6):1787–1794. [DOI] [PubMed] [Google Scholar]
- 32. Kim SS, Kang HG, Kim NH, Lee HC, Lee HH. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum Reprod. 2005;20(9):2502–2508. [DOI] [PubMed] [Google Scholar]
- 33. Imthurn B, Cox SL, Jenkin G, Trounson AO, Shaw JM. Gonadotrophin administration can benefit ovarian tissue grafted to the body wall: implications for human ovarian grafting. Mol Cell Endocrinol. 2000;163(1-2):141–146. [DOI] [PubMed] [Google Scholar]
- 34. Israely T, Dafni H, Nevo N, Tsafriri A, Neeman M. Angiogenesis in ectopic ovarian xenotransplantation: multiparameter characterization of the neovasculature by dynamic contrast-enhanced MRI. Magn Reson Med. 2004;52(4):741–750. [DOI] [PubMed] [Google Scholar]
- 35. Li SH, Hwu YM, Lu CH, Chang HH, Hsieh CE, Lee RK. VEGF and FGF2 improve revascularization, survival, and oocyte quality of cryopreserved, subcutaneously-transplanted mouse ovarian tissues. Int J Mol Sci. 2016;17(8):1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Henry L, Labied S, Fransolet M, Kirschvink N, Blacher S, Noel A, Foidart JM, Nisolle M, Munaut C. Isoform 165 of vascular endothelial growth factor in collagen matrix improves ovine cryopreserved ovarian tissue revascularisation after xenotransplantation in mice. Reprod Biol Endocrinol. 2015;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mahmoodi M, Soleimani Mehranjani M, Shariatzadeh SM, Eimani H, Shahverdi A. Effects of erythropoietin on ischemia, follicular survival, and ovarian function in ovarian grafts. Reproduction. 2014;147(5):733–741. [DOI] [PubMed] [Google Scholar]
- 38. Saber M, Eimani H, Soleimani Mehranjani M, Shahverdi A, Momeni HR, Fathi R, Tavana S. The effect of Verapamil on ischaemia/reperfusion injury in mouse ovarian tissue transplantation. Biomed Pharmacother. 2018;108:1313–1319. [DOI] [PubMed] [Google Scholar]
- 39. Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu MY, Yiang GT, Liao WT, Tsai APY, Cheng YL, Cheng PW, Li CY, Li CJ, Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46(4):1650–1667. [DOI] [PubMed] [Google Scholar]
- 41. Durackova Z., Some current insights into oxidative stress. Physiol Res. 2010;59(4):459–469. [DOI] [PubMed] [Google Scholar]
- 42. Sengul O, Ferah I, Polat B, Halici Z, Bayir Y, Yilmaz M, Kilic N, Keles ON. Blockade of endothelin receptors with bosentan limits ischaemia/reperfusion-induced injury in rat ovaries. Eur J Obstet Gynecol Reprod Biol. 2013;170(2):458–463. [DOI] [PubMed] [Google Scholar]
- 43. Eken MK, Ersoy GS, Kaygisiz EI, Devranoglu B, Takir M, Cilingir OT, Cevik O. Etanercept protects ovarian reserve against ischemia/reperfusion injury in a rat model. Arch Med Sci. 2019;15(4):1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forman HJ, Zhang HQ, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30(1-2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garcia-Ramallo E, Marques T, Prats N, Beleta J, Kunkel SL, Godessart N. Resident cell chemokine expression serves as the major mechanism for leukocyte recruitment during local inflammation. J Immunol. 2002;169(11):6467–6473. [DOI] [PubMed] [Google Scholar]
- 46. Li J, Shu Y, Hao T, Wang Y, Qian Y, Duan C, Sun H, Lin Q, Wang C. A chitosan-glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials. 2013;34(36):9071–9081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-cll-10.1177_0963689721997151 for Protective Effects of Reduced Glutathione and Ulinastatin on Xeno-transplanted Human Ovarian Tissue Against Ischemia and Reperfusion Injury by Yubin Li, Yue Hu, Shunye Zhu, Ying Tuo, Bin Cai, Tengfei Long, Wen Zhao, Xiaoxin Ye, XiaoFang Lu and Lingli Long in Cell Transplantation