Abstract
Aims:
The study aimed to compare and analyze the outcomes of high-flow nasal cannula (HFNC) and noninvasive positive-pressure ventilation (NPPV) in the treatment of patients with acute hypoxemic respiratory failure (AHRF) who had extubation after weaning from mechanical ventilation.
Methods:
A total 120 patients with AHRF were enrolled into this study. These patients underwent tracheal intubation and mechanical ventilation. They were organized into two groups according to the score of Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II); group A: APACHE II score <12; group B: 12⩽ APACHE II score <24. Group A had 72 patients and patients given HFNC were randomly assigned to subgroup I while patients given NPPV were assigned to subgroup II (36 patients in each subgroup). Group B had 48 patients and patients given HFNC were randomly assigned to subgroup I while patients given NPPV were assigned to subgroup II (24 patients in each subgroup). General information, respiratory parameters, endpoint event, and comorbidities of adverse effect were compared and analyzed between the two subgroups.
Results:
The incidence of abdominal distension was significantly higher in patients treated with NPPV than in those treated with HFNC in group A (19.44% versus 0, p = 0.005) and group B (25% versus 0, p = 0.009). There was no significant difference between the HFNC- and NPPV-treated patients in blood pH, oxygenation index, partial pressure of carbon dioxide, respiratory rate, and blood lactic acid concentration in either group (p > 0.05). Occurrence rate of re-intubation within 72 h of extubation was slightly, but not significantly, higher in NPPV-treated patients (p > 0.05).
Conclusion:
There was no significant difference between HFNC and NPPV in preventing respiratory failure in patients with AHRF with an APACHE II score <24 after extubation. However, HFNC was superior to NPPV with less incidence of abdominal distension.
The reviews of this paper are available via the supplemental material section.
Keywords: acute hypoxemic respiratory failure, Acute Physiologic Assessment and Chronic Health Evaluation II, high-flow nasal cannula, noninvasive positive-pressure ventilation
Introduction
Acute hypoxemic respiratory failure (AHRF) is common in patients with severe pneumonia, cardiogenic pulmonary edema, acute respiratory distress syndrome, and sepsis, and is the main cause of admission to the intensive care unit (ICU).1 Oxygen therapy is an important aspect of supportive care to avoid re-intubation, and currently there are three different noninvasive ventilation options that could increase the oxygenation index (OI) in patients with AHRF, including routine oxygen inhalation, high-flow nasal cannula (HFNC), and noninvasive positive-pressure ventilation (NPPV).2,3 Conventionally, NPPV is often used as it can provide positive end-expiratory pressure (PEEP) and positive airway pressure, and can improve heart/lung function and OI. However, compared with HFNC, NPPV is associated with higher tidal volumes, which increase the risk of ventilator-induced lung injury. In addition, a patient’s tolerance to NPPV is low and comorbidity of adverse effect with NPPV (such as abdominal distension, aspiration, and sputum accumulation) is high.
HFNC is a novel therapeutic method that has recently been used in patients with AHRF after weaning from mechanical ventilation, which delivers optimal oxygen through a nasal cannula and generates low-level positive pressure. The humidified gas can improve cilia movement on the mucosal surface of the airway and enhance lung compliance. In addition, studies have shown that the effectiveness of clearing the dead space in nasal cavities has a linear positive dependency with nasal high-flow treatment, and that high-flow pressure increases the elimination of dead space gas during expiration, which results in improving OI and carbon dioxide clearance.4–6 However, HFNC application for patients who have had extubation following weaning from mechanical ventilation and have a score of >12 on Acute Physiologic Assessment and Chronic Health Evaluation II (APACHE II), remains to be further evaluated.7 It has been reported that patients with >15 APACHE II score have a higher risk of nosocomial infection and death.8 The current study was, therefore, designed to compare and evaluate the effect of HFNC and NPPV in the treatment of respiratory failure after weaning from mechanical ventilation and extubation for patients with AHRF by grouping patients into two groups based on APACHE II score: group A: APACHE II score <12; group B: 12⩽ APACHE II score <24.
Materials and methods
Patient enrollment
This study was approved by the Ethics Committee of Zhongnan Hospital of Wuhan University. Informed consent was obtained from all the study subjects before enrollment. The study was performed in accordance with the Helsinki II declaration. A patient was enrolled into the study if he/she was not re-intubated within 24 h of extubation and met the following criteria: (a) patients were conscious with a Glasgow score ⩾13, cough reflex, and sputum excretion; (b) acute respiratory failure (severe acute hypoxemia with PaO2/Fraction of inspiration O2 (FIO2) ratio <300 and a high respiratory drive reflected by clinical signs of respiratory distress); (c) hemoglobin >80 g/L; (d) no contraindication to the application of NPPV.
Criteria for exclusion included: patients with hypercapnia during the spontaneous breathing trial (SBT); inconsistent blood flow (systolic blood pressure <80 mmHg); insufficient blood flow as indicated by electrocardiogram (ECG) (flattened or inverted T wave, or ST segment depression); severe arrhythmia; severe cardiac failure [left ventricular ejection fraction (LVEF) <25%]; unconsciousness; high risk for aspiration or no cough reflex; complicated with other organ function failure (e.g. shock, digestive tract perforation, severe bleeding, or brain diseases); nontreated pneumothorax or mediastinal emphysema; re-intubated within 24 h of extubation; sepsis.9
Patients and grouping
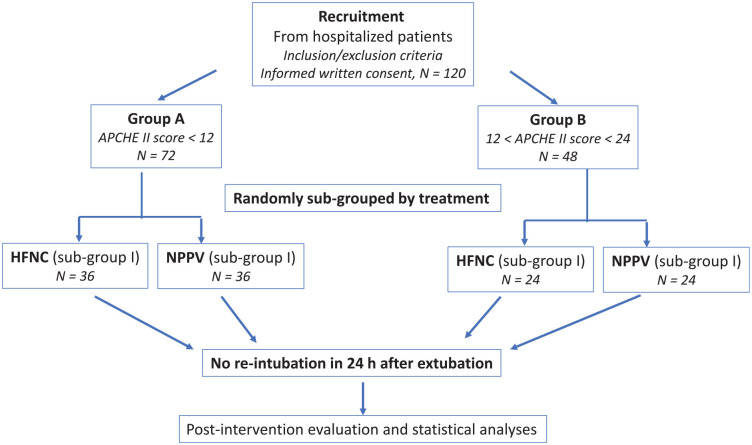
As shown in Figure 1, a total of 120 patients with AHRF treated with mechanical ventilation at the Department of Critical Care, Wuhan No. 1 Hospital, from December 2014 to November 2018, were enrolled into this study. Based on the APACHE II score, they were grouped as follows: group A: a low-risk group with APACHE II score <12 on the day of extubation; group B: a high-risk group with 12⩽ APACHE II score <24 on the day of extubation. Using a random-numbers table, patients in each group were randomly assigned into two subgroups: patients in subgroup I were treated with HFNC while patients in subgroup II were treated with NPPV. An informed and signed consent form was obtained from the patients or their legal guardians. The study protocol was registered (Registration #: 2014-P-07).
Figure 1.
Schematic flowchart of patient enrollment and grouping.
APACHE II, Acute Physiologic Assessment and Chronic Health Evaluation II; HFNC, high-flow nasal cannula; NPPV, noninvasive positive-pressure ventilation.
Weaning from mechanical ventilation
Hamilton Medical G5 and C2 ventilators (Bonaduz, Switzerland) were used in the current study. The weaning protocol included daily assessment of patients according to the following criteria. The mechanical ventilation was set at spontaneous breathing mode with FiO2 ⩽0.4 and PEEP <8 cm H2O. If the patient’s diaphragm movement was >10 mm and OI >150 without the following issues during a 120 min observation period,10 weaning was initiated: (a) respiratory rate (RR) >35 times/min or <8 times/min, which lasted for 5 min or longer; (b) altered level of consciousness; (c) ECG signs of myocardial ischemia; (d) acute arrhythmia; (e) two or more signs of respiratory distress including tachycardia (>130 beats/min), bradycardia (<60 beats/min), accessary muscle recruitment, paradoxical breathing, diaphoresis, and severe shortness of breath; (f) need for high doses of vasoactive drugs to maintain blood pressure.11 Patients fulfilling these criteria underwent a SBT, either with a T-tube or 7 cm H2O of pressure support for 30 min.
Patients who passed the SBT were randomized to receive either HFNC or NPPV.
Treatment option
Patients in the HFNC subgroup (subgroup I) were treated using a device from Fisher & Paykel (Guangzhou, China). Initial flow was 40 L/min, 37°C temperature, and adjustable FiO2 to ensure >90% blood oxygen saturation. HFNC was given for 24 h followed by conventional oxygen inhalation if necessary.
Patients in the NPPV subgroup (subgroup II) were treated using V60 ventilator (Philips, Cambridge MA, USA), S/T mode, 10–12 cm H2O inspiratory positive airway pressure, 4–6 expiratory positive airway pressure, 1.5–2.0 inspiration/expiration ratio, 0.5–1.0 s of pressure increase time, and adjustable FiO2 to ensure >90% blood oxygen saturation. NPPV was given for 24 h followed by conventional oxygen inhalation if necessary.
Parameters and study termination criteria
The following general information was collected: age, gender, body mass index (BMI), smoking status, APACHE II score, cause of respiratory failure, heart rate (HR), mean blood pressure (MBP), RR, Oxygen saturation (SpO2), body temperature, LVEF, arterial pH, blood lactate acid concentration, PaCO2, and OI. Patients had insertion of a cannula needle into the radial artery in order to monitor artery pressure as well as to analyze artery blood gas. Blood pH, PaO2, FiO2, PaCO2, blood lactic acid, and LVEF were recorded before the intervention as well as at 1 h and 6–12 h after the intervention. Re-intubation rate, abdominal distension, facial skin damage (facial skin turned red or broken), atelectasis, 28-day mortality, time of oxygen therapy, length of ICU stay, and in-ICU mortality were also recorded.
Abdominal distension was defined as patients feeling abdominal tension with an abdominal bulge that was higher than the chest, percussion revealed mid-tone or high-tone drum sound, and auscultation revealed bowel sounds decreased or disappeared.
The study was terminated if a patient had a second intubation within 72 h of extubation.
Statistics
SPSS 17.0 statistical software was used. Data were expressed as the mean ± SD and statistical comparisons were performed using the Students t-test for the normal distributed data. The Wilcoxon rank-sum test was used for the non-normally distributed data analysis. The chi-squared test was used for the comparison of categorical variables.
Results
A total 120 patients with AHRF were enrolled into this study. Of these, 71 were men and 49 women, with average age of 67.11 ± 5.60 years. As shown in Table 1, there were no significant differences between the patients treated with NPPV or HFNC with regard to age, gender ratio, BMI, smoking status, cause of respiratory failure, HR, MBP, RR, SpO2, body temperature, LVEF, arterial pH, blood lactate, PaCO2, and OI (p > 0.05). Similarly, there were no significant differences in those parameters in group A (p > 0.05) (Table 2) or group B (p > 0.05) (Table 3) patients treated with either NPPV or HFNC.
Table 1.
Baseline comparison of patients treated with NPPV or HFNC.
| NPPV (n = 60) | HFNC (n = 60) | p value | |
|---|---|---|---|
| Age (years) | 66.87 ± 6.92 | 67.35 ± 4.85 | 0.659 |
| Gender (male/female) | 39/21 | 32/28 | 0.194 |
| Body mass index (kg/m2) | 22.49 ± 2.10 | 22.35 ± 1.69 | 0.677 |
| Smoking (case) | 30 | 31 | 0.855 |
| Cause of respiratory failure | |||
| Pulmonary infection (case) | 38 | 35 | 0.575 |
| Cardiogenic pulmonary edema (case) | 11 | 14 | 0.500 |
| Specific | 3 | 1 | 0.309 |
| Other identified causes (case) | 7 | 8 | 0.782 |
| Not identified causes (case) | 1 | 2 | 0.559 |
| Parameters at baseline | |||
| Heart rate (beats/min) | 93.12 ± 15.83 | 87.70 ± 16.92 | 0.073 |
| Mean blood pressure (mmHg) | 93.72 ± 13.29 | 92.50 ± 11.60 | 0.594 |
| Respiratory rate (breaths/min) | 23.67 ± 3.50 | 22.88 ± 3.49 | 0.222 |
| Body temperature (°C) | 37.02 ± 0.74 | 37.23 ± 0.79 | 0.138 |
| Left ventricular ejection fraction | 51.65 ± 5.56 | 53.81 ± 6.53 | 0.053 |
| Arterial pH | 7.36 ± 0.04 | 7.37 ± 0.04 | 0.135 |
| Blood lactate (mmol/L) | 2.23 ± 0.68 | 2.03 ± 0.80 | 0.143 |
| PaO2 (mmHg) | 51.75 ± 4.29 | 52.12 ± 5.60 | 0.688 |
| PaCO2 (mmHg) | 39.92 ± 4.87 | 40.30 ± 5.07 | 0.673 |
| FiO2 (%) | 0.45 ± 0.08 | 0.43 ± 0.06 | 0.111 |
| Oxygenation index (mmHg) | 118.87 ± 23.66 | 121.38 ± 20.70 | 0.536 |
| Mechanical ventilation time (hours) | 69.22 ± 12.53 | 70.83 ± 13.61 | 0.502 |
FiO2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; NPPV, noninvasive positive-pressure ventilation; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
Table 2.
General characteristics of the patients in group A.
| NPPV (n = 36) | HFNC (n = 36) | p value | |
|---|---|---|---|
| Age (years) | 66.25 ± 6.97 | 67.64 ± 5.07 | 0.337 |
| Gender (male) | 23 | 20 | |
| Body mass index | 22.24 ± 2.51 | 22.37 ± 1.70 | 0.806 |
| Smoking | 17 | 18 | |
| Cause of respiratory failure | |||
| Respiratory infection | 22 | 20 | 0.633 |
| Cardiogenic pulmonary edema | 7 | 9 | 0.571 |
| Specific | 2 | 1 | 0.555 |
| Other identified causes | 4 | 6 | 0.496 |
| Not identified causes | 1 | 0 | 0.314 |
| Parameters at baseline | |||
| Heart rate (beats/min) | 92.75 ± 10.95 | 87.73 ± 13.33 | 0.085 |
| Mean blood pressure (mmHg) | 92.83 ± 12.90 | 91.72 ± 9.92 | 0.683 |
| Respiratory rate (breaths/min) | 23.95 ± 3.29 | 22.67 ± 3.89 | 0.137 |
| Body temperature (°C) | 37.17 ± 0.83 | 37.28 ± 0.80 | 0.575 |
| Left ventricular ejection fraction | 52.00 ± 4.88 | 54.19 ± 4.77 | 0.058 |
| Arterial pH | 7.35 ± 0.05 | 7.37 ± 0.04 | 0.133 |
| Blood lactate (mmol/L) | 2.23 ± 0.69 | 2.02 ± 0.78 | 0.216 |
| PaO2 (mmHg) | 51.97 ± 3.85 | 51.06 ± 4.77 | 0.137 |
| PaCO2 (mmHg) | 40.11 ± 4.74 | 40.67 ± 4.96 | 0.628 |
| FiO2 (%) | 0.45 ± 0.09 | 0.43 ± 0.06 | 0.214 |
| Oxygenation index (mmHg) | 120.47 ± 26.37 | 118.11 ± 17.19 | 0.654 |
| Mechanical ventilation time (hours) | 68.57 ± 14.32 | 68.22 ± 11.53 | 0.909 |
FiO2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; NPPV, noninvasive positive-pressure ventilation; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
Table 3.
General characteristics of the patients in group B.
| NPPV (n = 24) | HFNC (n = 24) | p value | |
|---|---|---|---|
| Age (years) | 67.79 ± 6.89 | 66.92 ± 4.58 | 0.607 |
| Gender (male) | |||
| Body mass index | 22.87 ± 1.20 | 22.32 ± 1.70 | 0.202 |
| Smoking | 13 | 13 | 1.000 |
| Cause of respiratory failure | |||
| Respiratory infection | 16 | 15 | 0.763 |
| Cardiogenic pulmonary edema | 4 | 5 | 0.712 |
| Specific | 1 | 0 | 0.312 |
| Other identified causes | 3 | 2 | 0.637 |
| Not identified causes | 0 | 2 | 0.149 |
| Parameters at baseline | |||
| Heart rate (beats/min) | 94.00 ± 13.26 | 87.42 ± 9.70 | 0.056 |
| Mean blood pressure (mmHg) | 95.04 ± 14.02 | 93.67 ± 13.89 | 0.734 |
| Respiratory rate (breaths/min) | 23.25 ± 3.84 | 23.21 ± 2.84 | 0.966 |
| Body temperature (°C) | 36.80 ± 0.53 | 37.16 ± 0.77 | 0.067 |
| Left ventricular ejection fraction | 50.67 ± 6.03 | 53.67 ± 4.23 | 0.052 |
| Arterial pH | 7.38 ± 0.04 | 7.38 ± 0.04 | 0.597 |
| Blood lactate (mmol/L) | 2.23 ± 0.67 | 2.03 ± 0.69 | 0.313 |
| PaO2 (mmHg) | 51.42 ± 4.94 | 53.71 ± 6.42 | 0.173 |
| PaCO2 (mmHg) | 39.63 ± 5.14 | 39.75 ± 5.29 | 0.934 |
| FiO2 (%) | 0.45 ± 0.04 | 0.43 ± 0.05 | 0.298 |
| Oxygenation index (mmHg) | 116.46 ± 19.16 | 126.29 ± 24.65 | 0.130 |
| Mechanical ventilation time (hours) | 70.17 ± 13.81 | 72.45 ± 14.21 | 0.576 |
FiO2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; NPPV, noninvasive positive-pressure ventilation; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
Incidence of abdominal distension, the primary outcome of this study, was significantly higher in the patients treated with NPPV (21.67%) than in patients treated with HFNC (0%) (p < 0.001) (Table 4). However, there were no significant differences in other parameters between patients treated with NPPV or HFNC (Table 4).
Table 4.
Comparison of parameters in all patients treated with NPPV or HFNC.
| Parameters | 1 h | 6–12 h | ||||
|---|---|---|---|---|---|---|
| NPPV (n = 60) | HFNC (n = 60) | p value | NPPV (n = 60) | HFNC (n = 60) | p value | |
| Heart rate (beats/min) | 82.40 ± 10.26 | 82.42 ± 8.20 | 0.992 | 78.60 ± 7.76 | 78.60 ± 7.71 | 1.000 |
| Mean blood pressure (mmHg) | 89.52 ± 11.04 | 87.70 ± 10.49 | 0.357 | 90.18 ± 12.88 | 86.00 ± 13.14 | 0.081 |
| Respiratory rate (breaths/min) | 20.95 ± 3.91 | 20.63 ± 3.31 | 0.633 | 18.00 ± 2.77 | 18.90 ± 2.56 | 0.067 |
| FiO2 (%) | 0.42 ± 0.07 | 0.43 ± 0.05 | 0.843 | 0.43 ± 0.07 | 0.42 ± 0.07 | 0.196 |
| Body temperature (°C) | 37.03 ± 0.61 | 37.26 ± 0.73 | 0.064 | 36.82 ± 0.56 | 36.61 ± 0.65 | 0.060 |
| Left ventricular ejection fraction (%) | 51.22 ± 5.48 | 53.10 ± 5.17 | 0.056 | 51.77 ± 6.20 | 53.85 ± 5.90 | 0.062 |
| Arterial pH | 7.38 ± 0.03 | 7.38 ± 0.03 | 0.860 | 7.38 ± 0.02 | 7.38 ± 0.02 | 0.434 |
| Blood lactate (mmol/L) | 2.32 ± 0.97 | 2.01 ± 0.86 | 0.060 | 1.69 ± 0.77 | 1.46 ± 0.49 | 0.053 |
| PaO2 (mmHg) | 65.75 ± 5.57 | 63.72 ± 6.26 | 0.066 | 73.76 ± 5.36 | 72.21 ± 6.39 | 0.153 |
| PaCO2 (mmHg) | 40.52 ± 3.12 | 40.17 ± 3.02 | 0.533 | 39.77 ± 2.13 | 39.70 ± 2.43 | 0.873 |
| Oxygenation index (mmHg) | 159.03 ± 29.54 | 152.25 ± 26.44 | 0.118 | 176.58 ± 31.33 | 169.72 ± 36.66 | 0.272 |
| NPPV (n = 60) | HFNC (n = 60) | p value | ||||
| Re-intubation rate (%) | 1 (1.66) | 0 | 0.315 | |||
| Abdominal distension (%) | 13 (21.67) | 0 | 0.000 | |||
| Facial skin damage (%) | 1 (1.66) | 0 | 0.315 | |||
| 28-day mortality (%) | 0 | 0 | – | |||
| Time of oxygen therapy (hours)* | 118.15 ± 41.56 | 120.43 ± 32.13 | 0.737 | |||
| Length of ICU stay (hours) | 150.57 ± 40.51 | 157.21 ± 26.13 | 0.288 | |||
| In-ICU mortality (%) | 0 | 0 | – | |||
During HFNC or NPPV treatment.
FiO2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; ICU, intensive care unit; NPPV, noninvasive positive-pressure ventilation; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
Comparison of the secondary outcomes between the patients treated with NPPV or HFNC in group A is shown in Table 5. It was found that re-intubation within 72 h of extubation occurred neither in the NPPV nor HFNC treatment subgroups (Table 5). Incidence of abdominal distension, however, was significantly higher in the patients treated with NPPV than in those treated with HFNC (19.44% versus 0, p = 0.005). Facial skin damage occurred in one patient (2.78%) treated with NPPV, but not in patients treated with HFNC (p > 0.05) (Table 5). Time of oxygen therapy was not significantly different between the patients treated with NPPV or HFNC [107.72 ± 39.31 h (interquartile range, 87–110) in NPPV versus 118.69 ± 23.85 h (interquartile range, 102–132) in HFNC; 95% confidence interval (CI): −26.26 to 4.31; p = 0.157]. No significant differences were found in the length of ICU stay, in-ICU mortality, and 28-day mortality between the patients treated with NPPV or HFNC (Table 5). Comparison of other outcomes including HR, MBP, RR, body temperature, LVEF, arterial pH, blood lactate, PaO2, PaCO2, and OI between patients treated with NPPV or HFNC at 1 h and 6–12 h is also showed in Table 5. There were no significant differences in these parameters.
Table 5.
Comparison of parameters after treatment in group A.
| Parameters | 1 h | 6–12 h | ||||
|---|---|---|---|---|---|---|
| NPPV (n = 36) | HFNC (n = 36) | p value | NPPV (n = 24) | HFNC (n = 24) | p value | |
| Heart rate (beats/min) | 81.06 ± 10.34 | 82.69 ± 8.53 | 0.466 | 75.31 ± 6.64 | 76.92 ± 6.85 | 0.314 |
| Mean blood pressure (mmHg) | 91.08 ± 9.40 | 87.39 ± 9.43 | 0.101 | 88.86 ± 8.94 | 85.36 ± 8.40 | 0.091 |
| Respiratory rate (breaths/min) | 19.36 ± 3.10 | 20.19 ± 4.06 | 0.331 | 17.53 ± 2.26 | 18.47 ± 2.82 | 0.122 |
| FiO2 (%) | 0.42 ± 0.08 | 0.43 ± 0.05 | 0.559 | 0.42 ± 0.07 | 0.44 ± 0.08 | 0.167 |
| Body temperature (°C) | 37.19 ± 0.59 | 37.35 ± 0.78 | 0.318 | 36.61 ± 0.32 | 36.66 ± 0.47 | 0.540 |
| Left ventricular ejection fraction | 51.39 ± 5.14 | 53.06 ± 4.55 | 0.150 | 52.17 ± 4.28 | 54.00 ± 3.84 | 0.060 |
| Arterial pH | 7.37 ± 0.03 | 7.38 ± 0.03 | 0.479 | 7.37 ± 0.02 | 7.38 ± 0.02 | 0.088 |
| Blood lactate (mmol/L) | 2.32 ± 0.70 | 2.03 ± 0.73 | 0.097 | 1.64 ± 0.54 | 1.41 ± 0.57 | 0.074 |
| PaO2 (mmHg) | 67.22 ± 4.38 | 65.11 ± 5.59 | 0.079 | 74.56 ± 4.81 | 72.39 ± 5.26 | 0.072 |
| PaCO2 (mmHg) | 40.78 ± 3.09 | 40.89 ± 2.39 | 0.865 | 40.11 ± 1.77 | 39.50 ± 2.77 | 0.269 |
| Oxygenation index (mmHg) | 164.47 ± 31.09 | 153.64 ± 25.25 | 0.109 | 183.17 ± 30.72 | 170.22 ± 32.86 | 0.089 |
| NPPV (n = 36) | HFNC (n = 36) | p value | ||||
| Re-intubation rate (%) | 0 | 0 | – | |||
| Abdominal distension (%) | 7 (19.44) | 0 | 0.005 | |||
| Facial skin damage (%) | 1 (2.78) | 0 | 0.314 | |||
| 28-day mortality (%) | 0 | 0 | – | |||
| Time of oxygen therapy (hours)* | 107.72 ± 39.31 | 118.69 ± 23.85 | 0.157 | |||
| Length of ICU stay (hours) | 138.03 ± 42.65 | 140.86 ± 26.07 | 0.735 | |||
| In-ICU mortality (%) | 0 | 0 | – | |||
During HFNC or NPPV treatment.
FiO2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; ICU, intensive care unit; NPPV, noninvasive positive-pressure ventilation; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
Comparison of the secondary outcomes between patients treated with NPPV or HFNC in group B is shown in Table 6. It was found that re-intubation within 72 h of extubation occurred in one patient (4.17%) in the NPPV group and none (0%) in the HFNC group (p = 0.312) (Table 6). Incidence of abdominal distension was significantly higher in the patients treated with NPPV than in those patients treated with HFNC (25% versus 0) (p = 0.009). Time of oxygen therapy was not significantly different between the two subgroups [133.88 ± 40.56 h (interquartile range, 108–164) in the patients treated with NPPV versus 122.38 ± 42.41 h (interquartile range, 95–123) in the patients treated with HFNC; 95% CI: −13.3 to 34.8; p = 0.342] (Table 6). Similarly, the length of ICU stays between the patients treated with NPPV (165.21 ± 41.08 h) or HFNC (172.56 ± 38.05 h) (p = 0.523) were not statistically different (Table 6). None of the patients in group B had facial skin damage or died within 28 days of extubation (28-day mortality) or during treatment in the ICU (in-ICU mortality). Comparison of other outcomes including HR, MBP, RR, body temperature, LVEF, arterial pH, blood lactate, PaO2, PaCO2, and OI between the patients treated with NPPV or HFNC at 1 h and 6–12 h are also showed in Table 6. There were no significant differences in these parameters.
Table 6.
Comparison of parameters after treatment in group B.
| Parameters | 1 h | 6–12 h | ||||
|---|---|---|---|---|---|---|
| NPPV (n = 24) | HFNC (n = 24) | p value | NPPV (n = 60) | HFNC (n = 60) | p value | |
| Heart rate (beats/min) | 84.42 ± 10.01 | 82.00 ± 7.83 | 0.356 | 83.54 ± 6.72 | 81.13 ± 8.36 | 0.275 |
| Mean blood pressure (mmHg) | 87.17 ± 12.99 | 88.17 ± 12.10 | 0.784 | 92.17 ± 8.60 | 87.83 ± 10.53 | 0.125 |
| Respiratory rate (breaths/min) | 23.33 ± 3.84 | 21.92 ± 1.69 | 0.105 | 19.42 ± 3.53 | 20.17 ± 3.31 | 0.451 |
| FiO2 (%) | 0.43 ± 0.04 | 0.42 ± 0.05 | 0.556 | 0.45 ± 0.05 | 0.44 ± 0.05 | 0.195 |
| Body temperature (°C) | 36.93 ± 0.72 | 37.24 ± 0.71 | 0.130 | 37.16 ± 0.68 | 37.00 ± 0.70 | 0.441 |
| Left ventricular ejection fraction | 50.67 ± 6.03 | 53.38 ± 3.62 | 0.066 | 51.08 ± 4.07 | 52.58 ± 3.11 | 0.158 |
| Arterial pH | 7.39 ± 0.03 | 7.38 ± 0.04 | 0.294 | 7.38 ± 0.03 | 7.38 ± 0.02 | 0.443 |
| Blood lactate (mmol/L) | 2.32 ± 0.58 | 1.98 ± 0.90 | 0.131 | 1.73 ± 0.62 | 1.49 ± 0.75 | 0.227 |
| PaO2 (mmHg) | 63.21 ± 5.63 | 60.75 ± 5.95 | 0.148 | 68.96 ± 5.42 | 71.63 ± 4.19 | 0.063 |
| PaCO2 (mmHg) | 40.13 ± 3.18 | 39.08 ± 3.55 | 0.290 | 39.25 ± 2.52 | 40.00 ± 1.81 | 0.244 |
| Oxygenation index (mmHg) | 139.88 ± 29.21 | 143.50 ± 30.19 | 0.674 | 154.33 ± 24.57 | 167.29 ± 25.48 | 0.079 |
| NPPV (n = 24) | HFNC (n = 24) | p value | ||||
| Re-intubation rate (%) | 1 (4.17) | 0 | 0.312 | |||
| Abdominal distension (%) | 6 (25) | 0 | 0.009 | |||
| Facial skin damage (%) | 0 | 0 | – | |||
| 28-day mortality (%) | 0 | 0 | – | |||
| Time of oxygen therapy (hour)* | 133.88 ± 40.56 | 122.38 ± 42.41 | 0.342 | |||
| Length of ICU stay (hours) | 165.21 ± 41.08 | 172.56 ± 38.05 | 0.523 | |||
| In-ICU mortality (%) | 0 | 0 | – | |||
During HFNC or NPPV treatment.
FiO2, fraction of inspired oxygen; HFNC, high-flow nasal cannula; ICU, intensive care unit; NPPV, noninvasive positive-pressure ventilation; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen.
Discussion
AHRF is characterized by severe acute hypoxemia (OI <300) and causes a high respiratory drive reflected by clinical signs of respiratory distress.12 Highly labored breathing usually results in hyperventilation and hypocapnia. For patients with impending respiratory muscle fatigue, hypercapnia may be induced. The therapeutic approach for patients with AHRF is to treat etiological factors such as infection, cardiac insufficiency, or fluid overload as well as to provide supportive care while awaiting resolution. Oxygen therapy, including conventional oxygen therapy, HFNC, and NPPV, is an important aspect of supportive care.13 However, it is not clear which oxygen therapy is more appropriate for patients at different risk who have undergone extubation.
Here, patients with AHRF were randomly assigned into two groups by APACHE II score, and outcomes of HFNC and NPPV in the treatment of patients with AHRF who had extubation after mechanical ventilation due to acute exacerbation, were compared and analyzed. APACHE II is a severity-of-disease classification system, which is often used to predict risk of death for severely ill patients. APACHE II has also been used to predict prognosis of acute exacerbation of AHRF14 and especially, APACHE II score could be used for making decisions on the application of mechanical ventilation in acute exacerbation of AHRF.15 In this study, patients with acute exacerbation of chronic obstructive pulmoriary disease (COPD) were assigned to group A: <12 APACHE II score and group B: 12⩽ APACHE II score <24. We found that there were no differences in HR, MBP, RR, body temperature, LVEF, arterial pH, blood lactate, PaO2, PaCO2, and OI in patients treated with HFNC or NPPV regardless of whether they were in group A or group B. However, incidences of abdominal distension and facial skin damage were higher in subgroup II (treated with NPPV) than in subgroup I (treated with HFNC), suggesting HFNC was superior to NPPV in patients with AHRF after weaning from mechanical ventilation.
In this study, 13 of the NPPV-treated patients had abdominal distension, and 1 patient had respiratory failure after re-intubation. However, the patient with respiratory failure did not have abdominal distension, suggesting abdominal distension might not contribute to the development of respiratory failure.
AHRF, which is often caused by airway infection, results in lung inflation, increased RR, respiratory fatigue, and decreased blood oxygen.16 Sequential mechanical ventilation from invasive to noninvasive is an effective method for the treatment of respiratory failure resulting from AHRF exacerbation.17 In this regard, NPPV is often used in the ICU for patients with AHRF after weaning from mechanical ventilation and extubation. However, a patient’s tolerance to NPPV is low and thus, approximately 29% of patients switch to re-intubation due to intolerance to NPPV.18 In contrast, HFNC provides a warm and humidified gas-flow delivered via nasal prongs, which is more comfortable and easier for patients to accept. Compliance is an important determining factor in order for a patient to have effective oxygen therapy by maximizing tolerance to the therapy.19 In addition, HFNC preserves high FiO2 and generates the PEEP counterbalances auto-PEEP due to a high flow of gas, which also provides washout of the upper airway dead space. Continuous positive airway pressure can also lead to inflation of the alveoli, supply of fresh oxygen, and enhanced gas exchange in the alveoli, which results in continuous elimination of CO2.5
Re-intubation due to post-extubation respiratory failure (PERF) is associated with increased duration of ICU stay, hospital stay, and mortality. Previous studies on the clinical effects of HFNC in post-extubation patients demonstrated that HFNC was superior to NPPV with lower length of stay in the ICU and lower rate of PERF with hypoxygen. However, for hypercapnic respiratory failure, there is limited and controversial evidence for the role of HFNC therapy in managing hypercapnia except for the mechanism of dead space washout. In this regard, Hernández et al. reported a higher rate of hypercapnia after extubation in the NPPV group than in the HFNC group (6.7% versus 3.8%). In the current study, patients who developed hypercapnia during SBT were excluded.20
A limitation of the current study was that it was a single-center study with a limited number of cases. With accumulation of case numbers, we plan next to further compare and analyze the outcomes of HFNC and NPPV on the comorbidities of lung infection, liver and kidney function alteration, and interval between extubation and re-intubation. In addition, without sample size power analysis and stratified blocked randomization, type II error (false negative) in the outcomes of HFNC and NPPV treatment in the two groups, which remains to be addressed in the future study, might exist in this study.
Taken together, the current study indicated that HFNC seems to be superior to NPPV for the treatment of patients with AHRF after weaning from mechanical ventilation.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666211004235 for Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation by Xiaoke Shang and Yanggan Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211004235 for Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation by Xiaoke Shang and Yanggan Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211004235 for Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation by Xiaoke Shang and Yanggan Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211004235 for Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation by Xiaoke Shang and Yanggan Wang in Therapeutic Advances in Respiratory Disease
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81873507, 82070348, and 81420108004).
ORCID iD: Yanggan Wang  https://orcid.org/0000-0002-8258-5633
https://orcid.org/0000-0002-8258-5633
Data availability statement: The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Xiaoke Shang, Department of Internal Medicine, Zhongnan Hospital of Wuhan University, Wuhan University, Wuhan, China.
Yanggan Wang, Department of Internal Medicine, Zhongnan Hospital of Wuhan University, Wuhan University, No.169 East Lake Road, Wuchang District, Wuhan, Hubei, 430071, China; Medical Research Institute of Wuhan University, Wuhan, China.
References
- 1. Vaschetto R, Longhini F, Persona P, et al. Early extubation followed by immediate noninvasive ventilation vs. standard extubation in hypoxemic patients: a randomized clinical trial. Intensive Care Med 2019; 45: 62–71. [DOI] [PubMed] [Google Scholar]
- 2. Papazian L, Corley A, Hess D, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med 2016; 42: 1336–1349. [DOI] [PubMed] [Google Scholar]
- 3. Coleman JM, Wolfe LF, Kalhan R. Noninvasive ventilation in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2019; 16: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 4. Lewis S, Martin CJ. Characteristics of the washout dead space. Respir Physiol 1979; 36: 51–63. [DOI] [PubMed] [Google Scholar]
- 5. Dysart K, Miller TL, Wolfson MR, et al. Research in high flow therapy: mechanisms of action. Respir Med 2009; 103: 1400–1405. [DOI] [PubMed] [Google Scholar]
- 6. Mündel T, Feng S, Tatkov S, et al. Mechanisms of nasal high flow on ventilation during wakefulness and sleep. J Appl Physiol (1985) 2013; 114: 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thille AW, Harrois A, Schortgen F, et al. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med 2011; 39: 2612–2618. [DOI] [PubMed] [Google Scholar]
- 8. Ma W, Wang L, Zhang JL. Relevance between APACHE II score and nosocomial infection in ICU patients: a comparative study. Chin J Nosocomiol 2010; 20: 183–186. [Google Scholar]
- 9. Pasquina P, Merlani P, Granier JM, et al. Continuous positive airway pressure versus noninvasive pressure support ventilation to treat atelectasis after cardiac surgery. Anesth Analg 2004; 99: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 10. Kim WY, Suh HJ, Hong SB, et al. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 2011; 39: 2627–2630. [DOI] [PubMed] [Google Scholar]
- 11. Stephan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA 2015; 313: 2331–2339. [DOI] [PubMed] [Google Scholar]
- 12. Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36: 585–599. [DOI] [PubMed] [Google Scholar]
- 13. Tinelli V, Cabrini L, Fominskiy E, et al. High flow nasal cannula oxygen vs. conventional oxygen therapy and noninvasive ventilation in emergency department patients: a systematic review and meta-analysis. J Emerg Med 2019; 57: 322–328. [DOI] [PubMed] [Google Scholar]
- 14. Wang CH, Lin HC, Chang YC, et al. Predictive factors of in-hospital mortality in ventilated intensive care unit. Medicine 2017; 96: e9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang H, He H, Zhang D. The application value of prognosis about APACHE II in AECOPD patient. China Modern Med 2011; 18: 41–42. [Google Scholar]
- 16. García-de-Acilu M, Patel BK, Roca O. Noninvasive approach for de novo acute hypoxemic respiratory failure: noninvasive ventilation, high-flow nasal cannula, both or none? Curr Opin Crit Care 2019; 25: 54–62. [DOI] [PubMed] [Google Scholar]
- 17. Stenberg K, Lundström M, Olofsson S, et al. Incorporation into nucleic acids of the antiherpes guanosine analog buciclovir, and effects on DNA and protein synthesis. Biochem Pharmacol 1988; 37: 1925–1931. [DOI] [PubMed] [Google Scholar]
- 18. Spoletini G, Alotaibi M, Blasi F, et al. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest 2015; 148: 253–261. [DOI] [PubMed] [Google Scholar]
- 19. Lu Z, Meng S-S, Zhang X, et al. Effect of high-flow nasal cannula oxygen therapy compared with conventional oxygen therapy in postoperative patients: a systematic review and meta-analysis. BMJ Open 2019; 9: e027523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernández G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA 2016; 316: 1565–1574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666211004235 for Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation by Xiaoke Shang and Yanggan Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_17534666211004235 for Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation by Xiaoke Shang and Yanggan Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_17534666211004235 for Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation by Xiaoke Shang and Yanggan Wang in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_17534666211004235 for Comparison of outcomes of high-flow nasal cannula and noninvasive positive-pressure ventilation in patients with hypoxemia and various APACHE II scores after extubation by Xiaoke Shang and Yanggan Wang in Therapeutic Advances in Respiratory Disease