Abstract
Background
Published reports on tocilizumab in COVID‐19 pneumonitis show conflicting results due to weak designs or heterogeneity in critical methodological issues.
Methods
This open‐label trial, structured according to Simon's optimal design, aims to identify factors predicting which patients could benefit from anti‐IL6 strategies and to enhance the design of unequivocal and reliable future randomized trials. A total of 46 patients with COVID‐19 pneumonia needing of oxygen therapy to maintain SO2 > 93% and with recent worsening of lung function received a single infusion of tocilizumab. Clinical and biological markers were measured to test their predictive values. Primary end point was early and sustained clinical response.
Results
Twenty‐one patients fulfilled pre‐defined response criteria. Lower levels of IL‐6 at 24 h after tocilizumab infusion (P = 0.049) and higher baseline values of PaO2/FiO2 (P = 0.008) predicted a favourable response.
Conclusions
Objective clinical response rate overcame the pre‐defined threshold of 30%. Efficacy of tocilizumab to improve respiratory function in patients selected according to our inclusion criteria warrants investigations in randomized trials.
Keywords: COVID‐19, IL‐6, pneumonia, severe acute respiratory syndrome, tocilizumab
Abbreviations
- AF
atrial fibrillation
- ALT
alanine aminotransferase
- CP
CPAP
- HRCT
high‐resolution chest CT scan
- HYQ
hydroxychloroquine
- ICU
intensive care units
- IL‐6
interleukin 6
- LMWH
low molecular weight heparin
- P/F
ratio of PaO2 to FiO2
- SO2
oxygen saturation
- SP‐D
surfactant protein D
- TCZ
tocilizumab
- TSS
Total Severity Score
- UNL
upper normal level
- V
Ventimask
- vWF
von Willebrand Factor
Introduction
Multifocal interstitial pneumonia represents the most common cause of admission in intensive care units (ICU) and death during SARS‐CoV‐2 infection. Available data highlight an intense ‘cytokine storm’ with a consequent inflammatory infiltrate of pulmonary interstitium and subsequent extended alveolar damage [1, 2].
Tocilizumab, an anti‐IL‐6 receptor monoclonal antibody, has proved effective in rheumatoid arthritis, as well as in diseases characterized by an intense systemic inflammatory activation [3, 4]. Moreover, small case series from China and Italy suggest that IL‐6 blockade induces rapid clinical improvement in patients affected by COVID‐19 interstitial pneumonia [5, 6, 7, 8].
However, conflicting results have been recently reported, in particular in patients affected by more severe disease.
Indeed, favourable effect of the drug on mortality and risk of intubation [9] has not been confirmed in other studies [10, 11]. In particular, in two recent press releases [12, 13], divergent results from COVACTA and EMPACTA phase 3 trials have been reported. Moreover, almost simultaneously, multiple studies showing favourable effect of tocilizumab on clinical relevant end points appeared [14, 15].
Different designs and heterogeneity in population and outcomes characteristics hamper a reliable comparison and interpretation of the results.
To investigate which patients could benefit from a single intravenous infusion of tocilizumab, we designed a trial with the goals:
to explore parameters useful to predict clinical response and select the population characterized by the higher benefit/risk ratio;
to acquire elements useful to design future unequivocal randomized trials.
Methods
Study design
The study was structured according to Simon's optimal two‐stage design [16] to test the hypothesis that an early single intravenous tocilizumab administration can reverse worsening of lung function in patients affected by severe COVID‐19 pneumonitis. Fourteen patients (variable: n1) entered the first stage of the trial. Enrolment would have been stopped and the drug rejected if < 10% of the patients (variable: P0) had met the primary end point 72 h after the administration of tocilizumab. Conversely, the study would have been continued until at least 46 patients were enrolled in total (variable: n). The drug would have been rejected in the case of a positive response rate < 30% (variable: P1) of the patients completing the study.
The values of variables n1, n, P0 and P1 were set to obtain a probability ≤ 0.01 of accepting a drug worse than P0 after the first stage and a probability ≤ 0.2 of rejecting a drug better than P1 at the end of the study.
Study population
Between 12 March and 26 March 2020, 46 consecutive patients were treated.
Patients have been enrolled and treated in the Department of Infectious Diseases (n°15), Department of Emergency Medicine (n°17) and Department of Respiratory Medicine (n°5) at Ospedali Riuniti‐Ancona and in Department of Emergency and Internal Medicine (n°6) of Ospedali Riuniti‐Marche Nord and in the Departments of Internal Medicine of Ospedale di Senigallia (n°2) and Ospedale di Fabriano (n°1), all in Italy.
Main selection criteria were as follows: patients 18–90 years of age; SARS‐CoV‐2 infection diagnosed by RT‐PCR, multifocal interstitial pneumonia confirmed by Rx or chest CT‐scan, need of oxygen therapy (FiO2 50%) to maintain peripheral oxygen saturation (SO2) >93%, recent (within the last 24 h) worsening of lung function, defined as a decrease of SO2 > 3% points and/or decrease of the ratio of PaO2 to FiO2 (P/F) >50% and/or P/F below 150 mm Hg−1, capability to sign written informed consent.
Drug administration
Tocilizumab was administered as a single infusion of 8 mg kg−1.
Concomitant therapies were allowed according to local protocols. No patient received systemic corticosteroids.
Assessment
Complete clinical evaluation, assessment of SO2, arterial blood gas analysis, blood count, ALT, D‐dimer and creatinine, recording of both FiO2 and type of ventilation were performed at baseline, and after tocilizumab infusion at 24 and 72 h and day 7.
CT‐scan
High‐resolution chest CT scan (HRCT) was performed at baseline in 33 cases and at day +7 in 21 patients.
Two independent experienced pulmonologists evaluated the images blinded for sequence, and final decision was reached by consensus.
Assessment of radiological pattern and definition of the extent of parenchymal involvement were performed and scored as described [17].
The extent of parenchymal involvement at HRCT was first scored by visually evaluating the percentage of lesions' involvement at lobar basis according to a 5‐point categorical scale. A Total Severity Score (TSS; range from 0 to 20) was obtained by summing the scores of the five lobes. A TSS ≥ 8 was assumed as threshold of severity.
Assays
Serum levels of IL‐6 (Immulite 2000, Siemens Medical Solution‐Diagnostic, Mt Olive, NJ USA), von Willenbrand Factor (vWF) (EHVWF, Human vWF ELISA Kit, Thermo Fisher Scientific Inc., Waltham, MA, USA), Surfactant protein D (SP‐D) (RD194059101‐ Human Surfactant Protein D ELISA Kit, BioVendor Research and Diagnostic Products, Brno, Czech Republic) and Thrombomodulin (ab46508–Thrombomodulin [CD141] Human ELISA Kit, ABCAM, Abcam plc, Cambridge Biomedical Campus, Cambridge, UK) were determined with commercially available enzyme‐linked immunosorbent assay following the manufacturers' recommendations.
Sera from 20 healthy sex‐ and age‐matched individuals were obtained and tested as controls (Fig 1).
Fig. 1.
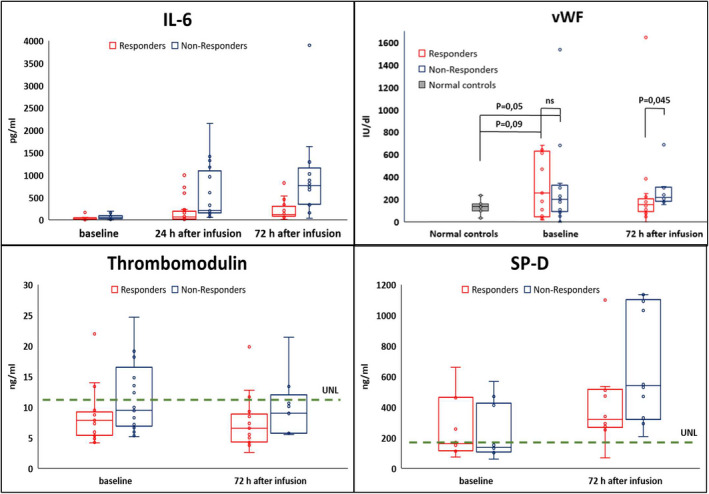
Upper normal limits of reference range at Ospedali Riuniti (Ancona) were IL‐6: 5.2 pg mL−1, thrombomodulin 11.2 ng mL−1 and SP‐D 190 ng mL−1; Using Mann–Whitney's U test; P‐value Il‐6 in responder vs non responder was 0.020, 0.001 and <0.001 at baseline, 24 and 72 h after infusion, respectively. Samples for IL‐6 at baseline were available in 36 patients, 40 patients at 24 h, and 35 patients at 72 h; vWf and thrombomodulin were tested 30 patients and for vWF in 20 normal controls; S‐PD was tested in 20 patients.
Response criteria
The primary outcome was the rate of responder patients. A patient was a‐priori defined as responder if fulfilling either criteria 1 or 2 AND criteria 3 of the ones listed below:
Improvement of oxygen saturation by more than 3% points and/or increase in P/F by 50% and/or increase P/F above 150 mmHg 72 h after tocilizumab AND persistence of this improvement at day 7;
No worsening of respiratory function as defined in the inclusion criteria at 72 h AND improvement of oxygen saturation by more than 3% points and/or increase in P/F > 50% and/or increase P/F above 150 mmHg at day 7;
No need of endotracheal ventilation for all or CP for those not requiring it at baseline.
Secondary outcomes were as follows: (i) rate of admission to intensive care unit for endotracheal intubation or evidence of multiple organ dysfunction; (ii) death; and (iii) rate of severe adverse events.
Although multiple score systems have been proponed to assess activity and severity of COVID‐19 patients, none of them has been validated so far [18, 19]. Consequently, we preferred to avoid adopting a scoring system, in order to prevent possible misguiding conclusions.
Statistical analysis
Data were expressed as median (and range or interquartile range) unless otherwise stated. Comparisons were made using Mann–Whitney U test or chi‐square test as appropriate. Two multivariate logistic regression analyses were performed using the primary outcome as dependent binary variable and the possible prognostic factors as independent variables. As possible prognostic variables, we considered age, sex, P/F at baseline, heparin and HYQ as co‐treatments, and either IL‐6 at baseline or IL‐6 at 24 h. A significance level alpha = 0.05 will be used for all the statistical analyses.
Results
Characteristics of Patients
Since five out of the first 14 patients met the primary outcome, enrolment was completed up to a total of 46 cases. Demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic, clinical, laboratory and radiological characteristics of patients enrolled in the experimental
| Median age (range) – years | 67.5 (34–89) |
| Sex M/F (%) | 33 (72)/13 (28) |
| Comorbidities N (%) | |
| Chronic heart failure | 2 (4) |
| Hypertension | 29 (63) |
| Diabetes | 5 (11) |
| BPCO | 0 (0) |
| Renal failure | 1 (2) |
| Renal failure with renal transplantation | 2 (4) |
| No comorbidity | 21 (46) |
| 1 comorbidity | 17 (37) |
| 2 comorbiditiesq | 6 (13) |
| 2+ comorbidities | 2 (4) |
| Smoke N (%) | |
| Actual | 0 (0) |
| Former | 4 (10) |
| na | 4 (9) |
| Time between onset of symptoms and TCZ infusion | |
| Days median (range) | 9.5 (2–21) |
| na (%) | 4 (9) |
| Time between onset of symptoms to hospital admission | |
| Days, median (range) | 7 (0–14) |
| na (%) | 4 (9) |
| Respiratory function at baseline N (%) | |
| Ventimask | 16 (35) |
| C‐PAP | 30 (65) |
| P/F ratio > 150 | 15 (37.5) |
| P/F ratio < 150 | 25 (62.5) |
| na | 6 (13) |
| Concomitant therapies N (%) | |
| Lopinavir‐ritonavir or darunavir‐cobicistat | 35 (78) |
| Hydroxychloquine | 41 (89) |
| Antibiotics | 30 (67) |
| Prophylactic LMWH | 18 (39) |
| Laboratory features at baseline | |
| IL‐6 pg mL−1 | 45.15 |
| Median (25–75 IQ range ) | (16.25–64.77) |
| na (%) | 10 (22) |
| Lymphocyte ×109/L | 0.635 |
| Median (25–75IQ range ) | (522.5–790) |
| na (%) | 4 (9) |
| ALT U/l | |
| Median (25–75IQ range) | 30 (12–158) |
| na (%) | 1 (2) |
| Extension of pulmonary involvement | |
| Chest CT‐scan Total Severity Score | 10 |
| Median (25–75 IQ range) | (7–12) |
| na (%) | 13 |
na, not available.
All the subjects were affected by pneumonitis requiring high‐flow oxygen therapy. Twenty‐five (63%) patients had severe respiratory failure, characterized by a P/F ratio < 150 mmHg, and 30 (65%) were on C‐PAP.
HRCT, available in 33 (72%) patients, revealed a diffuse pulmonary involvement, and 23 (69%) had a Total Severity Score (TSS) of eight or more, typical of the most severe patterns [17].
Treatment response
According to the a priori criteria, 72 h after tocilizumab 20 (43.5%) patients had an objective improvement and maintained the improvement in lung function at day 7. In 14 patients, improvement was already apparent 24 h after drug administration. One further patient was stable after 72 h and improved at day 7. Overall, 21 (45.6%) of the enrolled patients could be classified as responder. None of the responder patients underwent endotracheal intubation, admission to ICU or died.
In this subgroup, the median P/F significantly increased at 72 h compared to baseline value (median; IQ range: 250; 197–362 vs 163; 136–241, P = 0.009).
At day 7, twenty‐five patients were non‐responder, although three of them had shown a transient improvement after 72 h. Eleven (44%) of 25 were intubated a median of 2 (range 0–9) days after treatment and four died. Of the remaining 14 patients, three died, and 11 were still classified as non‐responder.
All responder patients were discharged after a median of 21 days (IQ range 17–27) after tocilizumab infusion, compared to 25.5 days (IQ range 20–34.25) of the non‐responder patients.
Patients with P/F > 150 mmHg at baseline (P = 0.003) and lower CT‐scan TSS (P = 0.008) were most likely to benefit from tocilizumab (Table 2).
Table 2.
Clinical, biological and radiological characteristics of responder and non‐responder patients
| Responders | Non‐Responders | P‐value a | |
|---|---|---|---|
| Number (%) | 21 (46) | 25 (54) | ns |
| Age median (range) | 68 (37–89) | 65 (34–89) | |
| Sex | |||
| M | 15 (45) | 18 (55) | ns |
| F | 6 (46) | 7 (54) | |
| Number of comorbidities | |||
| 0 | 11 (52) | 10 (48) | ns |
| 1 | 9 (53) | 8 (47) | |
| 2 or 2+ | 1 (13) | 7 (88) | |
| PaO2/FiO2 at baseline | |||
| >150 mm Hg−1 | 12 | 3 | χ2 (df1, N 40) = 8.64 P = 0.003 |
| <150 mm Hg−1 | 8 | 17 | |
| mm Hg−1 median (IQ range; na) | 163 (133–241; 1) | 102 (88–142; 5) | nap |
|
PaO2/FiO2 at 24 h median (IQ range; na) |
211 (140–352; 5) | 153 (121–177; 9) b | |
|
PaO2/FiO2 at 72 h median (IQ range; na) |
250 (197–362; 6) | 138 (77–179; 9) b | |
| Prophylactic LMWH | 6 | 13 | ns |
| HYQ | 18 | 23 | ns |
|
Extension of pulmonary involvement (Chest CT scan Total Severity Score) c |
8 | 11 | U‐MN = 61.5 P = 0.008 |
| median (IQ range) | (5.25–10) | (9.5–13) | |
| Discharged home (%) | 21 (100) | 16 d (64) | nap |
| ICU admission requiring intubation | 0 | 11 | |
| Death | 0 | 7 | |
nap, not applicable; ns, not significant.
χ2 = chi‐square test, U‐MN = Mann–Whitney U test.
Intubated patients were excluded.
CT scan images at baseline were available for 18 responder and 15 non‐responder patients assessed as in references [11, 12].
Five patients have been discharged after having treating in ICU.
Higher levels of IL‐6 at baseline were higher in non‐responders and showed a greater increase after 24 and 72 h (Fig 1).
No differences regarding to different CT‐scan patterns, nor comorbidities number or type, emerged from the comparison between responders and non‐responders.
We could compare chest CT scan taken at baseline and after 7 days in 21/46 patients. Changes in CT scan poorly correlate with clinical course.
Markers of endothelial and alveolar damage
Increased levels of vWF were detected in sera at baseline and 72 h after drug infusion, particularly in non‐responder group. Thrombomodulin levels remained stable within the normal limits at baseline and after tocilizumab, in both groups.
The serum levels of SP‐D, a glycoprotein secreted by type II pneumocytes and a potential surrogate marker of alveolar damage, were elevated at baseline in 35% of patients, and the concentrations rose overtime, particularly in non‐responder patients (Fig 1).
In univariate analysis, baseline levels of the above‐mentioned biomarkers did not correlate with outcome.
Multivariate analysis
In a multivariable model, lower IL‐6 levels measured at 24 h (P = 0.049), and higher baseline values of P/F (P = 0.008) were associated with better response to the drug. In the alternative model, IL‐6 levels at baseline did not emerge as independent variable.
Safety
Adverse events were reported in 29 (63%) of patients. Ten patients reported a 5‐fold increase in alanine aminotransferase at day 7, with a subsequent normalization within 14 days. No case of hepatic failure was observed.
In three patients, significant neutropenia (<1 × 109/L) appeared more than 7 days after drug infusion. One of these was a non‐responder and had a severe infection 1 month after tocilizumab infusion and despite normal neutrophil count.
Six bacterial infections were reported in six patients after admission to the ICU. The relationship with the experimental drug was considered uncertain by the investigators. Briefly, one patient was diagnosed with urinary infection caused by Enterococcus Faecium and Candida albicans. Three patients were found positive for methicillin‐resistant Staphylococcus aureus in their bronchial aspirate culture. In a further case, a pulmonary infection caused by Burkholderia gladioli was diagnosed. Finally, a case of Pseudomonas aeruginosa pneumonia was reported.
Three patients, on hydroxychloroquine and lopinavir‐ritonavir combination, suffered clinically relevant arrhythmias. One patient had a new episode of atrial fibrillation (AF) soon after tocilizumab infusion, with a spontaneous return to normal sinus rhythm within 24 h. A second patient developed AF 24 h after tocilizumab, and since this occurred with the worsening of respiratory function, he was transferred to the ICU and intubated. The third patient developed AF within 24 h after infusion and underwent successful pharmacological cardioversion.
After the detection of pulmonary embolism 9 days after tocilizumab, one non‐responder patient was anticoagulated and was discharged home once clinically improved.
No adverse reaction has been reported during or after parenteral administration of tocilizumab.
Discussion
The present study shows that 7 days after a single dose of tocilizumab administered within 24 h from evidence of clinical worsening, 21 patients with severe pneumonia fulfilled the responder criteria and none required admission to ICU or died. These patients were part of the 23 patients' subgroup who had improved at 72 h. The responder rate (46%) overcame the predefined threshold of 30%, and thus, the results justify future phase III randomized trials.
All 23 patients who did not improve at 72 h were also non‐responder at day 7. All the patients who died were in the non‐responder subgroup.
These data indicate that the response obtained 24–72 h after the administration of tocilizumab to patients with severe pneumonia foreshadows the likely prognosis.
At variance with what reported by others [20], high baseline values of IL‐6 did not predict the clinical outcome of our patients, rather more informative were the serum levels at 24 h which better reflected the actual generation of IL‐6 and thus the severity of the inflammation, as previously described in chronic inflammatory diseases [21]. The greater increase of the cytokine serum levels in patients who did not respond to tocilizumab supports the hypothesis that IL‐6 plays a major role in the pathogenesis of organ damage in this infection, as also reported by others [22].
In addition, we investigated correlations between serum levels of three markers of endothelial or alveolar damage and clinical deterioration or response to the treatment.
In particular, in COVID‐19 pneumonitis, increased plasma levels of thrombomodulin and vWF following endothelial apoptosis and necrosis lead to further endothelial damage [23].
Similarly, SP‐D levels increase in respiratory distress syndromes triggered by septic shock as well as in patients affected by severe COVID‐19 alveolar disruption [24].
In our experience, while the increasing levels of SP‐D accurately illustrate the ongoing lung damage, vWF and thrombomodulin performed differently, the former higher in more severely affected patients at 72 h, the latter stable and within the normal limits at all time points. Since vWF is produced constitutively in endothelium, while thrombomodulin is an integral membrane protein expressed on the surface of endothelial cells, our findings suggest that at least an abnormal breakdown of vWF may be involved in the microvascular damage in COVID‐19 patients.
Thus, the conclusions that can be drawn from these set of data are as follows: (i) a significant portion of severely ill and early deteriorating patients responded quickly to tocilizumab infusion; (ii) lower levels of IL‐6, particularly 24 h after drug infusion and higher P/F ratio correlated with a better response; and (iii) patients not improving after 24–72 h, as well as those with unfavourable prognostic factors, should either receive a second administration of tocilizumab, or switched to other treatment regimen.
In our study, failure of tocilizumab in reducing mortality probably relies on its insufficient activity (at the scheduled dose) in the most severe cases. However, the rapid improvement observed in responsive patients is a very relevant end point, on a clinical and organizational ground, reducing the use of mechanical ventilation and making semi‐intensive care beds more available for other patients.
Moreover, our data shed some light on conflicting results coming from randomized trials. Negative results of COVACTA trial, that is, could be attributed to the differences in many critical points, as severity of the disease, timing in tocilizumab administration and tardive end points' evaluation. According to our data, better results from EMPACTA trial could be linked to a less severe population at baseline and to the particular outcome chosen (cumulative proportion of participants requiring mechanical ventilation).
Several adverse events occurred in our patients. The three episodes of AF, which occurred within 24 h from tocilizumab, seem to point to a direct relationship with the medication. However, it is difficult to consider tocilizumab the sole culprit since: (i) arrhythmias are frequent in COVID‐19 patients; (ii) tocilizumab seems to reduce the incidence of disorders of cardiac rhythm and cardiac events in inflammatory diseases [25]; and iii all three patients' co‐treatments could have, at least, contributed to the rhythm abnormalities.
Our study has some weaknesses. First, it was a small, open‐label trial without a control group. It cannot be ruled out that the improvement observed in some patients was due to a milder disease. However, in our opinion, the enrolment of patients with severe pneumonia and recent worsening of lung function in the previous 24 h, and appraising as responder only those who rapidly ameliorated after treatment, mitigated this problem. Second, due to the frantic days of the pandemic, there was quite a number of missing data, albeit equally distributed between the responder and non‐responder subgroups. Complete reports were, however, available from two‐thirds of the enrolled patients. Third, the small study group makes the multivariate analysis exposed to the risk of ‘random effects’; thus, the data should be compared with those of ongoing prospective studies.
Conclusion
A prompt single infusion of 8 mg kg−1 of tocilizumab appears a promising strategy to rapidly improve respiratory function in patients with early severe and rapidly worsening COVID‐19 lung disease, particularly with a P/F ratio > 150 mm kg−1 and lower generation of IL‐6. This population should be considered the ideal target for future randomized trials.
Conflict of interest statement
The authors declare that they have no competing interests.
Funding
This study was supported by Azienda Ospedaliera Universitaria Ospedali Riuniti, Ancona, Italy.
Authors' contributions
AG, GP and AF contribute in study design, acquisition and interpretation of data and were major contributors in writing the manuscript. MR contributes to statistical analysis of data. MM, SS and MP perform biological X analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was authorized on 12 March 2020 by the Internal Ethical Board of Ospedali Riuniti‐Ancona (Italy).
Consent for publication
Not applicable.
Acknowledgements
Not applicable.
Pomponio G, Ferrarini A, Bonifazi M, Moretti M, Salvi A, Giacometti A, Tavio M, Titolo G, Morbidoni L, Frausini G, Onesta M, Amico D, Rocchi MLB, Menzo S, Zuccatosta L, Mei F, Menditto V, Svegliati S, Donati A, D'Errico MM, Pavani M, Gabrielli A (Ospedali Riuniti di Ancona; SOD Medicina di Laboratorio Ospedali Riuniti di Ancona; Ospedali Riuniti Marche Nord, Pesaro/Fano; Ospedale di Senigallia, Senigallia; Ospedale di Fabriano, Fabriano; Università di Urbino, Urbino; DISCLIMO, Università Politecnica delle Marche, Ancona, Italy). Tocilizumab in COVID‐19 interstitial pneumonia (Brief Report). J Intern Med 2021;289:738–746.
Trial registration: NCT 04315480
Data availability statement
Not applicable.
References
- 1. Ashour HM, Elkhatib WF, Rahman MM et al. Insights into the recent 2019 Novel Coronavirus (SARS‐CoV‐2) in light of past human coronavirus outbreaks. Pathogens 2020; 9: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39: 529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheppard M, Laskou F, Stapleton PP et al. Tocilizumab (Actemra). Hum Vaccin Immunother 2017; 13: 1972–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL‐6 blockade for cytokine storm. Immunotherapy 2016; 8: 959–70. [DOI] [PubMed] [Google Scholar]
- 5. Simon R, Wittes RE, Ellenberg SS. Randomized phase II Clinical Trials. Cancer Treat Rep 1985; 69: 1375–81. [PubMed] [Google Scholar]
- 6. Piva S, Filippini M, Turla F et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in Brescia, Italy. J Crit Care 2020; 58: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sciascia S, Aprà F, Baffa A et al. Pilot prospective open, single‐arm multicentre study on off‐label use of tocilizumab in patients with severe COVID‐19. Clin Exp Rheumatol 2020; 38: 529–32. [PubMed] [Google Scholar]
- 8. Colaneri M, Bogliolo L, Valsecchi P et al. Tocilizumab for treatment of severe COVID‐19 patients: preliminary results from SMAtteo COVID19 REgistry (SMACORE). Microorganisms 2020; 8: 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guaraldi G, Meschiari M, Cozzi‐Lepri A et al. Tocilizumab in patients with severe COVID‐19: a retrospective cohort study. Lancet Rheumatol 2020; 2: e474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quartuccio L, Sonaglia A, McGonagle D et al. Profiling COVID‐19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol 2020; 129: 104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rojas‐Marte GR, Khalid M, Mukhtar O et al. Outcomes in patients with severe COVID‐19 disease treated with tocilizumab ‐ a case‐ controlled study. QJM 2020; 113: 546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. https://www.roche.com/investors/updates/inv‐update‐2020‐07‐29.htm.
- 13. https://www.roche.com/investors/updates/inv‐update‐2020‐09‐18.htm.
- 14. Okoh AK, Bishburg E, Grinberg S, Nagarakanti S. Tocilizumab use in COVID‐19 associated pneumonia. J Med Virol 2020; 93: 1023–8. 10.1002/jmv.26471 [DOI] [PubMed] [Google Scholar]
- 15. Guaraldi G, Meschiari M, Cozzi‐Lepri A et al. Tocilizumab in patients with severe COVID‐19: a retrospective cohort study. Lancet Rheumatol 2020; 2: e474–84. 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon R. Optimal two stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 17. Li K, Fang Y, Li W et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID‐19). Eur Radiol 2020; 30: 4407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Izcovich A, Ragusa MA, Tortosa F et al. Prognostic factors for severity and mortality in patients infected with COVID‐19: a systematic review. PLoS One 2020; 15: e0241955. 10.1371/journal.pone.0241955. PMID: 33201896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu FY, Sun XL, Zhang Y et al. Evaluation of the risk prediction tools for patients with coronavirus disease 2019 in Wuhan, China: a single‐centered, retrospective. Observational study. Crit Care Med 2020; 48(11): e1004–11. 10.1097/CCM.0000000000004549. PMID: 32897668; PMCID: PMC7448719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toniati P, Piva S, Cattalini M et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020; 19: 102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishimoto N, Terao K, Mima T et al. Mechanisms and pathologic significances in increase in serum interleukin‐6 (IL‐6) and soluble IL‐6 receptor after administration of an anti‐IL‐6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008; 112: 3959–64. [DOI] [PubMed] [Google Scholar]
- 22. Perricone C, Triggianese P, Bartoloni E et al. The anti‐viral facet of anti‐rheumatic drugs: lessons from COVID‐19. J Autoimmun 2020; 111: 102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang J, Tecson KM, McCullough PA. Endothelial dysfunction contributes to COVID‐19‐associated vascular inflammation and coagulopathy. Rev Cardiovasc Med 2020; 21: 315–9. 10.31083/j.rcm.2020.03.126. PMID: 33070537. [DOI] [PubMed] [Google Scholar]
- 24. Kerget B, Kerget F, Koçak AO, et al. Are serum Interleukin 6 and surfactant protein D levels associated with the clinical course of COVID‐19? Lung. 2020; 198: 777–84. 10.1007/s00408-020-00393-8. Epub 2020 Sep 12. PMID: 32918573; PMCID: PMC7486805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lazzerini PE, Acampa M, Capecchi PL et al. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected QT interval by controlling systemic inflammation. Arthritis Care Res 2015; 67: 332–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


