Abstract
Objective:
Compare the effects of preoperative embolization for carotid body tumor resection on surgical outcomes to carotid body tumor resections without preoperative embolization.
Methods:
Single-center retrospective review of all consecutive patients who underwent carotid body tumor resection from 2001 to 2019. Surgical outcomes with emphasis on operative time (estimated blood loss and cranial nerve injury) of patients undergoing carotid body tumor resection following preoperative embolization were compared to those undergoing resection alone using unpaired Student’s t-test and Fisher’s exact test.
Results:
Forty-six patients (15% male, mean age 50 ± 15 years) underwent resection of 49 carotid body tumors. Patients undergoing preoperative embolization (n = 20 (40%)) had larger mean tumor size (4.0 ± 0.7 vs 3.2 ± 1 cm, p = 0.006), increased Shamblin II/III tumor classification (18 (90%) vs 22 (76%), p < 0.001), operative time (337 ± 195 vs 199 ± 100 min, p = 0.004), and cranial nerve injuries overall (8 (40%) vs 2 (10%), p = 0.01) compared to patients undergoing resection without preoperative embolization (n = 29 (60%)). In subgroup analysis of Shamblin II/III classification tumors (n = 40), preoperative embolization (n = 18) was associated with increased tumor size (4.1 ± 0.6 vs 3.5 ± 0.9 cm, p = 0.01), operative time (351 ± 191 vs 244 ± 105 min, p = 0.02), and cranial nerve injury overall (8 (44%) vs 2 (9%), p = 0.03) compared to resections alone (n = 19). In further subgroup analysis of large (⩾ 3 cm) tumors (n = 37), preoperative embolization (n = 18) was associated with increased operative time (350 ± 191 vs 198 ± 99 min, p = 0.006) and cranial nerve injury overall (8 (44%) vs 2 (11%), p = 0.03) compared to resections alone (n = 19). There were no significant differences in estimated blood loss, transfusion requirement, or hematoma formation between any of the embolization and non-embolization subgroups.
Conclusion:
After controlling for tumor Shamblin classification and size, carotid body tumor resections following preoperative embolization were associated with increased operative time and inferior surgical outcomes compared to those tumors undergoing resection alone. Nonetheless, such results remain susceptible to the confounding effects of individual tumor characteristics often used in the decision to perform preoperative embolization, underscoring the need for prospective studies evaluating the utility of preoperative embolization for carotid body tumors.
Keywords: Carotid body tumor, Shamblin classification, preoperative embolization, cranial nerve injury, high-altitude
Introduction
Carotid body tumors (CBTs) are rare neuroendocrine tumors with a reported incidence of 1:30,000 to 1:100,000.1–4 Due to the risk of malignancy, CBT resection is recommended in all healthy patients.5–7 Although surgical adjuncts such as preoperative embolization have been utilized in CBT resection, their effect on clinical outcomes remains poorly defined.6–10
The etiology of CBTs is sporadic, familial, or hyperplastic, with sporadic being the most common.8,11–13 The familial etiology of this disease is associated with germline mutations and account for 10% of tumors.2,9 The hyperplastic etiology is thought to be related to chronic hypoxia due to factors such as chronic lung disease and high altitude.12,14
High-altitude environments have unique CBT epidemiology with increased overall incidence, increased female predominance, and a decreased proportion of bilaterality.15,16 At 5700 feet above sea level, New Mexico has the fourth highest mean altitude in the United States.17 The University of New Mexico Health Sciences Center in Albuquerque, New Mexico, manages a significant proportion of CBTs occurring in our region.
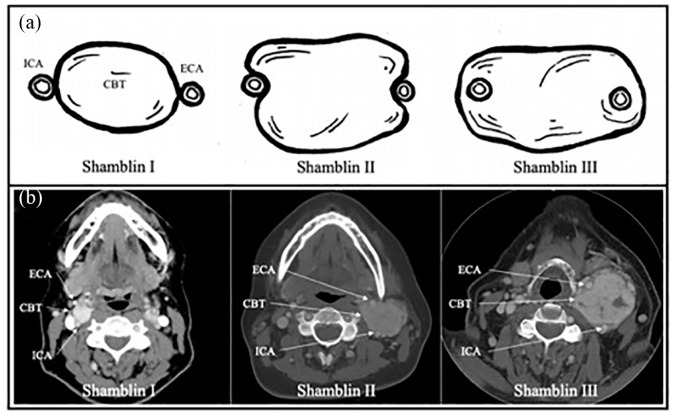
CBT resection carries a moderate risk for complications, with cranial nerve injury (CNI) being the most common, followed by hematoma, perioperative stroke, and wound infection.1 In recent metanalysis of 4418 patients and 4743 CBTs, the overall incidence of CNI was 25.4% with 11.5% of patients developing permanent CNIs (symptoms ⩾ 30 days).1 In addition, tumor size and the tumor’s anatomic relationship to the carotids, known as Shamblin classification, are associated with increased operative time, estimated blood loss (EBL), CNI, and perioperative stroke.1,10,18,19 In 1971, Shamblin and associates20 first described a classification system for the difficulty of surgical resection which characterized CBTs into three groups based on their relationship to the internal and external carotid arteries. Group 1 tumors are localized and with minimal attachment to the carotid vessels. Group 2 tumors are generally larger with moderate arterial attachment. Group 3 tumors are also large but encase the carotid vessels, presenting the greatest operative challenge. A representative diagram of the each Shamblin classification group is given in Figure 1(a).
Figure 1.
Shamblin classification: (a) Diagram of Shamblin classification of difficulty of CBT resection. Group 1 tumors are localized with minimal attachment to the carotid vessels. Group 2 tumors are generally larger with moderate arterial attachment. Group 3 tumors are also large and encase the carotid vessels, thus presenting the greatest operative challenge and (b) Examples of radiographic Shamblin classification of CBT.
CBT: carotid body tumor; ICA: internal carotid artery; ECA: external carotid artery.
In an effort to facilitate resection of challenging CBTs as well as decrease tumor size and minimize intraoperative blood loss, preoperative embolization was first employed by Schick et al.21 in 1980. The blood supply to the carotid body is primarily provided by the ascending pharyngeal branch of the external carotid artery (ECA), which plays a central role in preoperative embolization.7,21 The technique involves injection of an embolic agent, typically polyvinyl alcohol, directly into CBT feeding vessels under fluoroscopic guidance. Successful embolization is characterized by cessation of contrast opacification to the target artery and tumor.4,6,11
Whether preoperative embolization in CBT resection improves surgical outcomes remains controversial.6,7,9,10 A meta-analysis by Texakalidis et al.10 reviewed 25 studies involving 1326 patients and found that preoperative embolization for CBT resection decreased both operative time and EBL. However, no significant differences were found in the rates of CNI, stroke, or length of hospital stay.10 However, Power et al.,6 in a large single-center study, found no differences in operative time, EBL, or CNI when comparing patients undergoing preoperative embolization with those who did not.
The aim of this study is to compare the effects of preoperative embolization for CBT resection on surgical outcomes to CBT resections without preoperative embolization, with particular emphasis on operative time, EBL, and rate of CNI.
Methods
A single-center retrospective review was performed on all consecutive patients undergoing CBT resection from 26 September 2001 to 12 July 2019 at University of New Mexico Health Science Center in Albuquerque, New Mexico. The CPT code 60605 for carotid body tumor resection was utilized as inclusion criteria to identify subjects. Patients who had not undergone CBT resection were excluded from the study. Institutional review board approval from the University of New Mexico Health Science Center Human Research Protection Office (HRPO) was obtained for the study (#19-147). Clinical data regarding subjects were abstracted from the electronic medical record system including laboratory results, operative reports, pathology reports, radiographic imaging, and clinical notes.
Patients undergoing CBT resection with preoperative embolization (EMB group) were compared to patients undergoing CBT resection without preoperative embolization (NEMB group). To control for tumor factors that may influence the decision for embolization, both Shamblin II/III classification and large tumors ⩾ 3 cm underwent comparative subgroup analysis. The above tumor Shamblin and size thresholds were chosen as they were thought to be most representative of described criteria for embolization in the literature.6,22,23 Hence, patients with Shamblin II/III CBTs with undergoing resection with preoperative embolization (SII/III-EMB group) were compared to subjects with Shamblin II/III CBTs undergoing resection without preoperative embolization (SII/III-NEMB group). In addition, patients with large CBTs (⩾3 cm) undergoing resection with preoperative embolization (L-EMB group) were compared to patients with large CBTs (⩾3 cm) undergoing resection without preoperative embolization (L-NEMB group). Tumor Shamblin classification and size (maximal diameter) was determined for each patient by research team via blinded review of each patient’s preoperative imaging. Examples of radiographic Shamblin classifications are shown in Figure 1(b).
Statistical analyses
All clinical data were evaluated with descriptive analysis. Outcomes between groups were compared using unpaired Student’s t-test and Fisher’s exact test for continuous and categorical data, retrospectively, using GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA). All tests with a two-sided p value of < 0.05 were considered significant.
Carotid body tumor resection
CBT resection was performed by a total of 10 surgeons from the Divisions of Vascular Surgery and Otolaryngology. Patients with bilateral tumors initially underwent resection of the larger tumor. The contralateral tumor was either followed clinically or underwent interval resection.
All patients underwent CBT resection under general anesthesia. Use of electroencephalogram (EEG) monitoring was determined by surgeon preference. Cervical incision was performed in either oblique or transverse fashion. Subplatysmal flaps were then created and the sternocleidomastoid muscle was mobilized to expose the carotid sheath. Both monopolar and bipolar electrocautery were employed for surgical dissection. The cerebrovascular structures were routinely identified and controlled. Feeding vessels to the carotid body tumor were ligated and divided. Cranial nerves, including the vagus and hypoglossal, were routinely identified and protected from injury. Need for ICA clamping was based on patient anatomy and surgical necessity. If ICA clamping was performed, heparin was administered to achieve ACT of >250 s. When necessary, cerebrovascular reconstruction was performed according to surgeon preference and anatomical needs. Cranial nerves were resected en bloc with the tumor if complete nerve encasement was observed. Selective lymph node dissection was performed based on lymph node appearance and surgeon practice pattern. Wound closure and use of subcutaneous surgical drains were carried out according to surgeon preference. Technical success of CBT resection was defined as no gross tumor remaining following resection.
Preoperative carotid body tumor embolization
Historically, our institution does not have a universal selection criterion for preoperative embolization. Rather, the decision to perform preoperative embolization of CBT was determined by the operating surgeon based on practice patterns or individual tumor characteristics. Lack of a specific institutional embolization criterion creates a unique data pool where CBTs undergoing preoperative embolization can be compared to CBTs undergoing resection alone with otherwise similar tumors characteristics.
All patients that underwent preoperative embolization were admitted to the ICU for neuromonitoring. Embolization was performed under procedural sedation by an interventional neuroradiologist or neurosurgeon using transfemoral access. The distal ascending pharyngeal artery was fluoroscopically interrogated to determine feeding vessels to the CBT. A 1:1 mixture of contrast solution and polyvinyl alcohol particle solution (300–500 micrometers) was then injected into the target vessel until no further forward flow was identified. Completion arteriogram was then performed to verify technical success and ensure no distal embolic complications. Technical success was considered to be apparent angiographic devascularization > 50% of the CBT.
Results
Patient profile
From 26 September 2001 to 12 July 2019 a total of 46 patients underwent resection of 49 CBTs (Table 1). There were 7 males (15%) and 39 females (85%) with mean age of 50 ± 15 years at time of surgery. Resected CBT laterality was 20 (41%) right and 29 (59%) left. Six patients (13%) presented with bilateral tumors. Of these, three (7%) patients underwent resection of both tumors in an interval fashion with resection of the larger tumor first. Mean time from initial tumor resection to contralateral tumor resection was 30 (2 –121) months.
Table 1.
Demographics, presentation, investigations, and tumor characteristics.
| Variable | |
|---|---|
| Demographics | |
| Total patients | 46 |
| Total CBT resections | 49 |
| Age, years | |
| Mean | 50 ± 15 |
| Gender | |
| Male | 7 (15%) |
| Female | 39 (85%) |
| BMI | 31.7 ± 7.7 |
| Concurrent contralateral CBT | 3 (7%) |
| Family history of CBT | 4 (9%) |
| SDH mutation | 2 (4%) |
| Presentation | |
| Neck mass | 33 (72%) |
| Cranial nerve palsy | 3 (7%) |
| Dysphagia | 3 (7%) |
| Headache | 4 (9%) |
| Presyncope or syncope | 4 (9%) |
| Vertigo | 2 (4%) |
| CVA | 2 (4%) |
| Incidental finding | 10 (22%) |
| Multiple symptoms | 16 (35%) |
| Functional studies | |
| Performed | 25 (54%) |
| Functional tumor | 2 (4%) |
| Imaging | |
| CT arteriogram | 42 (86%) |
| MRI | 18 (37%) |
| Ultrasound | 26 (53%) |
| Radionucleotide scan | 4 (8%) |
| Tumor | |
| Maximal diameter, cm | 3.5 ± 0.9 |
| Laterality of resected tumor | |
| Right | 20 (41%) |
| Left | 29 (59%) |
| Shamblin classification | |
| Shamblin I | 9 (18%) |
| Shamblin II | 28 (57%) |
| Shamblin III | 12 (24%) |
CBT: carotid body tumor; BMI: body mass index; SDH: succinate dehydrogenase; CVA: cerebrovascular accident; CT: computed tomography; MRI: magnetic resonance imaging.
Categorical variable are presented as a number (n, %). Continuous variables are presented as a mean ± standard deviation or median (range).
The most common presenting symptom was a neck mass, seen in 30 patients (65%). In 10 cases (22%), the CBT was detected incidentally on an imaging study obtained for unrelated reasons. Other presenting symptoms are listed in Table 1. Median time from symptom presentation to CBT diagnosis was 1.7 (0–10) years. Once CBT diagnosis was obtained, median time to surgical resection was 0.4 (0–5) years.
Diagnosis and workup
CBT diagnosis was determined with the following imaging modalities: CT arteriogram in 40 patients (86%), ultrasound in 24 (53%), MRI in 27 patients (37%), and radionucleotide scan (123I-metaiodobenzylguanidine scintigraphy) in 4 (8%), with 32 (70%) undergoing multiple imaging modalities. Tumor size was obtained by measurement of largest tumor dimension with mean size of 3.5 ± 0.9 cm. Preoperative imaging was utilized for determination of radiographic CBT Shamblin classification. Nine tumors (18%) were Shamblin I, 28 (57%) were Shamblin II, and 12 (24%) were Shamblin III.
Functional tumor studies with 24-h urine catecholamine and metanephrines were obtained in 25 patients (54%) due the presence of clinical hypersecretion symptoms or surgeon practice-pattern. Two patients (4%) were found to have functional tumors and preoperative adrenergic blockade was utilized in one (50%) of those patients. Four patients (9%) had family history of CBTs. Genetic testing was obtained in all patients with family history of CBTs and in specific patient scenarios. A total of five patients (9%) underwent genetic testing, with succinate dehydrogenase (SHD) gene mutations found in two patients (4%).
Preoperative CBT embolization
Twenty-three patients (47%) underwent preoperative CBT embolization with technical success achieved in 20 patients (87%) (EMB group) (Table 2). Within the EMB group, the majority of patients underwent preoperative embolization the day prior to CBT resection, (median = 1 (0–14) days). Of the patients who underwent attempted preoperative embolization, one (4%) patient required emergent intubation following preoperative embolization. No other complications directly related to embolization were noted, including periprocedural stroke or arterial access complications such as hematoma or pseudoaneurysm formation.
Table 2.
Preoperative embolization.
| Variable | |
|---|---|
| Attempted | 23 (47%) |
| Successful | 20 (87%) |
| Time prior to resection, days | 1 (0–14) |
Categorical variable are presented as a number (n, %). Continuous variables are presented as a mean ± standard deviation or median (range).
CBT resection
Technical success was achieved in all 49 CBT resections (100%) (Table 3). Mean operative time for CBT resection was 256 ± 160 min with a median estimated blood loss of 150 (5–1200) mL. Five patients (10%) required blood transfusion during the resection. Selective lymph node dissection was performed in 38 operations (78%). The majority of CBT resections did not require any carotid artery management (n = 34 (78%)). Tumor extension necessitated ECA ligation in 11 resections (22%) and ICA repair or reconstruction in six resections (12%), with either patch angioplasty (n = 3 (6%)) or interposition graft (n = 3 (6%)). Conduits for interposition grafts utilized included Dacron graft in one patient (2%) and reverse saphenous vein graft in two patients (4%). A surgical drain was placed in 35 patients (71%) at the end of operation with a mean duration of drainage of 2.8 ± 2.3 days. The majority of patients were observed in the ICU postoperatively (n = 32 (65%)) with one patient (2%) remaining intubated following CBT resection. Mean hospital length of stay was 2.9 ± 2.5 days with home discharge disposition for all patients. There were no perioperative mortalities.
Table 3.
Carotid body tumor resection.
| Variable | |
|---|---|
| Operative time, min | 256 ± 160 |
| Estimated blood loss, mL | 150 (5–1200) |
| Transfusion requirementa | 5 (10%) |
| Carotid artery management | |
| Tumor resection alone | 38 (78%) |
| ECA ligation | 11 (22%) |
| ICA repair, any | 6 (12%) |
| Patch angioplasty | 3 (6%) |
| Interposition graft | 3 (6%) |
| Reverse saphenous vein | 2 (4%) |
| Dacron | 1 (2%) |
| Selective lymph node dissection | 38 (78%) |
| Surgical drain placement | 35 (71%) |
| Duration of surgical drain, days | 2.8 ± 2.3 |
ECA: external carotid artery; ICA: internal carotid artery.Categorical variable are presented as a number (n, %). Continuous variables are presented as a mean ± standard deviation or median (range).
Transfusion requirement for ⩾1 unit pRBC.
Outcomes and complications
The rate of overall complications following CBT resection was 31%, with CNI overall being the most common, occurring in 10 patients (20%) (Table 4). CNIs were identified by either direct laryngoscopy demonstrating ipsilateral vocal cord hypomobility or immobility, presence of ipsilateral tongue weakness or paresis, or new postoperative dysphagia which was confirmed by fluoroscopic swallow study. CNIs were differentiated into transient (n = 5 (10%)) resolving within 30 days, or permanent (n = 5 (10%)) persisting ⩾ 30 days postoperatively. Distribution of specific nerve injuries were seven (14%) recurrent laryngeal nerve injuries, six (12%) hypoglossal nerve injuries, and three (6%) glossopharyngeal nerve injuries. Two patients (4%) developed postoperative hematomas with one patient (2%) requiring re-exploration. Surgical site infection (SSI) developed in one (2%) patient and required incision and drainage. Two (4%) patients developed perioperative stroke, however neither patient had persistent sequelae on follow-up. Following CBT resection, there were no occurrences deep venous thrombosis (DVT) or pulmonary embolism (PE) in our series. Pathology revealed malignant CBT with microscopic lymph node involvement in one patient (2%). There were no 30-day mortalities. Median duration of patient follow-up was 7.4 (0.1–100.8) months. During the follow-up period, imaging studies were obtained in 24 patients (52%), which included ultrasound in 16 (35%), MRI in 7 (15%), and CT arteriogram in 5 (11%). Over the follow-up period, no patients were noted to have a clinical tumor recurrence.
Table 4.
Pathology, hospital course, complications, and follow-up.
| Variable | |
|---|---|
| Pathology | |
| Malignant | 1 (2%) |
| Positive margins | 20 (41%) |
| Positive lymph nodes | 1 (2%) |
| Hospital course | |
| Length of stay, days | 2.9 ± 2.5 |
| ICU length of stay, days | 1.5 ± 1.7 |
| Complications | |
| 30-day mortality | 0 (0) |
| Overall complication | 15 (31%) |
| Stroke | 2 (4%) |
| Hematoma | 2 (4%) |
| Surgical site infection | 1 (2%) |
| Cranial nerve injury | |
| CNI overall | 10 (20%) |
| Transient CNI | 5 (10%) |
| Permanent CNI | 5 (10%) |
| Distribution of CN injuries | |
| Recurrent laryngeal | 7 (14%) |
| Hypoglossal | 5 (10%) |
| Glossopharyngeal | 3 (6%) |
| Follow-up | |
| Duration of follow-up, months | 7.4 (0.1–100.8) |
| Recurrence | 0 (0%) |
CNI: cranial nerve injury.
Categorical variable are presented as a number (n, %). Continuous variables are presented as a mean ± standard deviation or median (range).
Effects of preoperative embolization
Surgical outcomes were compared between the EMB group (n = 20 (40%)) and NEMB group (n = 29 (60%)) and are detailed in Table 5. The groups did not differ in age, sex ratio, comorbidities, family history, or presenting symptomatology. Patients in the EMB group had a larger mean tumor size (4.0 ± 0.7 vs 3.2 ± 1 cm, p = 0.006), increased Shamblin II/III tumor classification (18 (90%) vs 22 (76%), p < 0.001), longer operative time (337 ± 195 vs 199 ± 100 min, p = 0.004), increased length of hospital stay (3.8 ± 2.9 vs 2.3 ± 2.1, p = 0.04), increased occurrence of overall complications (10 (50%) vs 5 (17%), p = 0.03), CNIs overall (8 (40%) vs 2 (10%), p = 0.01), and transient CNIs (5 (25%) vs 0 (0%), p = 0.035) compared to patients in the NEMB group. There were no significant differences in EBL (242 ± 178 vs 242 ± 320 mL, p > 0.99), transfusion requirement (3 (15%) vs 2 (7%), transfusions, p = 0.39), need for ECA ligation (6 (30%) vs 5 (17%), p = 0.32), need for ICA repair or reconstruction (3 (15%) vs 3 (10%) repairs or reconstructions, p = 0.68), development of permanent CNIs (3 (15%) vs 2 (7%), p = 0.39), postoperative hematomas (2 (10%) vs 0 (0%), p = 0.16), or SSIs (1 (5%) vs 0 (0%), p = 0.41) between the EMB and NEMB groups.
Table 5.
Comparison of EMB and NEMB groups.
| Variable | EMB | NEMB | p value |
|---|---|---|---|
| Demographics | |||
| Total patients | 20 | 28 | |
| Total CBT resections | 20 | 29 | |
| Age, years | |||
| Mean | 47 ± 16 | 52 ± 14 | 0.33 |
| Gender | >0.99 | ||
| Male | 3 (15%) | 5 (18%) | |
| Female | 17 (85%) | 23 (82%) | |
| BMI | 31.3 ± 8.5 | 32.4 ± 7.2 | 0.65 |
| Tumor | |||
| Maximal diameter, cm | 4.0 ± 0.7 | 3.2 ± 1 | 0.006 * |
| Shamblin class II or III | 18 (90%) | 22 (76%) | <0.001 * |
| Operative | |||
| Operative time, min | 337 ± 195 | 199 ± 100 | 0.004 * |
| Estimated blood loss, mL | 242 ± 178 | 242 ± 27 | >0.99 |
| Transfusion requirementa | 3 (15%) | 2 (7%) | 0.39 |
| Carotid artery management | |||
| Tumor resection alone | 14 (70%) | 24 (82%) | 0.32 |
| ECA ligation | 6 (30%) | 5 (17%) | 0.32 |
| ICA repair, any | 3 (15%) | 3 (10%) | 0.68 |
| Patch angioplasty | 1 (5%) | 2 (7%) | >0.99 |
| Interposition graft, any | 2 (10%) | 1 (3%) | 0.56 |
| Hospital course | |||
| Length of stay, days | 3.8 ± 2.9 | 2.3 ± 2.1 | 0.04 * |
| ICU length of stay, days | 2.5 ± 2.1 | 0.8 ± 0.7 | <0.001 * |
| Complications | |||
| 30-day mortality | 0 (0%) | 0 (0%) | >0.99 |
| Overall complication | 10 (50%) | 5 (17%) | 0.03 * |
| Stroke | 1 (5%) | 1 (3%) | >0.99 |
| Hematoma | 2 (10%) | 0 (0%) | 0.16 |
| Surgical site infection | 1 (5%) | 0 (0%) | 0.41 |
| Cranial nerve injury (CNI) | |||
| CNI overall | 8 (40%) | 2 (7%) | 0.01 * |
| Transient CNI | 5 (25%) | 0 (0%) | 0.01 * |
| Permanent CNI | 3 (15%) | 2 (7%) | 0.39 |
CBT: carotid body tumor; ECA: external carotid artery; ICA: internal carotid artery; CNI: cranial nerve injury.
Categorical variable are presented as a number (n, %). Continuous variables are presented as a mean ± standard deviation or median (range).
Bold and “*” represent statistically significant values where p values < 0.05.
Transfusion requirement for ⩾1 unit pRBC.
Surgical outcomes were then compared between patients with Shamblin II/III tumors undergoing preoperative embolization (SII/III-EMB group, n = 18) to patients with Shamblin II/III tumor undergoing CBT resection alone (SII/III-NEMB group, n = 22), detailed in Table 6. The groups did not differ in age, sex ratio, comorbidities, family history, or presenting symptomatology. Despite controlling for high tumor Shamblin classification (II/III), CBTs in the SII/III-EMB group were significantly larger than those tumors in the SII/III-NEMB group (4.1 ± 0.6 vs 3.5 ± 0.9 cm, p = 0.01). In addition, patients in the SII/III-EMB group had longer operative time (351 ± 191 vs 244 ± 105 min, p = 0.02), increased rate of overall complications (10 (56%) vs 4 (18%), p = 0.02), increased rate of CNI overall (8 (44%) vs 2 (9%), p = 0.03), and increased rate of transient CNI (5 (28%) vs 0 (0%), p = 0.01) compared to patients in the SII/III-NEMB group. However, there were no significant differences in the rates of permanent CNI (3 (17%) vs 2 (9%), p = 0.64) or the remaining complications listed in Table 6 between the SII/III-EMB and SII/III-NEMB groups. Furthermore, there were no differences between the SII/III-EMB and SII/III-NEMB groups in respect to EBL (244 ± 183 vs 296 ± 356 mL, p = 0.59), transfusion requirements (3 (17%) vs 2 (9%), p = 0.64), or carotid artery management.
Table 6.
Comparison of SII/III-EMB and SII/III-NEMB groups.
| Variable | SII/III-EMB | SII/III-NEMB | p value |
|---|---|---|---|
| Demographics | |||
| Total patients | 18 | 21 | |
| Total CBT resections | 18 | 22 | |
| Age, years | |||
| Mean | 46.2 ± 14.9 | 49.1 ± 15 | 0.54 |
| Gender | >0.99 | ||
| Male | 3 (17%) | 3 (14%) | |
| Female | 15 (83%) | 18 (86%) | |
| BMI | 31.2 ± 8.7 | 32.5 ± 8.4 | 0.65 |
| Tumor | |||
| Maximal diameter, cm | 4.1 ± 0.6 | 3.5 ± 0.9 | 0.01* |
| Operative | |||
| Operative time, min | 351 ± 191 | 244 ± 105 | 0.02* |
| Estimated blood loss, mL | 244 ± 183 | 296 ± 356 | 0.59 |
| Transfusion requirementa | 3 (17%) | 2 (9%) | 0.64 |
| Carotid artery management | |||
| Tumor resection alone | 12 (67%) | 17 (77%) | 0.5 |
| ECA ligation | 6 (33%) | 5 (23%) | 0.5 |
| ICA repair, any | 3 (17%) | 3 (13%) | >0.99 |
| Patch angioplasty | 1 (6%) | 2 (9%) | >0.99 |
| Interposition graft, any | 2 (11%) | 1 (5%) | 0.59 |
| Hospital course | |||
| Length of stay, days | 3.9 ± 3 | 2.7 ± 2.3 | 0.15 |
| ICU length of stay, days | 2.6 ± 2.2 | 0.9 ± 0.8 | 0.003* |
| Complications | |||
| 30-day mortality | 0 (0%) | 0 (0%) | >0.99 |
| Overall complication | 10 (56%) | 4 (18%) | 0.02* |
| Stroke | 1 (6%) | 1 (5%) | >0.99 |
| Hematoma | 2 (11%) | 0 (0%) | 0.2 |
| Surgical site infection | 1 (6%) | 0 (0%) | 0.45 |
| Cranial nerve injury (CNI) | |||
| CNI overall | 8 (44%) | 2 (9%) | 0.03* |
| Transient CNI | 5 (28%) | 0 (0%) | 0.01* |
| Permanent CNI | 3 (17%) | 2 (9%) | 0.64 |
CBT: carotid body tumor; ECA: external carotid artery; ICA: internal carotid artery; CNI: cranial nerve injury.
Categorical variable are presented as a number (n, %). Continuous variables are presented as a mean ± standard deviation or median (range).
Transfusion requirement for ⩾1 unit pRBC.
Finally, patients with large tumors (⩾ 3 cm) undergoing preoperative embolization (L-EMB group, n = 18) were compared to patients with large tumors (⩾3 cm) undergoing CBT resection alone (L-NEMB group, n = 19), detailed in Table 7. Between the L-EMB and L-NEMB groups, there was no significant difference in tumor size (4.1 ± 0.6 vs 3.8 ± 0.7 cm, p = 0.12) or proportion of Shamblin II/III tumor classification (18 (100%) vs 17 (89%), p = 0.49). In addition, the groups did not differ in age, sex ratio, comorbidities, family history, or presenting symptomatology. The L-EMB group was found to have significantly longer operative time (351 ± 191 vs 198 ± 99 min, p = 0.006), increased overall complications (10 (56%) vs 3 (18%), p = 0.02), incidence of overall CNIs (8 (44%) vs 2 (11%), p = 0.03), and transient CNIs (5 (28%) vs 0 (0%), p = 0.02) compared to the L-NEMB group. Although, the incidence of permanent CNIs (3 (17%) vs 2 (11%), p = 0.66) and other complication listed in Table 7 were comparable between the L-EMB and L-NEMB groups. Furthermore, EBL (244 ± 183 vs 295 ± 373 mL, p = 0.61), rates of transfusion requirements (3 (17%) vs 2 (11%), p = 0.66), and carotid artery management were similar between the L-EMB and L-NEMB groups.
Table 7.
Comparison of L-EMB and L-NEMB groups.
| Variable | L-EMB | L-NEMB | p value |
|---|---|---|---|
| Demographics | |||
| Total patients | 18 | 18 | |
| Total CBT resections | 18 | 19 | |
| Age, years | |||
| Mean | 46 ± 15 | 48.6 ± 16 | 0.63 |
| Gender | >0.99 | ||
| Male | 3 (17%) | 4 (22%) | |
| Female | 15 (83%) | 14 (78%) | |
| BMI | 31.2 ± 8.7 | 32.1 ± 7.9 | 0.77 |
| Tumor | |||
| Maximal diameter, cm | 4.1 ± 0.6 | 3.8 ± 0.7 | 0.12 |
| Shamblin class II or III | 18 (100%) | 17 (89%) | 0.49 |
| Operative | |||
| Operative time, min | 350 ± 191 | 198 ± 99 | 0.006* |
| Estimated blood loss, mL | 244 ± 183 | 295 ± 375 | 0.61 |
| Transfusion requirementa | 3 (17%) | 2 (11%) | 0.66 |
| Carotid artery management | |||
| Tumor resection alone | 12 (67%) | 15 (79%) | 0.48 |
| ECA ligation | 6 (33%) | 4 (21%) | 0.48 |
| ICA repair, any | 3 (17%) | 2 (11%) | 0.66 |
| Patch angioplasty | 1 (6%) | 1 (5%) | >0.99 |
| Interposition graft, any | 2 (11%) | 1 (5%) | 0.6 |
| Hospital course | |||
| Length of stay, days | 3.9 ± 3 | 2.4 ± 2.2 | 0.08 |
| ICU length of stay, days | 2.6 ± 2.2 | 0.9 ± 0.8 | 0.004* |
| Complications | |||
| 30-day mortality | 0 (0%) | 0 (0%) | >0.99 |
| Overall complication | 10 (56%) | 3 (16%) | 0.02* |
| Stroke | 1 (6%) | 1 (5%) | >0.99 |
| Hematoma | 2 (11%) | 0 (0%) | 0.23 |
| Surgical site infection | 1 (6%) | 0 (0%) | 0.49 |
| Cranial nerve injury (CNI) | |||
| CNI overall | 8 (44%) | 2 (11%) | 0.03* |
| Transient CNI | 5 (28%) | 0 (0%) | 0.02* |
| Permanent CNI | 3 (17%) | 2 (11%) | 0.66 |
CBT: carotid body tumor; ECA: external carotid artery; ICA: internal carotid artery; CNI: cranial nerve injury.
Categorical variable are presented as a number (n, %). Continuous variables are presented as a mean ± standard deviation or median (range).
Transfusion requirement for ⩾1 unit pRBC.
Discussion
This study had several key findings. First, in our single-centered retrospective review, those patients who underwent preoperative embolization for CBT resection had greater proportions of Shamblin II/III classification tumors and the tumors were larger in size, compared to those patients who underwent resection alone. Furthermore, such patients undergoing CBT resection with preoperative embolization had increased operative time and inferior surgical outcomes compared to those patients undergoing resection alone. After controlling for tumor Shamblin classification and tumor size, preoperative embolization was still associated with increased operative time and inferior surgical outcomes compared to patients undergoing resection alone.
Although there exists no universal criterion for preoperative embolization of CBT, generally anatomic features and size of the tumor are taken into consideration.6,22,23 Our institution does not universally adhere to a specific tumor size threshold with regard to preoperative embolization, nonetheless we found patients whom had undergone preoperative embolization had greater proportions of Shamblin II/III tumors as well larger tumors. This correlation of tumor size and likelihood to undergo preoperative embolization is corroborated in the literature.6,7,22,24–26 Furthermore, as both increased size and higher Shamblin classification can independently increase operative difficulty and worsen surgical outcomes,1,10,18,19 a need to control for such tumor characteristic was imperative to determine the effect of preoperative embolization. Hence, subgroups with tumor size of ⩾3 cm and Shamblin II/III classification were chosen for analysis as they were thought to be most representative of described criteria for embolization.6,22,23
After controlling for tumor Shamblin classification and tumor size, we found that preoperative embolization for CBT resection was associated with increased operative time, overall complications, CNIs overall, and transient CNIs. In addition, we observed no significant decreases in EBL, transfusion rates, or hematoma formation in any of the EMB subgroups when compared to their respective NEMB subgroup. The detrimental effects of preoperative embolization observed here may be explained by the resultant inflammation obscuring the surgical planes leading to paradoxically increased dissection difficulty thus increasing operative time and the likelihood for iatrogenic nerve injury.
Compared to a recent meta-analysis by Robertson et al.,1 we demonstrate similar rates of overall (20% vs 25.4%) and permanent CNIs (10% vs 11.2%). However, we demonstrate a reduced rate of transient CNIs (10% vs 20.4%). This apparent discrepancy in rates of transient CNIs may reflect differences in the clinical definitions of CNI and detection methodology for occult nerve injuries between studies. Moreover, this raises questions as to the clinical significance of transient cranial nerve dysfunction observed during the postoperative period.
Our study has several limitations. First, with a sample size of 49 CBT resections, our study is sufficiently robust for a single-center retrospective series, but nonetheless susceptible to type II error. Second, the retrospective nature of the study itself and review period of 18 years renders our data prone to selection, reporting, and misclassification bias. Furthermore, disparate practice patterns between subspecialists and provider threshold for bedside laryngoscopy may influence the rate at which transient CNI is detected postoperatively, potentially confounding the true frequency CNIs in our study. In addition, the retrospective nature of the study prevents insight the rationale for selection preoperative embolization. It remains unclear if decision to perform preoperative embolization was based on provider practice patterns, individual tumor characteristics, or other factors. Finally, our results beg the question if our study adequately controlled for the effects tumor size or if the results remain confounded by tumor size. The limitations mentioned above underscore the need for prospective multicenter studies evaluating preoperative embolization for CBT resection which control for selection bias, tumor Shamblin classification, tumor size, and discrepancies in detection of occult CNIs. Furthermore, more robust multicenter or database studies may reveal additional predictors for the likelihood of suffering a CNIs during CBT resection. The results from our study currently do not support the use of preoperative embolization for CBT resection regardless of size of Shamblin classification. This study adds to the existing clinical equipoise in the literature regarding the advantages and disadvantages of preoperative embolization for CBT resection.6,7,9,10
Conclusion
This retrospective review indicates CBTs in patients undergoing preoperative embolization represent a distinct group that have higher proportions of Shamblin II/III classification and are larger in size. After controlling for tumor Shamblin classification and size, patients undergoing preoperative embolization for CBT resection had increased operative time, inferior surgical outcomes, and no difference in EBL compared to those undergoing resection alone. Nonetheless, such results remain susceptible to the confounding effects of individual tumor characteristics often used in the decision to perform preoperative embolization, underscoring the need for prospective studies evaluating the utility of preoperative embolization for CBT resection.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Institutional review board approval from the University of New Mexico Health Science Center Human Research Protection Office (HRPO) was obtained for the study (#19-147).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: The written informed consent and Health Insurance Portability and Accountability Act (HIPPA) authorization addendum were waived by the HRPO. There is no associated waiver number.
ORCID iDs: Robin Osofsky  https://orcid.org/0000-0002-4901-4097
https://orcid.org/0000-0002-4901-4097
Jaideep Das Gupta  https://orcid.org/0000-0003-3748-1568
https://orcid.org/0000-0003-3748-1568
References
- 1. Robertson V, Poli F, Hobson B, et al. A systematic review and meta-analysis of the presentation and surgical management of patients with carotid body tumours. Eur J Vasc Endovasc Surg 2019; 57(4): 477–486. [DOI] [PubMed] [Google Scholar]
- 2. Sajid MS, Hamilton G, Baker DM, et al. A multicenter review of carotid body tumour management. Eur J Vasc Endovasc Surg 2007; 34(2): 127–130. [DOI] [PubMed] [Google Scholar]
- 3. Oosterwijk JC, Jansen JC, van Schothorst EM, et al. First experiences with genetic counselling based on predictive DNA diagnosis in hereditary glomus tumours (paragangliomas). J Med Genet 1996; 33(5): 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davila VJ, Chang JM, Stone WM, et al. Current surgical management of carotid body tumors. J Vasc Surg 2016; 64(6): 1703–1710. [DOI] [PubMed] [Google Scholar]
- 5. Nora JD, Hallett JW, O’Brien PC, et al. Surgical resection of carotid body tumors: long-term survival, recurrence, and metastasis. Mayo Clin Proc 1988; 63: 348–352. [DOI] [PubMed] [Google Scholar]
- 6. Power AH, Bower TC, Kasperbauer J, et al. Impact of preoperative embolization on outcomes of carotid body tumor resections. J Vasc Surg 2012; 56(4): 979–989. [DOI] [PubMed] [Google Scholar]
- 7. Economopoulos KP, Tzani A, Reifsnyder T. Adjunct endovascular interventions in carotid body tumors. J Vasc Surg 2015; 61(4): 1081–1091.e2. [DOI] [PubMed] [Google Scholar]
- 8. Cobb AN, Barkat A, Daungjaiboon W, et al. Carotid body tumor resection: just as safe without preoperative embolization. Ann Vasc Surg 2018; 46: 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Liu J, Li Y, Yang L, et al. Surgical resection of carotid body tumors with versus without preoperative embolization: retrospective case-control study. Head Neck 2018; 40(12):2590–2595. [DOI] [PubMed] [Google Scholar]
- 10. Texakalidis P, Charisis N, Giannopoulos S, et al. Role of preoperative embolization in carotid body tumor surgery: a systematic review and meta-analysis. World Neurosurg 2019; 129: 503–513.e2. [DOI] [PubMed] [Google Scholar]
- 11. Ridge BA, Brewster DC, Darling RC, et al. Familial carotid body tumors: incidence and implications. Ann Vasc Surg 1993; 7(2): 190–194, https://www.sciencedirect.com/science/article/abs/pii/S0890509606605906?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 12. Saldana MJ, Salem LE, Travezan R. High altitude hypoxia and chemodectomas. Hum Pathol 1973; 4(2): 251–263, https://www.sciencedirect.com/science/article/abs/pii/S0046817773800127?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 13. Lotti LV, Vespa S, Pantalone MR, et al. A developmental perspective on paragangliar tumorigenesis. Cancers (Basel) 2019; 11(3): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pacheco-Ojeda LA. Carotid body tumors: surgical experience in 215 cases. J Cranio-Maxillofac Surg 2017; 45(9): 1472–1477. [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez-Cuevas S, López-Garza J, Labastida-Almendaro S. Carotid body tumors in inhabitants of altitudes higher than 2000 meters above sea level. Head Neck 1998; 20(5): 374–378. [DOI] [PubMed] [Google Scholar]
- 16. Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V, et al. Carotid body tumors: review of a 20-year experience. Oral Oncol 2005; 41: 56–61. [DOI] [PubMed] [Google Scholar]
- 17. Carpenter A, Provorse C. The world almanac of the U.S.A. Mahwah, NJ: PRIMEDIA Reference Inc., 1998, https://unm.on.worldcat.org/oclc/416207194 [Google Scholar]
- 18. Law Y, Chan YC, Cheng SW. Surgical management of carotid body tumor—is Shamblin classification sufficient to predict surgical outcome? Vascular 2016; 25(2): 184–189. [DOI] [PubMed] [Google Scholar]
- 19. Kruger AJ, Walker PJ, Foster WJ, et al. Important observations made managing carotid body tumors during a 25-year experience. J Vasc Surg 2010; 52(6): 1518–1523. [DOI] [PubMed] [Google Scholar]
- 20. Shamblin WR, ReMine WH, Sheps SG, et al. Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg 1971; 122: 732–739. [DOI] [PubMed] [Google Scholar]
- 21. Schick PM, Hieshima GB, White RA, et al. Arterial catheter embolization followed by surgery for large chemodectoma. Surgery 1980; 87(4): 459–464. [PubMed] [Google Scholar]
- 22. Kasper GC, Welling RE, Wladis AR, et al. A multidisciplinary approach to carotid paragangliomas. Vasc Endovascular Surg 2007; 40(6): 467–474. [DOI] [PubMed] [Google Scholar]
- 23. Kafie FE, Freischlag JA. Carotid body tumors: the role of preoperative embolization. Ann Vasc Surg 2001; 15: 237–242. [DOI] [PubMed] [Google Scholar]
- 24. Zhang TH, Jiang WL, Li YL, et al. Perioperative approach in the surgical management of carotid body tumors. Ann Vasc Surg 2012; 26(6): 775–782. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J, Fan X, Zhen Y, et al. Impact of preoperative transarterial embolization of carotid body tumor: a single center retrospective cohort experience. Int J Surg 2018; 54(2): 48–52. [DOI] [PubMed] [Google Scholar]
- 26. Zeitler DM, Glick J, Har-El G. Preoperative embolization in carotid body tumor surgery: is it required? Ann Otol Rhinol Laryngol 2010; 119(5): 279–283. [DOI] [PubMed] [Google Scholar]