Dear Editors,
The ongoing and relentless worldwide diffusion of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is jeopardizing the response capacity of most healthcare systems. This includes the capability to provide diagnostic tests to all people who need them for purposes of clinical diagnosis, contact tracing, and even for monitoring the progression of coronavirus disease 2019 (COVID‐19). 1 To this end, the availability of additional tests or algorithms which may anticipate the result of SARS‐CoV‐2 molecular testing would be welcomed for guiding the diagnostic process, thus preventing unnecessary waste of reagents and avoiding to further overwhelm already burdened clinical laboratories.
Convincing evidence has been provided that the monocyte distribution width (MDW), a simple laboratory parameter that can be automatically calculated and reported by specific hematological analyzers manufactured by Beckman Coulter (Beckman Coulter) along with the complete blood count, reflects some changes in monocyte biology (especially size) that are highly predictive of critical acute infections and/or viral sepsis. 2 , 3 Interestingly, significant differences in morphology and function of monocytes have been recently observed between COVID‐19 patients and healthy individuals. 4 Therefore, in this article, we aim to provide a pooled analysis of studies that have addressed the potential clinical utility of MDW for predicting SARS‐CoV‐2 infection at hospital admission.
An electronic search was carried out in Medline (PubMed interface), Scopus, and Web of Science, with the keywords “monocyte distribution width” OR “MDW” AND “coronavirus disease 2019” OR “COVID‐19” OR “SARS‐CoV‐2” without date (ie, up to December 5, 2020) and language restrictions, according to the protocol based on the transparent reporting of systematic reviews and meta‐analysis (PRISMA) (Appendix S1). The title, abstract, and full text of all articles that could be identified with these search criteria were systematically evaluated. Those studies reporting MDW values in patients with and without SARS‐CoV‐2 infection, diagnosed using nucleic acid amplification tests (NAATs) via nasopharyngeal or oropharyngeal swabs at hospital admission, were included in the pooled analysis. The reference list of these documents was also scrutinized with forward and backward citation tracking, to detect other potentially eligible studies. A pooled analysis was then performed, with estimation of weighted mean difference (WMD) and 95% confidence interval (95% CI) of MDW values in subjects with or without SARS‐CoV‐2 infection. A random‐effect model was used to adjust for potential heterogeneity arising due to different threshold values and sampling times across studies. Heterogeneity was assessed using chi‐square test and I 2 statistics. The pooled analysis was performed with MetaXL, software Version 5.3 (EpiGear International Pty Ltd.). This study was conducted in accordance with the Declaration of Helsinki and within the terms of the local legislation. The investigation was exempted from ethical committee approval as it is not locally required for pooled analyses, nor received any funding.
A total of 23 articles were initially detected with our search criteria, 20 of which were excluded after title, abstract, or full‐text screening, because they either failed to provide MDW values in patients with and without SARS‐CoV‐2 infection (n = 15) or MDW values were not measured in patients with COVID‐19 (n = 5). Thus, a total of three studies, with combined 452 subjects (143 with SARS‐CoV‐2 infection; 31.6%, range 6.0%‐60.0%), were finally included in our pooled analysis 5 , 6 , 7 (Table 1). One study compared MDW in COVID‐19 positive patients vs COVID‐19 negative controls, 7 one study compared MDW in COVID‐19 symptomatic and paucisymptomatic patients presenting to the emergency department, 6 and one study compared MDW in those with COVID‐19 as opposed to upper respiratory tract infections. 5 In all three studies, the cutoff was set at a similar threshold (≥20 in two studies and ≥20.1 in the third, respectively), and MDW values were higher in patients with SARS‐CoV‐2 infection than those without, as shown in Figure 1. Despite a relevant heterogeneity (I 2, 91%), the pooled analysis revealed that the MDW value was 14.4% higher in patients with SARS‐CoV‐2 infection than in those without (WMD, 3.95; 95% CI, 1.41‐6.49) (Figure 1).
TABLE 1.
Main characteristics of studies included in the pooled analysis
| Authors | Setting | Sample size | MDW cutoff | SARS‐CoV‐2 infection | MDW with or without SARS‐CoV‐2 infection |
|---|---|---|---|---|---|
| Lin HA et al, 2020 5 | Taiwan | 150 | ≥20 | 9 (6.0%) | 23.5 ± 2.1 vs 21.8 ± 5.4 |
| Ognibene A et al, 2020 6 | Italy | 147 | ≥20 | 41 (27.9%) | 27.3 ± 4.9 vs 20.3 ± 3.3 |
| Zeng X et al, 2020 7 | China | 155 | ≥20.1 | 93 (60.0%) | 22.1 ± 2.3 vs 18.9 ± 2.0 |
| Cumulative | ‐ | 452 | ‐ | 143 (31.6%) | 23.7 ± 3.0 vs 20.7 ± 4.0 |
Abbreviations: MDW, monocyte distribution width; SARS‐CoV‐2; severe acute respiratory distress syndrome 2.
FIGURE 1.
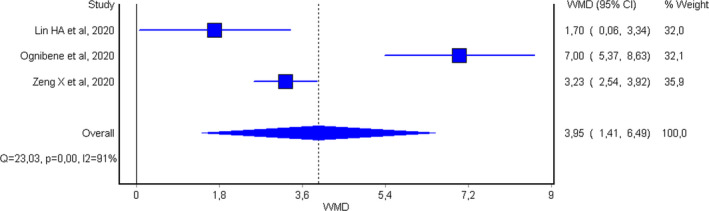
Weighted mean difference and 95% confidence interval (95% CI) of monocyte distribution width (MDW) values in patients with or without severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection at hospital admission
Despite the limited numbers of studies available so far and their lack of uniformity, the results of this pooled analysis suggest that MDW at hospital admission is higher in subjects with active SARS‐CoV‐2 infection than in those without. As reported by Lin et al, 5 MDW was significantly elevated in patients with COVID‐19, even in comparison with those with upper respiratory tract infections. This is not an unexpected finding, as monocytes biology appears considerably perturbed in patients with SARS‐CoV‐2 infection. 8 This may be due to direct cytopathic effect of SARS‐CoV‐2 on this cell lineage, as well as to direct or indirect cell activation by circulating cytokines and/or immunocomplexes. 9 Regardless of the underlying mechanisms, further studies should be planned to define the role of MDW within diagnostic algorithms for rationing SARS‐CoV‐2 diagnostics and for predicting disease progression and complications, especially the hyperinflammatory syndrome, which frequently characterize severe or critical COVID‐19 illness. 10
CONFLICT OF INTEREST
The authors have no competing interests.
AUTHOR CONTRIBUTIONS
Giuseppe Lippi designed the project, collected and analyzed data, and wrote the manuscript. Fabian Sanchis‐Gomar and Brandon M. Henry supervised the project, analyzed data, and revised the manuscript.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
Supporting information
Appendix S1
REFERENCES
- 1. Iacobucci G Covid‐19: Government faces criticism over pound500m plan to pilot mass testing. BMJ. 2020;370:m3482. [DOI] [PubMed] [Google Scholar]
- 2. Crouser ED, Parrillo JE, Seymour C, et al. Improved early detection of sepsis in the ED with a novel monocyte distribution width biomarker. Chest. 2017;152:518‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crouser ED, Parrillo JE, Seymour CW, et al. Monocyte distribution width: a novel indicator of sepsis‐2 and sepsis‐3 in high‐risk emergency department patients. Crit Care Med. 2019;47:1018‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karimi Shahri M, Niazkar HR, Rad F COVID‐19 and hematology findings based on the current evidences: a puzzle with many missing pieces. Int J Lab Hematol. 2020. 10.1111/ijlh.13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin HA, Lin SF, Chang HW, et al. Clinical impact of monocyte distribution width and neutrophil‐to‐lymphocyte ratio for distinguishing COVID‐19 and influenza from other upper respiratory tract infections: a pilot study. PLoS One. 2020;15:e0241262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ognibene A, Lorubbio M, Magliocca P, et al. Elevated monocyte distribution width in COVID‐19 patients: the contribution of the novel sepsis indicator. Clin Chim Acta. 2020;509:22‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng X, Xing H, Wei Y, et al. Monocyte volumetric parameters and lymph index are increased in SARS‐CoV‐2 infection. Int J Lab Hematol. 2020;42:e266‐e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frater JL, Zini G, d'Onofrio G, Rogers HJ COVID‐19 and the clinical hematology laboratory. Int J Lab Hematol. 2020;42(Suppl 1):11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinez FO, Combes TW, Orsenigo F, Gordon S Monocyte activation in systemic Covid‐19 infection: assay and rationale. EBioMedicine. 2020;59:102964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G Hyperinflammation and derangement of renin‐angiotensin‐aldosterone system in COVID‐19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data available on request from the authors.


