Abstract
Study Design:
In vitro cadaveric biomechanical study.
Objective:
Biomechanically characterize a novel lateral lumbar interbody fusion (LLIF) implant possessing integrated lateral modular plate fixation (MPF).
Methods:
A human lumbar cadaveric (n = 7, L1-L4) biomechanical study of segmental range-of-motion stiffness was performed. A ±7.5 Nċm moment was applied in flexion/extension, lateral bending, and axial rotation using a 6 degree-of-freedom kinematics system. Specimens were tested first in an intact state and then following iterative instrumentation (L2/3): (1) LLIF cage only, (2) LLIF + 2-screw MPF, (3) LLIF + 4-screw MPF, (4) LLIF + 4-screw MPF + interspinous process fixation, and (5) LLIF + bilateral pedicle screw fixation. Comparative analysis of range-of-motion outcomes was performed between iterations.
Results:
Key biomechanical findings: (1) Flexion/extension range-of-motion reduction with LLIF + 4-screw MPF was significantly greater than LLIF + 2-screw MPF (P < .01). (2) LLIF with 2-screw and 4-screw MPF were comparable to LLIF with bilateral pedicle screw fixation in lateral bending and axial rotation range-of-motion reduction (P = 1.0). (3) LLIF + 4-screw MPF and supplemental interspinous process fixation range-of-motion reduction was comparable to LLIF + bilateral pedicle screw fixation in all directions (P ≥ .6).
Conclusions:
LLIF with 4-screw MPF may provide inherent advantages over traditional 2-screw plating modalities. Furthermore, when coupled with interspinous process fixation, LLIF with MPF is a stable circumferential construct that provides biomechanical utility in all principal motions.
Keywords: lumbar, degenerative disc disease, fusion, lumbar interbody fusion, fixation, PEEK cages, biomechanics, cadaver, XLIF, sagittal balance
Introduction
Lateral lumbar interbody fusion (LLIF) is an effective intervention when treating pain secondary to disc degenerative and segmental instability.1,2 The retroperitoneal transpsoas approach (lateral access) preserves the anterior and posterior stabilizing structures, while affording liberal disc removal and placement of a wide cage spanning the apophyseal ring. Given such inherent structural benefits, it has been proposed that extensive and/or invasive posterior fixation could be unnecessary with LLIF.3-18
Accordingly, increased consideration has been given to alternative, less invasive means to achieving stability with the lateral access technique. Two techniques of particular interest have been lateral plating and interspinous process fixation (ISPF).3-5,7-9,14,16,18-33 Lateral plating does not extend the intraoperative footprint as the plate is placed through the same surgical corridor as the interbody cage and provides immediate rigidity to the anterior column in the axial and coronal planes. ISPF requires an additional posterior midline incision at the index level (though less invasive than that required for pedicle screw fixation), provides robust stability in the sagittal plane, and largely preserves the posterior paraspinal structures. While both lateral plating and ISPF techniques have demonstrated utility when used independently, the most advantageous biomechanical outcomes have been observed when used in conjunction.8,9,18 The favorable rigidity of the plate in the axial and coronal planes, coupled with the sagittal stability of ISPF, creates a synergistic circumferential construct.
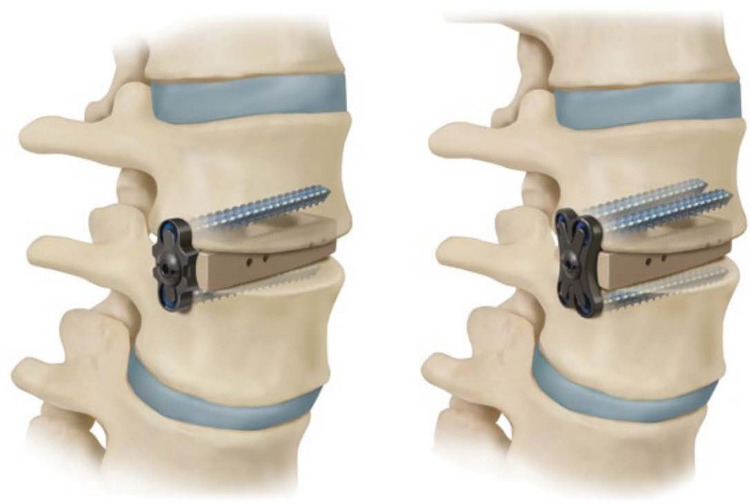
Recent introduction of a novel LLIF cage possessing integrated lateral modular plate fixation (MPF) may further enhance the structural rigidity (Figure 1). MPF, which consolidates the cage and plate into a singular entity, creates a continuous rigid body about the index level. However, the extent to which this continuous design facilitates segmental rigidity is not yet understood in the literature.
Figure 1.
Lateral lumbar interbody fusion cage with integrated modular plate fixation (2-screw—left; 4-screw—right).
The purpose of this biomechanical study was to characterize MPF in LLIF, with and without supplemental ISPF, utilizing traditional LLIF with bilateral pedicle screw fixation (BPSF) as a control. The primary objective was to determine whether LLIF + MPF + ISPF can provide circumferential rigidity comparable to that of LLIF+BPSF, and a secondary objective was to assess LLIF + MPF as a stand-alone construct.
Materials and Methods
Specimen Preparation
Seven (n = 7) fresh human cadaveric lumbar spines (L1-L4) were included in this study (3 females, 4 males; age 29-62 years, mean 46 ± 11 years; body mass index 18 to 37 kg/m2, mean 26 ± 6 kg/m2). Anatomical structural integrity was confirmed via standard anteroposterior and lateral radiographs. Any specimens exhibiting previous lumbosacral surgery or anatomical discrepancy were excluded. Bone mineral density (BMD) evaluations were performed by dual-energy X-ray absorptiometry (DEXA) scans. Mean BMD was 0.969 g/cm2 (range: 0.77-1.07 g/cm2).
Each spine was thawed at room temperature, the L1-L4 column was isolated, and all residual musculature and adipose tissue were removed while preserving all ligamentous structures. Of note, the decision for a 3-level specimen was done in accordance with the recommendation of Wilke et al.34 Doing so would allow for the cranial and caudal potting to be applied to vertebral bodies that would receive no instrumentation and allow one adjacent native intervertebral disc above and below the instrumented segment. The cephalad and caudal vertebrae of each specimen were potted for subsequent attachment to the test apparatus. Standard wood screws were placed in the L1 and L4 vertebral bodies and anchored within high-strength resin. The specimens were hydrated, wrapped in saline-soaked gauze, sealed in air-tight plastic bags, and frozen at –20°C until approximately 10 hours before testing, at which time they were thawed at room temperature (∼25°C). At this time, specimens were instrumented with screws at each vertebral body (L1-L4) to which optoelectronic triad markers would later be affixed. Placement of these screws was performed to ensure that screw trajectories of the subsequent constructs would be uninhibited. Prior to instrumentation, the kinematics of all intact specimens were characterized via biomechanical testing in a spine simulator (described further in the section Testing and Motion Analysis).
A fellowship-trained spinal surgeon performed all discectomy and instrumentation procedures at L2-L3. It should be noted that the instrumentation level of L2-3 was selected to ensure that all available test specimens offered preserved and intact adjacent level intervertebral discs and vertebral bodies. The decision to select a specimen length and instrument level that ensured optimal adjacent level integrity was consistent with recommendations by Wilke et al.34 However, the authors acknowledge that the level of L2-3 is a less common operative level in lumbar fusion and possesses its own inherent biomechanical behavior in comparison with adjacent vertebral segments. Hence, consideration of study outcomes should be done within this context. Future evaluation of biomechanical performance at lower lumbar segments is warranted.
For each discectomy, the access window was centered in the anterior half of the disc space, the posterior annulus was left intact, and the endplates were preserved. Five sequential instrumentation iterations were performed following discectomy and include: LLIF cage only (LCage) (Timberline MPF Lateral Fusion System; Zimmer Biomet Spine, Westminster, CO, USA); LLIF+2-screw MPF (2S-MPF) (Figure 2); LLIF + 4-screw MPF (4S-MPF) (Figure 3); 4S-MPF + interspinous process fixation (4S-MPF + ISPF) (Figure 4); and LCage + bilateral pedicle screw fixation (LLIF + BPSF). A single MPF-compatible LCage was used in all instrumentation iterations for a given specimen, and the profile and height of each LCage was selected to best fit each specimen’s anatomy. Of note, the lateral cage utilized in this study was made of PEEK-OPTIMA polymer (Invibio; West Conshohocken, PA, USA). At the time of study, available cage footprints existed in 18 and 22 mm widths, with accompanying lengths of 45, 50, 55, and 60 mm, and heights of 8, 10, 12, and 14 mm. Built-in cage lordosis of 0°, 8°, and 14° was available in all cage profiles. A standard cage lordosis of 8° was chosen in this study for consistency sake. The cage possessed no expandable capabilities.
Figure 2.
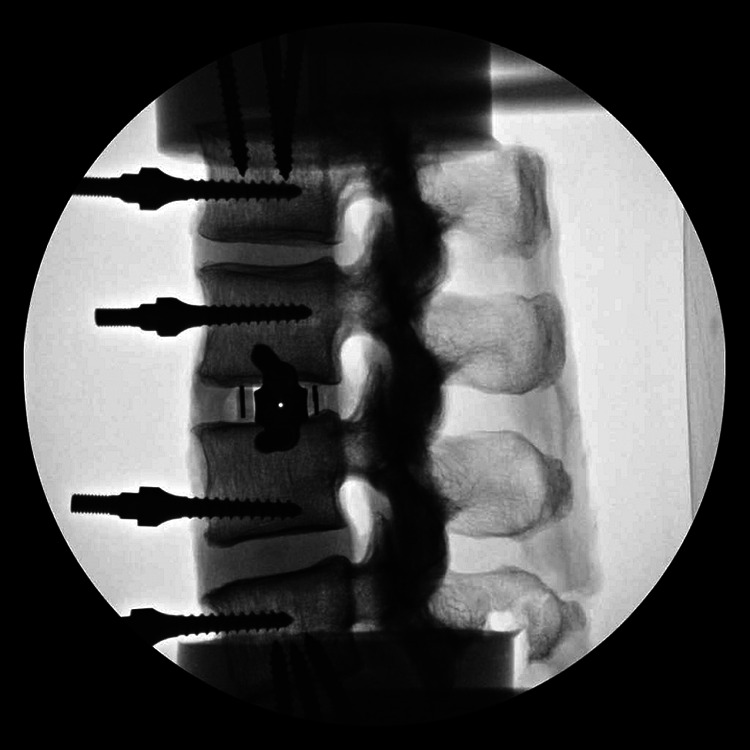
Lateral lumbar interbody fusion cage with 2-screw integrated modular plate fixation (lateral fluoroscopic image).
Figure 3.
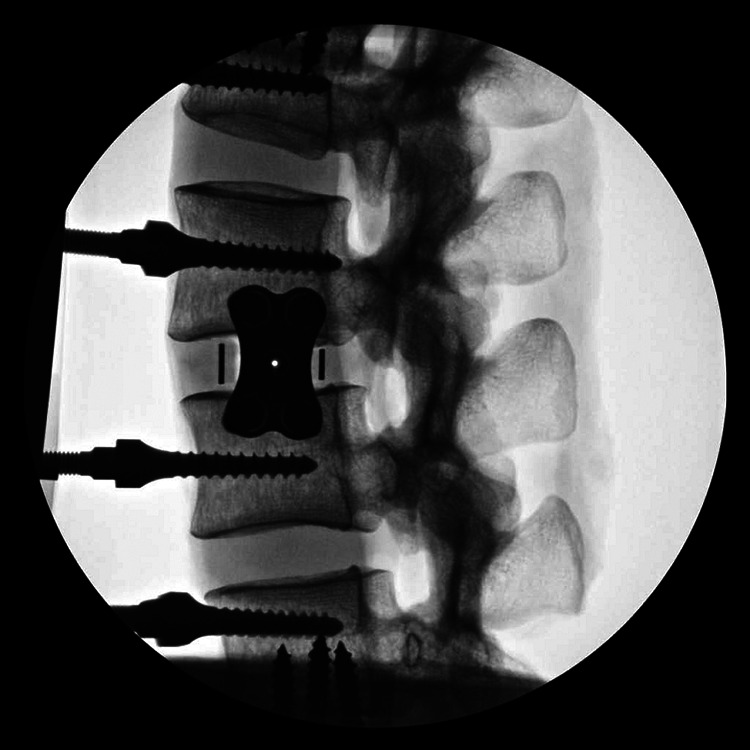
Lateral lumbar interbody fusion cage with 4-screw integrated modular plate fixation (lateral fluoroscopic image).
Figure 4.
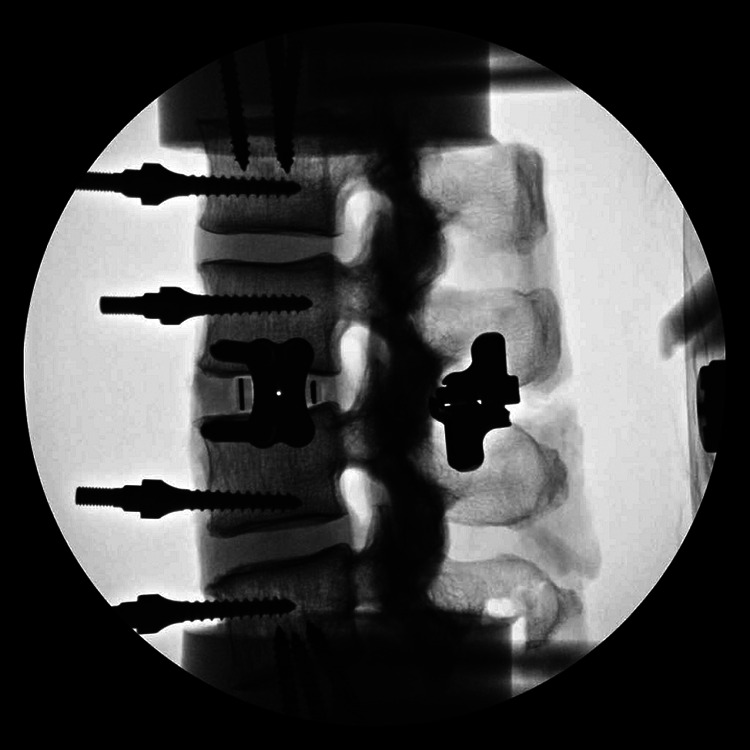
Lateral lumbar interbody fusion cage with 4-screw integrated modular plate fixation and interspinous process fixation (lateral fluoroscopic image).
MPF screw holes (2- and 4-S) were prepared using the system’s standard drill and awl technique (Timberline MPF Lateral Fusion System; Zimmer Biomet Spine, Westminster, CO, USA). Drill guides were used to ensure appropriate screw advancement and trajectory, and bicortical screw purchase was always obtained if possible.
The ISPF device (Alpine XC Adjustable Fusion System; Zimmer Biomet Spine, Westminster, CO, USA) was placed anteriorly within the interspinous space to grip the laminar junction per surgical technique. Both ISPF and MPF would then be removed to allow for BPSF instrumentation. However, the lateral screws were maintained during the initial establishment of pedicle screw trajectory such that overlapping trajectories were prevented. Doing so would ensure completely new bone stock for pedicle screw purchase. Final rod placement and fixation was then performed once lateral plate and screws was removed. BPSF was performed via standard technique (Silverton Spinal Fixation System; Zimmer Biomet Spine, Westminster, CO, USA). The authors would like to acknowledge that the iterative nature of the testing sequence may possess inherent limitation in the preservation of specimen quality, particularly the quality available for final pedicle screw fixation. To best ensure that specimen quality was preserved for each iterative instrumentation cycle, the authors employed protective/preventative measures. First, as previously noted, all specimens were screened for bone quality prior to testing via DEXA scan. By ensuring healthy bone in all specimens, the authors believed that all screw trajectories could be achieved without subsequent influence or failure. Second, as previously noted, pedicle screw placement was done under fluoroscopic guidance while the lateral screws were still intact, such that their trajectories would not overlap and diminish pedicle screw purchase by utilizing previously tapped bone stock. Last, the authors believe that by utilizing a modest pure-loading moment of 7.5 Nċm, at a loading rate of 1 deg/s, soft tissue and boney integrity would be preserved during each iterative cycle. Wilke et al34 assert that under these conditions specimens can be deformed without a considerable effect on the results.
Anteroposterior and lateral fluoroscopic imaging was employed during all device implantations.
Testing and Motion Analysis
A 6 degree-of-freedom (6-DOF) kinematic spine simulator (Bionix Spine Kinematics System, MTS Corporation, Eden Prairie, MN, USA) was used to apply nonconstraining, nondestructive, pure-moment loading in the three principal motion directions: flexion/extension (FE), left/right lateral bending (LB), and axial rotation (AR) (Figure 5).34 After each surgical intervention, specimens were mounted within the test apparatus at the L1 and L4 pots. The caudal pot attachment afforded translation in the X-Y plane via a translating table. A maximum loading moment of ±7.5 Nċm was applied at rate of 1 deg/s for 3 consecutive cycles of FE, then LB, and finally AR.34
Figure 5.
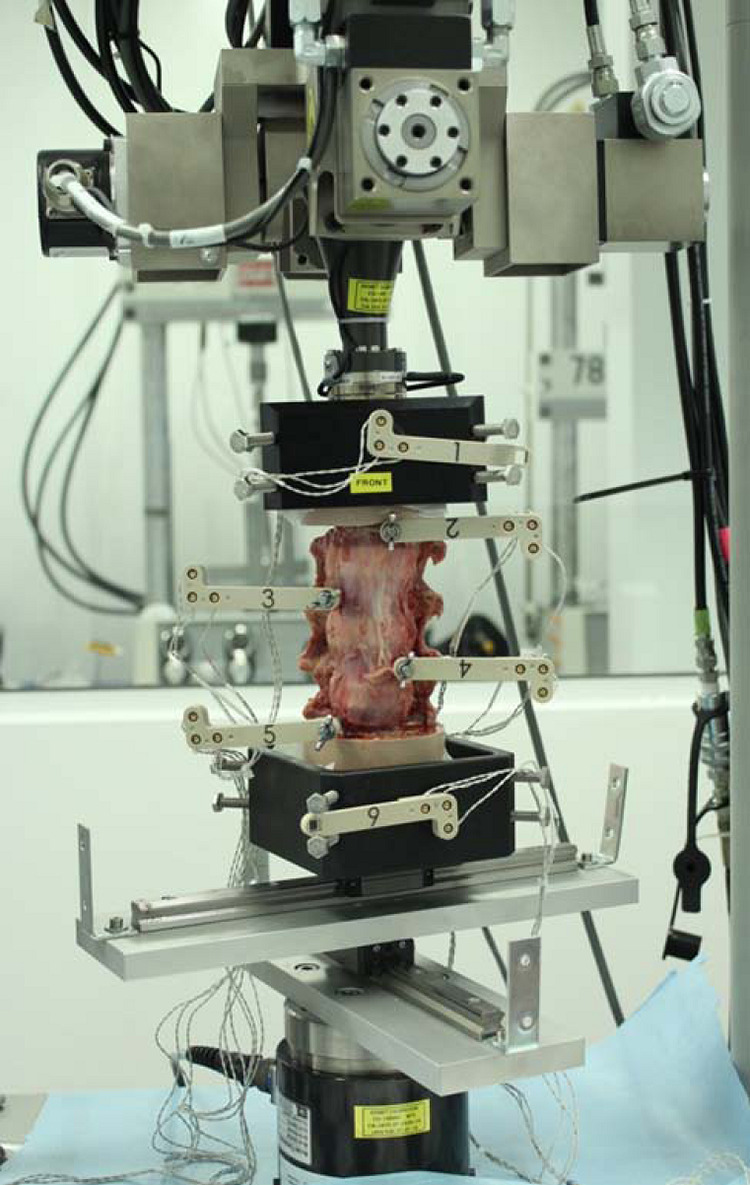
Six degree-of-freedom kinematic testing machine (Bionix Spine Kinematics System, MTS Corporation, Eden Prairie, MN, USA) with intact specimen attached.
Three-dimensional motion of each vertebral body was recorded, in all cycles, relative to their adjacent levels (L1-L2, L2-L3, L3-L4), as well as the cumulative specimen (L1-L4) using an optoelectronic motion measurement system (Optotrak Certus Motion Capture System; Northern Digital Inc, Waterloo, Ontario, Canada). Each optoelectronic triad maker was coupled to its respective level to establish a local coordinate system. Additionally, 2 optoelectronic markers were rigidly attached to the static test frame to define the +X and +Y axes, and subsequently the +Z axis. Data acquired during the third test cycle was used for statistical analyses.34 Range of motion (ROM) reduction relative to intact conditions were subsequently calculated.
Statistical Analysis
Of note, A pretest power analysis was not performed given a lack of historical data and clinical rationale for choosing an effect size. A repeated-measures analysis of variance and Bonferonni post hoc tests (P < .05) were performed to determine significance in ROM reductions between constructs. Pairwise comparisons were made between all constructs.
Results
ROM outcomes are summarized in Table 1. Please note that all ROM values reported at specific to the single instrumented segment.
Table 1.
Range-of-Motion Measurements.
| Range of Motion (deg) Mean ± SD | |||
|---|---|---|---|
| Construct | Flexion/Extension | Lateral Bending | Axial Rotation |
| Intact | 4.1 ± 3.1 | 5.0 ± 1.4 | 1.4 ± 0.6 |
| LCage | 1.5 ± 1.1 | 2.0 ± 1.6 | 1.1 ± 0.4 |
| 2S-MPF | 1.3 ± 1.0 | 0.7 ± 0.5 | 0.8 ± 0.3 |
| 4S-MPF | 1.0 ± 0.8 | 0.6 ± 0.4 | 0.7 ± 0.4 |
| 4S-MPF + ISPF | 0.3 ± 0.3 | 0.5 ± 0.4 | 0.6 ± 0.2 |
| LLIF + BPSF | 0.3 ± 0.3 | 0.6 ± 0.5 | 0.7 ± 0.2 |
Abbreviations: LLIF, lateral lumbar interbody fusion; LCage, traditional LLIF cage; 2S-MPF, LLIF with 2-screw modular plate fixation; 4S-MPF, LLIF with 4-screw modular plate fixation; 4S-MPF + ISPF, LLIF with 4-screw modular plate fixation and interspinous process fixation; LLIF + BPSF, LLIF cage with bilateral pedicle screw fixation.
Flexion/Extension
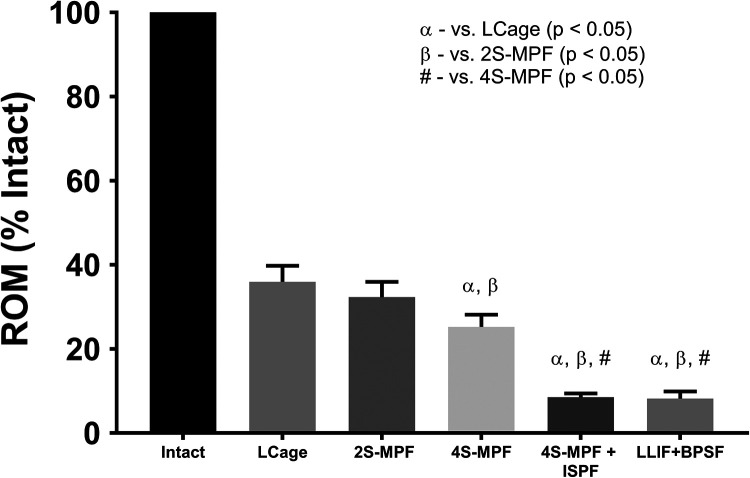
All constructs demonstrated significant reductions in ROM (Figure 6) in FE when compared with the intact state (P < .0001) (% intact: LCage 36%; 2S-MPF 32%; 4S-MPF 25%; 4S-MPF + ISPF 8%; LCage + BPSF 8%). When comparing fixation constructs, significant differences were observed between all but 2 iterative ROM. The FE ROM reductions of the LCage (36%) and 2S-MPF (32%) constructs were not significantly different (P = .7); nor were the reductions of the 4S-MPF + ISPF (8%) and LCage + BPSF constructs (8%) (P = 1.0).
Figure 6.
Mean range of motion (ROM), relative to intact conditions, when loaded in flexion-extension under a pure moment of 7.5 Nċm. Bars represent the mean and error bars are standard deviation. Symbols denote significant differences (P < .05) between groups according to a repeated-measures analysis of variance with Bonferonni’s correction for multiple comparisons.
Lateral Bending
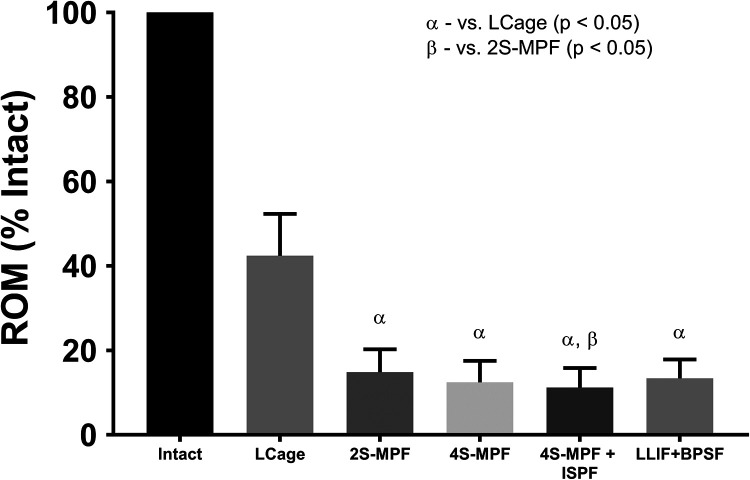
All constructs demonstrated significant reductions in ROM (Figure 7) in LB than the intact state (P < .0001) (% intact: LCage 42%; 2S-MPF 15%; 4S-MPF 12%; 4S-MPF + ISPF 11%; LCage + BPSF 13%). When comparing fixation constructs, significant differences in ROM were observed between LCage construct and all subsequent iterations (P < .0001), as well as between the 2S-MPF (15%) and 4S-MPF + ISPF (11%) constructs (P < .03).
Figure 7.
Mean range of motion (ROM), relative to intact conditions, when loaded in lateral bending under a pure moment of 7.5 Nċm. Bars represent the mean and error bars are standard deviation. Symbols denote significant differences (P < .05) between groups according to a repeated-measures analysis of variance with Bonferonni’s correction for multiple comparisons.
Axial Rotation
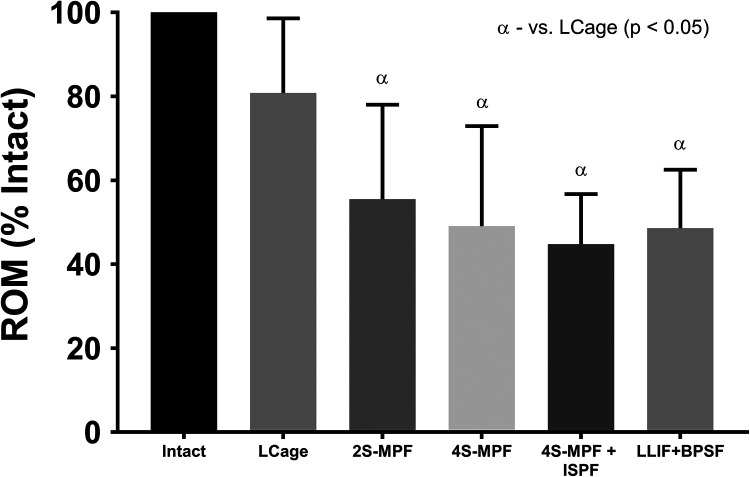
All MPF and BPSF constructs demonstrated significant ROM reduction in AR (Figure 8) when compared with the intact state (P < .0001); however, ROM of LCage (81%) was not significantly different from intact conditions (P = 1.0) (% intact: LCage 81%; 2S-MPF 56%; 4S-MPF 49%; 4S-MPF + ISPF 45%; LCage + BPSF 49%). When comparing fixation constructs, significant differences in ROM were observed between the LCage construct and all subsequent iterations (P ≤ .01).
Figure 8.
Mean range of motion (ROM), relative to intact conditions, when loaded in axial rotation under a pure moment of 7.5 Nċm. Bars represent the mean and error bars are standard deviation. Symbols denote significant differences (P < .05) between groups according to a repeated-measures analysis of variance with Bonferonni’s correction for multiple comparisons.
Discussion
The lateral access technique has received growing consideration as an effective minimally disruptive procedure in treating the symptomatic lumbar spine.35 While early accounts of the technique were largely synonymous with use of supplemental BPSF, a furthered understanding of the inherent structural benefits of the technique has resulted in diverse philosophy in the use, or nonuse, of adjunctive fixation.
A multitude of cadaveric biomechanical assessments have been performed to better characterize the various alternative constructs available within the LLIF model. Adjunctive unilateral PSF (UPSF), facet screw fixation (FSF), facet wedging, integrated lateral screws, lateral plating, and ISPF have all been studied.3-18 The latter 2 fixation modalities, lateral plating and ISPF, were of particular interest in this study given their synergistic capabilities. While Fogel et al8,9 and Reis et al18 have previously assessed LLIF + ISPF in vitro; plating was traditional independent fixation only. The aim of this study was to characterize the biomechanical utility of a novel integrated MPF LLIF construct, with and without adjunctive ISPF.
ROM Reductions
The LCage provided significant reductions in ROM in both the sagittal and coronal planes; however, limited ROM resistance in the axial plane was readily apparent. This is consistent with the literature, in which diminished AR rigidity is common with a stand-alone LCage.3-8,10-18 However, given the modest amount of axial angular motion inherent to the lumbar spine, ROM reduction in AR is typically of secondary concern. While the authors acknowledge the validity of such assertions around axial plane rigidity, consideration must still be given to the potential for cage subsidence and the amount of slip reduction necessary when performing stand-alone LLIF.19,36-38 Accordingly, characterization of stand-alone LLIF utility was deemed outside the scope of this article. The LCage construct was included to provide a baseline for subsequent instrumented iterations.
In considering supplemental fixation, the authors first assessed MPF without posterior instrumentation. Both the 2S- and 4S-MPF constructs achieved significant reductions in ROM relative to intact conditions in all directions. Furthermore, both iterations significantly exceeded the stand-alone LCage with regard to ROM reduction in LB and AR, exemplifying the inherent benefit of lateral plating in the axial and coronal planes. While similar trends have been captured in the literature with lateral plating, no study has characterized a plated LLIF construct capable of LB motion reduction greater than 85%.3-5,16,18 The 2S- and 4S-MPF constructs achieved LB ROM reductions of 85.8% and 88.4%, respectively. Only Hartensauer et al,12 Kretzer et al,13 and Nayak et al16 have characterized LLIF constructs with LB ROM rigidity greater than the stand-alone 4S-MPF iteration, all of which included posterior instrumentation.
While not as unprecedented as the outcomes in LB, ROM reduction of the stand-alone MPF constructs in AR were also of particular note (2S-MPF 44%; 4S-MPF 51%), comparing favorably to reported traditional lateral plate constructs (single-level ROM reduction: 40.5% to 55.6%) and similarly to an integrated lateral screw stand-alone construct (single-level ROM reduction: 56.8%).3-5,18 The stand-alone MPF iterations also performed notably well in comparison to the LLIF + BPSF study control (ROM reduction: 51%), demonstrating an inherent benefit of direct anterior column fixation. Greater reductions in AR ROM have been captured in the literature with LLIF + BPSF (single-level ROM reduction: 15% to 72.1%); although, such values are still comparable to those in this study.3-7,11-13 Not explored in this study, facet fixation has been shown to be advantageous when seeking rigidity in the axial plane, achieving reductions of 26.7% to 81.9%.3,6,12,13 These trends can be largely attributed to the robust locking of the middle column with facet fixation.
Outcomes in FE with the stand-alone MPF constructs were also clinically advantageous (ROM Reduction: 2S 67.9%; 4S 74.9%). Compared with the literature, FE outcomes with stand-alone MPF appeared favorable to LLIF independent plated (only) constructs (single-level ROM reduction: 54.2% to 72.9%).3-5,8,18 Only Basra et al3 have characterized a stand-alone lateral construct (LCage + integrated lateral screws) demonstrating FE ROM reduction (74.9%) equal to that of the 4S-MPF iteration. These similarities further the ideal that integrated fixation modalities may enhance the rigidity of independent or isolated LLIF constructs in the axial and coronal planes.
Pertaining to the primary study comparison, the addition of ISPF to the 4S-MPF construct proved to be particularly beneficial in FE, enhancing sagittal ROM (ROM reduction: 92%) of the 4S-MPF construct by 17%.
Clinical Implications
Both stand-alone (instrumented) and circumferential LLIF applications come with respective advantages. Stand-alone application avoids the posterior structures entirely, providing indirect decompression and disc height restoration, while circumferential application offers furthered sagittal stability and correction.
In this study, MPF demonstrated inherent benefits that can further both stand-alone and circumferential LLIF techniques. By providing comparable rigidity in AR and LB to that of BPSF, the MPF iterations significantly diminished the need for posterior fixation in those respective planes. These capabilities are particularly beneficial when sagittal correction is limited or unnecessary.
Whether assembled prior to insertion or in situ, the integrated design of the MPF construct may also support intraoperative ease of plate placement and plate alignment optimization not seen with traditional independent plates. In characterizing perioperative outcomes with LLIF + MPF, DenHaese et al23 reported operative time, fluoroscopy time, and blood loss with MPF that was not differing from those outcomes associated with placing a traditional cage alone.23 While these outcomes may not be intuitive, given that use of additional hardware is associated with greater intraoperative resources, consideration must be given to the singular entity design of MPF in providing early anterior column stability and ensuring optimal cage placement/advancement with minimal imaging.
In further considering the potential marginal differences in intraoperative demand, the utility of ISPF as an adjunct to 4S-MPF becomes largely apparent. While circumferential rigidity was shown to be comparable between 4S-MPF + ISPF and LLIF + PSF, the less invasive and less demanding nature of ISPF over PSF makes the novel technique potentially more advantageous.23 Accordingly, both DenHaese et al23 and Kim et al29 have demonstrated greater improvements in patient reported Oswestry Disability Index scores at 1.5, 3, 6, and 12 months postoperatively with 4S-MPF + ISPF and LLIF + ISPF as compared with LLIF + PSF.
Last, consideration must be given to the anatomical and ergonomic value of stand-alone MPF and MPF + ISPF techniques. If adjacent level posterior instrumentation is already present, the ability to use PSF may be limited. However, if the spinous processes are intact, ISPF can be readily placed without obstruction by adjacent level hardware. If the adjacent spinous processes have been removed or compromised, the stand-alone MPF construct can still be leveraged, preserving/avoiding a secondary posterior approach. Use of ISPF in LLIF also allows for the subject to remain in the lateral decubitus position without a need for repositioning.
Study Limitations
Study protocol limitations were consistent with multiple reports in the literature. The iterative testing sequence was not randomized. This was done to avoid excessive removal and reinsertion of fixation screws. Given that a singular lateral cage was used throughout, coupled with a small loading-moment, tissue fatigue and/or unwanted intervertebral distraction was diminished across iterations. Additionally, the cadaveric model did account for degenerative changes or instability. While this was done to ensure consistency across specimens, future work may be warranted in which such variables are built into the model. Finally, while not an inherent limitation of study design, the exclusion of a traditional independent/stand-alone lateral plate iteration does diminish the comparative nature of the outcomes. A traditional plate was not included in the current study given concern for additional screw tapping and placement that could conflict/compromise with the screw placement of the modular plate. Future evaluation in which a direct comparison between plate designs is warranted.
Conclusion
This study demonstrated that a LLIF + MPF construct can provide significant ROM reduction and segmental stability in all motion directions. Trends observed with the LLIF + MPF device demonstrated inherent stabilization capabilities not seen previously with traditional stand-alone techniques. Additionally, supplementation of the LLIF + MPF construct with ISPF proved to be highly synergistic, performing on the same magnitude as LLIF + BSPF. The ability to provide circumferential stability while avoiding the exposure associated with internal posterior screw fixation is clinically advantageous. In conclusion, the authors assert that the LLIF + MPF + ISPF technique may present as a less-invasive, yet structurally robust, alternative to circumferential LLIF + BPSF in single-level application. Clinical validation of these results with regards to the patient quality-of-life outcomes and fusion rates is necessary to further facilitate robust clinical recommendations
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Zimmer Biomet Spine (funding source) manufactures one or more the devices examined in this study. Three authors (AG, CF, and SF) are salaried employees of the funding source. One author (RD) has received royalties (specific to one of the devices examined in this study) from the funding source. Two authors (RD, RP) have received consulting fees from the funding source.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Zimmer Biomet Spine.
ORCID iD: Chris Ferry, MS  https://orcid.org/0000-0002-5594-088X
https://orcid.org/0000-0002-5594-088X
References
- 1. Joseph JR, Smith BW, La marca F, Park P. Comparison of complication rates of minimally invasive transforaminal lumbar interbody fusion and lateral lumbar interbody fusion: a systematic review of the literature. Neurosurg Focus. 2015;39:E4. [DOI] [PubMed] [Google Scholar]
- 2. Lehmen JA, Gerber EJ. MIS lateral spine surgery: a systematic literature review of complications, outcomes, and economics. Eur Spine J. 2015;24(suppl 3):287–313. [DOI] [PubMed] [Google Scholar]
- 3. Basra S, Bucklen B, Muzumdar A, Khalil S, Gudipally M. A novel lateral lumbar integrated plate-spacer interbody implant: in vitro biomechanical analysis. Spine J. 2015;15:322–328. [DOI] [PubMed] [Google Scholar]
- 4. Bess RS, Cornwall GB, Vance RE, et al. Biomechanics of lateral arthrodesis. In: Goodrich JA, Volcan IJ, eds. eXtreme Lateral Interbody Fusion (XLIF). St. Louis, MO: Quality Medical Publishing; 2008:31–40. [Google Scholar]
- 5. Cappuccino A, Cornwall GB, Turner AW, et al. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine (Phila Pa 1976). 2010;35(26 suppl):S361–S367. [DOI] [PubMed] [Google Scholar]
- 6. Chin KR, Newcomb AG, Reis MT, et al. Biomechanics of posterior instrumentation in L1-L3 lateral interbody fusion: pedicle screw rod construct vs. transfacet pedicle screws. Clin Biomech (Bristol, Avon). 2016;31:59–64. [DOI] [PubMed] [Google Scholar]
- 7. Doulgeris JJ, Aghayev K, Gonzalez-blohm SA, Lee WE, 3rd, Vrionis FD. Biomechanical comparison of an interspinous fusion device and bilateral pedicle screw system as additional fixation for lateral lumbar interbody fusion. Clin Biomech (Bristol, Avon). 2015;30:205–210. [DOI] [PubMed] [Google Scholar]
- 8. Fogel GR, Parikh RD, Ryu SI, Turner AW. Biomechanics of lateral lumbar interbody fusion constructs with lateral and posterior plate fixation: laboratory investigation. J Neurosurg Spine. 2014;20:291–297. [DOI] [PubMed] [Google Scholar]
- 9. Fogel GR, Turner AW, Dooley ZA, Cornwall GB. Biomechanical stability of lateral interbody implants and supplemental fixation in a cadaveric degenerative spondylolisthesis model. Spine (Phila Pa 1976). 2014;39:E1138–E1146. [DOI] [PubMed] [Google Scholar]
- 10. Gandhi A, Ferry C, DenHaese RP, Karahalios D, Chang S, Cappuccino A., Alpine XC. adjustable fusion system versus pedicle screw fixation: a biomechanical study of interbody cage load and segmental lordosis in LLIF. Paper presented at: Society for Minimally Invasive Spine Surgery Global Forum Meeting; September 19-21, 2014; Miami, FL. [Google Scholar]
- 11. Gonzalez-Blohm SA, Doulgeris JJ, Aghayev K, Lee WE, 3rd, Laun J, Vrionis FD. In vitro evaluation of a lateral expandable cage and its comparison with a static device for lumbar interbody fusion: a biomechanical investigation. J Neurosurg Spine. 2014;20:387–395. [DOI] [PubMed] [Google Scholar]
- 12. Hartensuer R, Riesenbeck O, Schulze M, et al. Biomechanical evaluation of the Facet Wedge: a refined technique for facet fixation. Eur Spine J. 2014;23:2321–2329. [DOI] [PubMed] [Google Scholar]
- 13. Molina C, Kretzer RM, Hu N, et al. A comparative biomechanical analysis of stand alone versus facet screw and pedicle screw augmented lateral interbody arthrodesis: an in vitro human cadaveric model. J Spinal Disord Tech. 2014;27:40–47. [DOI] [PubMed] [Google Scholar]
- 14. Laws CJ, Coughlin DG, Lotz JC, Serhan HA, Hu SS. Direct lateral approach to lumbar fusion is a biomechanically equivalent alternative to the anterior approach: an in vitro study. Spine (Phila Pa 1976). 2012;37:819–825. [DOI] [PubMed] [Google Scholar]
- 15. Mantell M, Cyriac M, Haines CM, Gudipally M, O’Brien JR. Biomechanical analysis of an expandable lateral cage and a static transforaminal lumbar interbody fusion cage with posterior instrumentation in an in vitro spondylolisthesis model. J Neurosurg Spine. 2016;24:32–38. [DOI] [PubMed] [Google Scholar]
- 16. Nayak AN, Gutierrez S, Billys JB, Santoni BG, Castellvi AE. Biomechanics of lateral plate and pedicle screw constructs in lumbar spines instrumented at two levels with laterally placed interbody cages. Spine J. 2013;13:1331–1338. [DOI] [PubMed] [Google Scholar]
- 17. Pimenta L, Turner AW, Dooley ZA, Parikh RD, Peterson MD. Biomechanics of lateral interbody spacers: going wider for going stiffer. Scientific World Journal. 2012;2012:381814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reis MT, Reyes PM, Bse, et al. Biomechanical evaluation of lateral lumbar interbody fusion with secondary augmentation. J Neurosurg Spine. 2016;25:720–726. [DOI] [PubMed] [Google Scholar]
- 19. Ahmadian A, Bach K, Bolinger B, et al. Stand-alone minimally invasive lateral lumbar interbody fusion: multicenter clinical outcomes. J Clin Neurosci. 2015;22:740–746. [DOI] [PubMed] [Google Scholar]
- 20. Alimi M, Hofstetter CP, Cong GT, et al. Radiological and clinical outcomes following extreme lateral interbody fusion. J Neurosurg Spine. 2014;20:623–635. [DOI] [PubMed] [Google Scholar]
- 21. Berjano P, Balsano M, Buric J, Petruzzi M, Lamartina C. Direct lateral access lumbar and thoracolumbar fusion: preliminary results. Eur Spine J. 2012;21(suppl 1):S37–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28:E8. [DOI] [PubMed] [Google Scholar]
- 23. DenHaese R, Hill C, Strenge B, et al. Lateral lumbar interbody fusion with modular plate fixation: initial experience with a novel technology. Intra-op and 1-year outcomes. Paper presented at: Society of Minimally Invasive Spine Surgery (SMISS) Global Forum; November 5-7, 2015; Las Vegas, NV. [Google Scholar]
- 24. Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976). 2010;35(26 suppl):S322–S330. [DOI] [PubMed] [Google Scholar]
- 25. Kepler CK, Sharma AK, Huang RC. Lateral transpsoas interbody fusion (LTIF) with plate fixation and unilateral pedicle screws: a preliminary report. J Spinal Disord Tech. 2011;24:363–367. [DOI] [PubMed] [Google Scholar]
- 26. Le TV, Smith DA, Greenberg MS, Dakwar E, Baaj AA, Uribe JS. Complications of lateral plating in the minimally invasive lateral transpsoas approach. J Neurosurg Spine. 2012;16:302–307. [DOI] [PubMed] [Google Scholar]
- 27. Le TV, Vivas AC, Dakwar E, Baaj AA, Uribe JS. The effect of the retroperitoneal transpsoas minimally invasive lateral interbody fusion on segmental and regional lumbar lordosis. Scientific World Journal. 2012;2012:516706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson RD, Valore A, Villaminar A, Comisso M, Balsano M. Pelvic parameters of sagittal balance in extreme lateral interbody fusion for degenerative lumbar disc disease. J Clin Neurosci. 2013;20:576–581. [DOI] [PubMed] [Google Scholar]
- 29. Kim K, DenHaese R, Hill C, et al. Interspinous process fixation versus pedicle screw fixation in circumferential fusion: outcomes from a prospective randomized multi-center trial. Paper presented at: European Academy of Neurosurgeons Annual Meeting; October 18-21, 2015; Madrid, Spain. [Google Scholar]
- 30. Malham GM, Ellis NJ, Parker RM, Seex KA. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. Scientific World Journal. 2012;2012:246989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phillips FM, Isaacs RE, Rodgers WB, et al. Adult degenerative scoliosis treated with XLIF: clinical and radiographical results of a prospective multicenter study with 24-month follow-up. Spine (Phila Pa 1976). 2013;38:1853–1861. [DOI] [PubMed] [Google Scholar]
- 32. Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion: clinical and radiographic outcomes at 1 year: a preliminary report. J Spinal Disord Tech. 2011;24:242–250. [DOI] [PubMed] [Google Scholar]
- 33. Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976). 2010;35(26 suppl):S302–S311. [DOI] [PubMed] [Google Scholar]
- 34. Wilke HJ, Wenger K, Claes L. Testing criteria for spinal implants: recommendations for the standardization of in vitro stability testing of spinal implants. Eur Spine J. 1998;7:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6:435–443. [DOI] [PubMed] [Google Scholar]
- 36. Tempel ZJ, Gandhoke GS, Okonkwo DO, Kanter AS. Impaired bone mineral density as a predictor of graft subsidence following minimally invasive transpsoas lateral lumbar interbody fusion. Eur Spine J. 2015;24(suppl 3):414–419. [DOI] [PubMed] [Google Scholar]
- 37. Nemani VM, Aichmair A, Taher F, et al. Rate of revision surgery after stand-alone lateral lumbar interbody fusion for lumbar spinal stenosis. Spine (Phila Pa 1976). 2014;39:E326–E331. [DOI] [PubMed] [Google Scholar]
- 38. Marchi L, Oliveira L, Amaral R, et al. Lateral interbody fusion for treatment of discogenic low back pain: minimally invasive surgical techniques. Adv Orthop. 2012;2012:282068. [DOI] [PMC free article] [PubMed] [Google Scholar]