Abstract
Study Design:
Systematic review.
Objective:
Spinal cord injuries (SCIs) resulting in motor deficits can be devastating injuries resulting in millions of health care dollars spent per incident. Nonsteroidal anti-inflammatory drugs (NSAIDs) are a potential class of drugs that could improve motor function after an SCI. This systematic review utilizes PRISMA guidelines to evaluate the effectiveness of NSAIDs for SCI.
Methods:
PubMed/MEDLINE, CINAHL, PsycINFO, Embase, and Scopus were reviewed linking the keywords of “ibuprofen,” “meloxicam,” “naproxen,” “ketorolac,” “indomethacin,” “celecoxib,” “ATB-346,” “NSAID,” and “nonsteroidal anti-inflammatory drug” with “spinal.” Results were reviewed for relevance and included if they met inclusion criteria. The SYRCLE checklist was used to assess sources of bias.
Results:
A total of 2960 studies were identified in the PubMed/MEDLINE database using the above-mentioned search criteria. A total of 461 abstracts were reviewed in Scopus, 340 in CINAHL, 179 in PsycINFO, and 7632 in Embase. A total of 15 articles met the inclusion criteria.
Conclusions:
NSAIDs’ effectiveness after SCI is largely determined by its ability to inhibit Rho-A. NSAIDs are a promising therapeutic option in acute SCI patients because they appear to decrease cord edema and inflammation, increase axonal sprouting, and improve motor function with minimal side effects. Studies are limited by heterogeneity, small sample size, and the use of animal models, which might not replicate the therapeutic effects in humans. There are no published human studies evaluating the safety and efficacy of these drugs after a traumatic cord injury. There is a need for well-designed prospective studies evaluating ibuprofen or indomethacin after adult spinal cord injuries.
Keywords: spinal cord injury, ibuprofen, indomethacin, NSAID, neuroprotective
Introduction
Spinal cord injuries (SCIs) have a global incidence of 10.5 cases per 100 000 people, with the most frequent cause being motor vehicle collision.1 The average cost of SCI depends on injury level and severity, with complete SCIs costing as much as 5 times more than American Spinal Injury Association Impairment Scale Grade D injuries2 (motor function deficit with power able to overcome gravity in over 50% of the muscles below the level of injury) with individual cases resulting in costs of up to US$5.4 million just for initial care.3 Therefore, interventions that can mitigate the severity and sequelae of SCI and improve outcomes have the potential to significantly enhance quality of life for thousands of individuals and save a tremendous amount in health care expenditures.
The mechanisms of spinal cord injury are believed to involve direct impact to the spinal cord and secondary inflammatory effects. The physical force imparted on the spinal cord is believed to be most indicative of the severity of the injury. After the primary mode of trauma, the injury response including the inflammatory cascade imparts an enlarged zone of injury through edema and hemorrhage. This is mediated by the inflammatory cascade where cells including macrophages, neutrophils, and T-cells invade the injury site causing disruption of the blood-brain barrier. This allows penetrance into spinal cord cerebrospinal fluid and the release of cytokines. The cytokines regulate matrix metalloproteinases, which lead to tissue breakdown and induced cell death. The cell death releases glutamate and aspartate from the apoptotic cells. These specific amino acids cause toxicity to surrounding cells, furthering cell death of glia and neurons. The apoptosis of oligodendrocytes, neurons, and glia then leads to demyelination and eventually cystic cavitation causing a large defect in the spinal cord.4 To mitigate the inflammatory component and enlargement of the zone of spinal cord injury, effective medical management could prove highly beneficial. However, there is no current consensus on the administration of any specific drug or regimen for treatment of SCI-associated inflammation.
The most common prophylactically administered drug after acute SCI has been methylprednisolone sodium succinate (MPSS). The Second National Acute Spinal Cord Injury Study provided initial promise for use of methylprednisolone in SCI with increased motor and sensory function recovery after 6 months reported for patients who were administered MPSS within 8 hours of the injury.5 However, its use continues to be controversial due to concerns regarding its effectiveness, complication profile, and timing of administration.6 Due to the controversy regarding MPSS administration after acute SCI, Fehlings et al7 authored a systematic review of available randomized trials and prospective studies comparing MPSS to controls addressing both safety and efficacy of MPSS continuous infusion. Based on 3 randomized trials and an additional prospective study with indiscriminate administration of MPSS, they found no improvement in total motor recovery or pinprick sensation. However, when 2 additional randomized and 1 prospective trial compared MPSS to control within 8 hours of SCI, there was a significant improvement in total motor scores in the MPSS group. Furthermore, there was no difference in complications including pneumonia, wound complications, or risk of death.7 Based on this systematic review, AOSpine offers treatment guidelines with 3 recommendations regarding MPSS, all are graded as weak recommendations. A 24-hour infusion of MPSS should not be given to patients who present 8 hours or more after an acute SCI; this recommendation has moderate evidence to support the guideline. A 24-hour infusion of MPSS is an option to offer to adult patients who present within 8 hours after an SCI; this recommendation also has moderate evidence. Finally, a continuous infusion of steroids should not be offered for 48 hours to any patient with an acute SCI.8
Nonsteroidal anti-inflammatory drugs (NSAIDs) are an alternative class of drugs with anti-inflammatory effects through cyclooxygenase (COX) inhibition. Additionally, a subset of NSAIDs, including ibuprofen and indomethacin, inhibit Rho-A.9 Rho-A inhibition is a promising therapeutic target in spinal cord injury because it has been shown to increase myelination of axons and promote axonal elongation and sprouting. However, inactivation of Rho-A must occur acutely after injury. Delayed administration does not show any increase in axonal sprouting or motor function.10
Despite the potential benefits for use of NSAIDs for management of SCI, they are not routinely used for this purpose. One potential hesitancy might be lack of published guidelines for safe yet efficacious doses of NSAIDs after an acute SCI. Ibuprofen and indomethacin are the most commonly studied NSAIDs and are easiest to extrapolate dosing for. The typical dose of ibuprofen in rat models is 60 to 70 mg/kg/day, while indomethacin is slightly more variable with doses ranging from 0.1 to 10.0 mg/kg/day. Based on previous human equivalency dosing estimates, rat doses can be divided by 6.2 to get an equivalent human dose.11 This would equate to 11.3 mg/kg for ibuprofen and 1.6 mg/kg for indomethacin. While this does not account for differences in diffusion across the blood-brain barrier due to plasma protein binding, the total plasma albumin and total plasma protein concentration between rats and humans closely approximate to within 25%.12 Based on these estimates there should be some efficacy found at doses as low as 1100 mg ibuprofen daily and 16 mg indomethacin. However, there is a clear dose response level with higher levels of NSAIDs being more efficacious with safe doses as high as 3200 mg ibuprofen and 200 mg indomethacin daily.13,14
There are likely multiple other reasons for physicians’ hesitance to prescribe NSAIDs after SCIs. Other hesitancies likely center on potential complications including gastric ulceration,15,16 nonunion after spinal fusion,17-23 poor penetrance across the blood-brain barrier into the cerebrospinal fluid (CSF),24-26 or increased bleeding risk.27,28 However, these potential complications are based on limited evidence and generalization of NSAIDs use without high-quality evidence related to SCI. For example, while NSAIDs are known to increase bleeding risk after some surgeries, the clinical relevance regarding increased bleeding risk following acute spinal cord injuries is unknown. As such, NSAID use for management of SCI has been primarily limited to animal studies and has not progressed to clinical evaluations of their potential therapeutic benefits and complications in patients suffering SCI.
To judiciously advance the care and treatment of SCI, the authors sought to critically review the available evidence for use of NSAIDs after SCI. Therefore, this study was designed as a systematic review of the peer-reviewed literature using PRISMA guidelines to evaluate the most commonly used COX inhibitors for safety and efficacy of use after SCI. The hypothesis to be tested was that one or more NSAIDs will be associated with an acceptable safety profile and documented evidence for neuroprotective benefits and regenerative effects for treatment after SCI in animal models such that clinical trials in patients are warranted.
Methods
Literature Review Search
Using methodology based on the PICO (problem, intervention, control, and outcome) method, we examined the problem related to evidence-based decision making regarding the use of an NSAID intervention compared to a control for improving outcomes after spinal cord injury. With this objective in mind, a literature review was conducted to locate published studies in the following databases: PubMed/MEDLINE, CINAHL, PsycINFO, Embase, and Scopus. The following words, “ibuprofen,” “meloxicam,” “naproxen,” “ketorolac,” “indomethacin,” “celecoxib,” “ATB-346,” “NSAID,” and “nonsteroidal anti-inflammatory drug,” were linked with “spinal” to form a key phrase for searching. For example, “ibuprofen AND spinal” was used in each database to find all relevant literature. Additionally, all references in the articles meeting acceptable search criteria were reviewed for inclusion in the systematic review. All titles were then reviewed by the primary author for relevance. All articles deemed to be relevant were then read to ensure proper inclusion.
Literature Inclusion
Only studies in the English language were considered for inclusion. Any articles not in the time frame of January 1980 to September 2018 were excluded. If the article was published in a supplemental section of a journal article as an abstract, it was excluded. The mechanism of injury accepted included either a sharp transection injury, an acute compression injury, or a single crush injury. If treatment groups had multiple variables or types of treatment compared to controls, it was excluded. In order for the study to meet inclusion criteria, the NSAID had to be compared to a control group. If the study had a control group and addressed one of the following questions, then the article was included: Do NSAIDs have any impact on intramedullary hemorrhage after injury? Is there a difference in edema of the spinal cord between control and NSAID groups? Do NSAIDs promote neuronal sprouting or nerve myelination? Do NSAIDs decrease cystic cavitation of the spinal cord? Does the use of NSAIDs decrease cell apoptosis after injury? Is there motor improvement compared to a control group after NSAID administration? What complications are associated with use of NSAIDs when used for spinal cord injury? Data from studies was categorized and summarized for presentation based on these questions.
Risk of Bias
We utilized the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) tool29 to evaluate all studies for bias. We found the allocation sequence was adequately generated and applied in 11 of the 15 studies with the remaining 4 studies not adequately disclosing the methods. All studies (n = 15) had similar baseline characteristics for the animals tested. It was unclear in the majority of the studies if the caregivers were blinded to the treatment designation of the animals. Most important, the outcome assessors were found to be blinded in 10 of the 15 studies. Three studies explicitly stated they were not blinded and 2 studies did not specify (Appendix A, available online).
Results
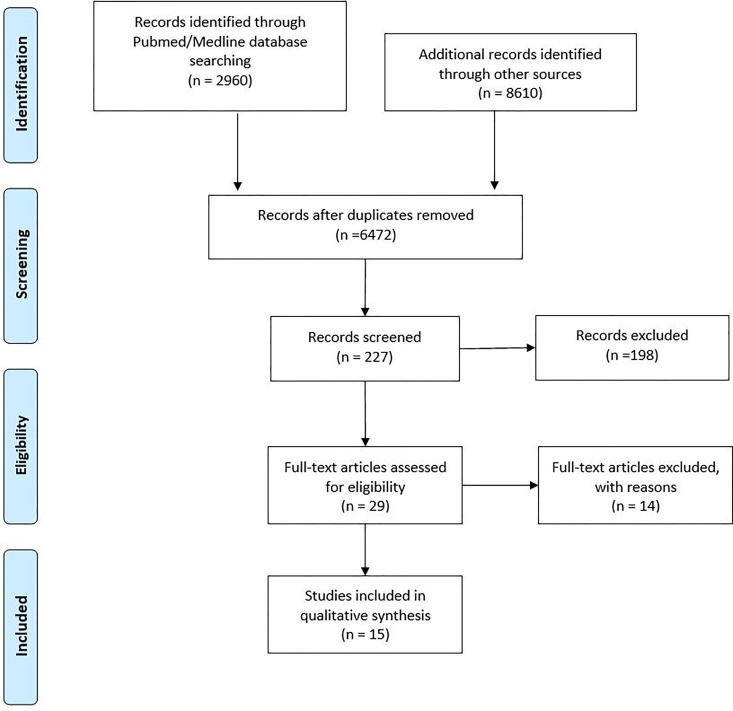
A total of 2960 studies were returned in the PubMed/MEDLINE database using the above-mentioned search criteria. An additional 461 articles were reviewed in Scopus, 340 in CINAHL, 179 in PsychINFO, and 7632 in Embase. Ultimately, all relevant articles in this review were found using the MeSH advanced search builder in PubMed. After review of 29 full text articles, 14 articles were excluded (Appendix B). A total of 15 studies met inclusion criteria and were included (Figure 1).
Figure 1.
PRISMA flow chart demonstrating articles identified, excluded, and included.
Seven studies evaluated indomethacin, 5 studied ibuprofen, 1 study evaluated ATB-346, 2 evaluated meloxicam, 1 studied NS-398, and 4 evaluated naproxen. The NSAID was administered post-SCI in 11 (73%) of the studies and pre-SCI in 4 (27%). The time from injury to evaluation ranged from 5 hours to 6 weeks across studies. No study specifically looked at intramedullary hemorrhage after SCI. Two studies compared mortality rate between the control group and NSAID after spinal cord injury. Seven studies looked at cord edema or inflammation of the spinal cord. Four studies evaluated neuronal sprouting or increased myelination after SCI. Five studies evaluated cystic cavitation, 3 of the studies looked at apoptosis of cells, and 10 studies evaluated the functional recovery of the animal after SCI (Table 1). Outcomes of studies focused on the following questions were reviewed to determine if the intervention had statistically superior results compared to controls.
Table 1.
List of All Included Studies in the Systematic Review. Type of Intervention (NSAID), Animal Model, Timing of Drug Administration and Injury Evaluation, and Mechanism of Injury.
| Study | NSAID Administered | Species (n = per Group) | Weight | Pre/Post Injury Drug Administration | Time From Injury to Evaluation | Mechanism of Injury |
|---|---|---|---|---|---|---|
| Pedram et al38 | Meloxicam | Male Wistar rats (n = 4-6/group) | 300-350 g | Post | 4 weeks | Compression injury |
| Campolo et al40,a | ATB-346, naproxen | Male CD1 mice (n = 15, 5 sacrificed day 1 for exam) | 25-30 g | Post | 1 day for histology, 10 days for functional recovery | Compression injury |
| Hakan et al30,a | Meloxicam | Sex unknown, Wistar Albino rats (n = 5-7 per group) | 250-300 g | Post | 8 days | Blunt impaction to spinal cord |
| Wang et al36,a | Ibuprofen, naproxen | Female Sprague-Dawley rats (n = 16 ibu, 15 naproxen and control), female C57/B16 mice (n = 22 ibu, 12 control) | 250-270 g | Post | 2 and 6.5 weeks | Blunt impaction to spinal cord, sharp transection |
| Sharp et al35,a | Ibuprofen | Female Sprague-Dawley rats (ibu = 34, control = 39) | N/A | Post | 6 weeks | Dorsal transection of spinal cord |
| Redondo-Castro and Navarro39,a | Ibuprofen | Female Sprague-Dawley rats (ibuprofen n = 10, saline n = 10; acute treatment group: ibuprofen n = 4, saline n = 4; histology at 3 days: ibuprofen n = 3, saline n = 3) | 250-300 g | Post | 6 weeks | Blunt impaction to spinal cord |
| Pantović et al41 | Indomethacin | Adult rabbits (n = 6) | 2.5-3.5 kg | Post | 9 days | Crush injury |
| Xing et al37,a | Ibuprofen, indomethacin, naproxen | Female Sprague-Dawley rats (n = 8 control, naproxen, ibuprofen, n = 10 indomethacin for apoptosis; n = 8 indomethacin and control; and n = 9 for naproxen and ibuprofen for myelination) | 180-250 g | Post | 7 days until analysis of apoptosis; 28 days for myelination analysis | Blunt impaction to spinal cord |
| Fu et al31,a | Ibuprofen, naproxen | Female Sprague-Dawley rats (n = 7 control, 7 naproxen, 12 ibu) | 180-250 g | Post | 4 weeks | Dorsal hemisection of cord, blunt impaction to spinal cord |
| Guth et al32 | Indomethacin | Rats not specified (n = 6 control, 5 ind, 7 LPS, and 7 LPS with ind) | 170-200 g | Post | 3-4 weeks | Crush injury with jewelers forceps |
| Simpson et al34 | Indomethacin | Sex not identified, Sprague-Dawley rats (n = 10 sham surgery control tx, n = 12 SCI control, n = 9 sham-ind, n = 10 SCI ind) | 250-350 g | Post | 6 weeks | Blunt impaction to spinal cord |
| Sharma et al13 | Indomethacin | Male Sprague-Dawley rats (n = 5 per group) | 200-300 g | Pre | 5 hours | Transection of right dorsal horn of spinal cord |
| Winkler et al51 | Indomethacin | Male Sprague-Dawley rats (n = 5 per group) | 350-400 g | Pre | 5 hours | Transection of dorsal horn of spinal cord |
| Sharma et al14 | Indomethacin | Wistar rats, sex unknown untraumatized group: (n = 17 in 5 mg/kg ind), n = 21 in 10 mg/kg ind; traumatized group: (n = 23 in 5 mg/kg ind and n = 35 in 10 mg/kg ind) | 200-300 g | Pre | 5 hours | Longitudinal incision made in right dorsal horn of spinal cord |
| Hains et al33 | NS-398 | Male Sprague-Dawley rates (n = 20 locomotor function, n = 10 immunohistochemistry, n = 10 at 7, 14, 21, and 28 days for histologic analysis) | 200-225 g | Pre | 4 weeks | Contusion injury with NYU impact injury device |
Abbreviations: NSAID, nonsteroidal anti-inflammatory drug; SCI, spinal cord injury; ind, indomethacin; ibu, ibuprofen.
a Data estimated from figures in the original article.
What Complications Are Associated With Use of NSAIDs When Used for Spinal Cord Injury?
No study specifically looked at complications of NSAID treatment after SCI. However, the study by Hakan et al30 and Fu et al31 did compare mortality rates. Hakan et al30 found that 10/27 of the SCI rats treated with vehicle died, while 8/26 meloxicam treated rats died after SCI. These results were not significantly different. The Fu et al31 study also found 4 deaths in the control group, 4 in the naproxen group, and 2 in the ibuprofen group. These results showed no significant difference in mortality rate.31
Is There a Difference in Edema of the Spinal Cord Between Control and NSAID Groups?
Seven studies quantitatively evaluated spinal cord edema or inflammation with 6 out of 7 showing significantly decreased spinal cord edema or inflammation after NSAID therapy. However, there was little standardization in the measurement of edema across studies. In some studies, myeloperoxidase (MPO) was used as a proxy for neutrophil infiltration. Additionally, some of the studies evaluated the histology of the spinal cord either quantitatively or qualitatively. The study by Guth et al32 did evaluate indomethacin versus a control, but the power was determined to be low and they restudied indomethacin with a larger sample size and with additional drugs. While the combination therapy showed decreased edema, indomethacin alone did not decrease edema compared to control.32
The study by Hakan et al30 showed a qualitative decrease in the amount of edema based on histologic analysis perhaps supporting their finding of a statistically significant decrease in MPO activity in the SCI group treated with meloxicam. The study by Hains et al33 demonstrated decreased inflammation of a selective COX-2 inhibitor (NS-398) as measured by decreased levels of prostaglandin E2 (PGE2). Four of the remaining studies demonstrated a statistically significant decrease in MPO activity or edema after an SCI was treated with a more commonly utilized NSAID (Table 2). The final remaining study by Simpson et al34 graded the amount of myelin edema on a scale of 1 to 4 with mild edema receiving a grade of 1 and marked edema receiving a grade of 4. While the amount of edema was a full grade lower for indomethacin compared to nifedipine or the control group, this study is not reproducible and the significance of the decreased edema is unclear based on the grading system. However, this study is in line with the majority of other studies showing decreased edema after treatment with a Rho-A inhibitor NSAID.
Table 2.
Compiled Data of Outcomes Including Cord Edema/Inflammation, Neuronal Sprouting, Cystic Cavitation, and Apoptosis Comparing Intervention to Control.
| Study | Outcomes Measured | Results—Control | Results—NSAIDs | Significance Level |
|---|---|---|---|---|
| Cord edema/inflammation | ||||
| Campolo et al40,a | Total myeloperoxidase activity | 300 mU/g | Nap: 250 mU/g, ATB-346: 125 mU/g | P < .05 with nap compared to control and P < .05 for ATB-346 compared to nap |
| TNF-α levels | 100 mU/g | Nap: 70 mU/g, ATB-346: 50 mU/g | P < .05 when nap compared to control and ATB-346 compared to nap | |
| Histological damage score based on number of eosinophilic neurons. 1 = 1-5 neurons, 2 = 5-10 neurons, 3 = >10, 1 = <1/3 of gray matter, 5 = 1/3-1/2 of gray matter, 6 = >1/2 of gray matter | 5 | Nap: 3, ATB-346: 1 | P < .05 when nap compared to control; P < .05 when ATB-346 compared to nap | |
| Hakan el al30,a | Myeloperoxidase activity (U/g) | No SCI: 3.8 U/g SCI control: 6.5 |
Mel: 4.0 | P < .001 with mel compared to saline |
| Guth et al32 | Total lesion size measuring edema 21 days after injury (mm2) | Control: 1.62 ± 0.11 LPS: 1.63 ± 0.14 Preg: 1.84 ± 0.1 DHEA: 1.68 ± 0.12 |
Ind: 1.63 LPS and ind: 1.63 ± 0.13 Preg, LPS, and ind: 1.14 ± 0.14 DHEA, LPS, and ind: 1.12 ± 0.08 |
P < .01 for preg, LPS, and ind P < .01 for DHEA, LPS and ind |
| Experiment rerun with larger sample sizes measuring edema (mm2) | Control: 1.89 ± 0.12 Preg and LPS: 1.77 ± 0.14 |
Preg, LPS, and ind: 1.33 ± 0.14 | P < .05 for preg, LPS, and ind compared to control | |
| Simpson et al34 | SCI evaluation based on scale of mild (1) to marked (4) as determined by the authors | 2.4 ± 0.5 | SCI-nifedipine: 2.1 ± 0.6 SCI-indomethacin: 0.8 ± 0.3 |
Unknown |
| Sharma et al13 | Spinal cord water content (%) using microscope for evaluation rostral to SCI | Non-SCI control: 71.56 ± 0.42 SCI control: 74.48 ± 0.38 |
SCI with 1 mg ind: 74.76 ± 0.53 | P > .05 |
| SCI with 5 mg ind: 74.68 ± 0.88 | P > .05 | |||
| SCI with 10 mg ind: 72.74 ± 0.32 | P < .05 | |||
| Spinal cord water content (%) using microscope for evaluation caudal to SCI | Non-SCI control: 71.38 ± 0.42 SCI control: 75.34 ± 0.42 |
SCI with 1 mg ind: 75.78 ± 0.63 | P > .05 | |
| SCI with 5 mg ind: 74.89 ± 0.61 | P > .05 | |||
| SCI with 10 mg ind: 72.64 ± 0.38 | P < .05 | |||
| Spinal cord water content (%) using microscope for evaluation of total spinal cord injury | Non-SCI control: 72.03 ± 0.38 SCI control:76.58 ± 0.34 |
SCI with 1 mg ind: 76.84 ± 0.76 | P > .05 | |
| SCI with 5 mg ind: 77.23 ± 0.67 | P > .05 | |||
| SCI with 10 mg ind: 74.48 ± 0.52 | P < .05 | |||
| Winkler et al51 | Water content in spinal cord (%) | Non-SCI control: 72.03 | SCI treated with ind: 73.31 | P < .05 with ind compared to control |
| SCI control: 76.23 | ||||
| Sharma et al14 | Water content of spinal cord (%) | Non-SCI control group (%): 67.64 ± 0.23 | Non-SCI group treated with 5 mg/kg ind: 66.58 ± 0.32 Non-SCI with 10 mg/kg ind: 66.74 ± 0.38 |
N/A |
| Control SCI: 70.18 ± 0.34 | 5 mg/kg ind with SCI: 69.43 ± 0.33 | P < .01 | ||
| Control SCI: 70.18 ± 0.34 | 10 mg/kg ind with SCI: 68.28 ± 0.18 | P < .01 | ||
| Hains et al33 | Inflammation evaluated by PGE2 immunohistochemistry at 12 hours postinjury | 6389 ± 784 | Staining intensity for NS-398: 2331 ± 1177 | P < .05 |
| Neuronal sprouting/myelination | ||||
| Wang et al36,a | Central horn 5-HT fiber length in complete transection model at 5 weeks (µm) | 0 | Ibu at 35 mg: 5, Ibu at 70 mg: 11 | P < .05 ibu; regeneration seen across transection in ibu group |
| Contusion model fiber length at 2 weeks (µm) | 75 | Nap: 100, ibu: 90 | P > .05 for nap and ibu | |
| Contusion model fiber length at 6 weeks (µm) | 50 | Nap: 4, ibu: 200 | P < .01 for ibu group | |
| Optical density of BDA-labeled CST fibers rostral to injury | 0.12 | Ibu: 0.7 | P < .05 for ibu group | |
| Sharp et al35,a | Axonal sprouting | 27 axons per high-powered section | Ibu: 25 axons per high-powered section | P > .05 |
| Xing et al37,a | Total oligodentrocytes 6-10 mm rostral to lesion 5 days after SCI injury | 1600 | Ibu: 1800, ind: 1800, nap: 1600 | P < .05 for ibu and ind |
| Total oligodentrocytes 6-10 mm caudal to lesion 5 days after SCI injury | 1500 | Ind: 2000, ibu: 1950, nap: 1450 | P < .05 for ibu and ind | |
| MBP-labeled myelin 3-4 mm caudal to lesion 6 weeks after SCI (units measured as arbitrary units) | 36 | Nap: 40, ibu: 100, ind 50 | Ibu P < .05; qualitatively increased myelination for ind and ibu groups seen on electron microscopy | |
| 3-4 mm caudal with Luxol fast blue stained myelin (units measured as arbitrary units) | 25 | Nap: 22, ibu: 100, ind: 75 | P < .01 for ibu and ind | |
| 3-4 mm rostral with MBP-labeled myelin (units measured as arbitrary units) | 42 | Nap: 38, ind: 75, ibu: 100 | P < .05 ibu; qualitatively increased myelination for ind and ibu groups seen on electron microscopy | |
| 3-4 mm rostral with LFB (units measured as arbitrary units) | 45 | Nap: 10, ind: 50, ibu: 105 | P < .05 for ibu group | |
| Fu et al31,a | CST sprouts/rat in dorsal over hemi-section rats 1 hour postinjury | 5 sprouts | Nap: 1, ibu: 31 | P < .05 for ibu group |
| CST axons/slide 7-10 mm distal to injury | 4 | Nap: 3, ibu: 9 | P < .05 for ibu group | |
| 5HT fiber length (mm) | 8 | Nap: 10, ibu: 17 | P < .01 for ibu group | |
| Serotonin fiber length (µm) | 13 | Ibu: 23 | P < .05 | |
| # CST fiber >200 µm 1-2 mm from SCI in gray matter and at 3-4 mm, 5-6 mm, 7-8 mm, and 9-10 mm | 0, 5, 5, 3, 0 | 15, 30, 31, 29, 25 | P < .05 | |
| Gray and white matter CST fibers/rat found at least 1 mm distal to lesion | 25 | 200 | P < .05 | |
| Cystic cavitation | ||||
| Pedram et al38 | Measured based on Rexel classification: 0 = normal with no vacuolization, 1 = mild with <10% cells injured, 2 = moderate 10-50%, 3 = severe with >50% | Ventral horn gray matter: 0 | Ventral horn gray matter for mel: 0 | P > .05 |
| Intermediate gray matter: 0.5 ± 0.57 | Intermediate gray matter: 0 | P > .05 | ||
| Dorsal horn gray matter: 0 | Dorsal horn gray matter: 0 | P > .05 | ||
| Wang et al36,a | Spared tissue 2 weeks after injury (µm) | 300 | Nap: 300, ibu: 550 | P < .05 for ibu group compared to control/nap and nap, P > .05 compared to control |
| Spared tissue 6 weeks after injury (µm) | 250 | Nap: 250, ibu: 450 | P < .05 for ibu group compared to control/nap and nap, P > .05 compared to control | |
| Redondo-Castro and Navarro39,a | Total amount of spared tissue in epicenter of cord injury (µm2) | 2.0 × 109 | Ibu: 1.7 × 109 | P > .05 |
| Tissue spared at periphery of lesion (µm2) | 4.0 × 109 | Ibu: 3.5 × 109 | P > .05 | |
| Myelinated tissue at different areas of the lesion (µm2) | 2 000 000 at −3000 µm from epicenter, none at epicenter | Ibu: 3 000 000 at −3000 µm | P < .05 ibu versus control | |
| Guth et al32 | Total lesion size (mm2) at 28 days | Control: 0.93 ± 0.1 LPS: 0.49 ± 0.1 |
Ind: 1.05 ± 0.128 mm2 LPS and ind: 0.11 ± 0.4 |
P < .01 for LPS P < .001 for LPS and ind |
| Total lesion size after crush injury at 28 days | Control: 0.94 ± 0.13 LPS: 0.63 ± 0.09 Preg: 1.00 ± 0.11 DHEA: 0.94 ± 0.1 |
Ind: 1.05 ± 0.13 LPS and ind: 0.49 ± 0.11 Preg, LPS, ind: 0.3 ± 0.04 DHEA, LPS, ind: 0.33 ± 0.3 |
P < .01 for preg, LPS, and ind group P > .05 for LPS and ind group P < .01 for DHEA, LPS, and ind group |
|
| Crush injury repeated with increased sample size (mm2) | Control: 0.99 ± 0.09 Preg and LPS: 0.94 ± 0.12 mm2 |
LPS and ind: 0.49 ± 0.08 mm2 | P < .01 for LPS and ind group | |
| Simpson et al34 | Total tissue loss based on author scale of mild to marked injury | Sham control: 0.0 | Sham ind: 0.0 | Unknown |
| SCI control: 1.1 ± 0.4 | SCI ind: 0.1 ± 0.1 | |||
| Hains et al33 | Total viable tissue loss (%) | 32.2 ± 2.7 | NS-398: 17.6 ± 3.21 | P < .05 |
| Apoptosis of cells | ||||
| Campolo et al40,a | TUNEL positive cells/field | 4.8 | Nap: 3 ATB-346: 1.8 |
Nap P < .001 compared to control, ATB P < .001 compared to nap |
| Densitometric data (OD m2) for antiapoptotic bcl2 | 100 | Nap: 1600 ATB-346: 3700 |
P < .001 for ATB346 compared to nap | |
| Proaopoptotic Bax units | 4600 | Nap: 1000 ATB-346: 700 |
P < .001 for nap and ATB-346 compared to control | |
| Hakan et al30,a | % DNA fragmentation | Control without SCI: 0.04 Control with SCI: 0.16 |
SCI + mel: 0.06 | P < .01 |
| Xing et al37,a | Total TUNEL (+) cells/section 6-10 mm rostral to lesion 5 days after injury | 70 | Nap: 70 Ibu: 45 Ind: 35 |
P < .01 for both ibu and ind; nearly all apoptotic cells were in white matter |
| Total TUNEL (+) cells/section 6-10 mm caudal to lesion 5 days after injury | 45 | Nap: 45 Ibu: 28 Ind: 27 |
P < .01 for both ibu and ind; nearly all apoptotic cells were in white matter | |
Abbreviations: NSAID, nonsteroidal anti-inflammatory drug; TNF-α, tumor necrosis factor-α; ind, indomethacin; ibu, ibuprofen; nap, naproxen; mel, meloxicam; preg, pregnenolone.
a Data estimated from figures in the original article.
Do NSAIDs Promote Neuronal Sprouting or Nerve Myelination?
Three studies quantitatively assessed axonal sprouting after sectioning the spinal cord. Two of the studies found a significant increase in sprouting, while one study found no significant difference between treatments with a Rho-A inhibitor NSAID compared to control. The Sharp et al35 study tried to exactly replicate the Fu et al31 protocol to recreate the degree of axonal sprouting caused by ibuprofen. In fact, they involved the senior author of the Fu et al31 study to try and replicate the experiments. However, while Fu et al31 found ibuprofen to be neuroprotective and to promote axonal sprouting, Sharp et al35 found no statistical difference in degree of serotonergic or corticospinal tract (CST) sprouting. They hypothesized, based on BDA labeling of the ventral CST, the dorsal axons caudal to the injury site are from the arborizing ventral CST and not from regeneration of the injured axons. Additionally, there was no difference between vehicle-treated and ibuprofen-treated rats in degree of ventral CST sprouting in the Sharp et al35 study, which is in contrast to the findings from Fu et al.31 Similar to the findings by Fu et al,31 Wang et al36 also concluded that ibuprofen leads to significantly greater axonal sprouting.
The study by Xing et al37 evaluated the degree of myelination after a contusion injury to the spinal cord. Treatment for 5 days with ibuprofen after an SCI led to increased oligodendrocytes compared to control. Additionally, 6 weeks after injury there was increased rostral and caudal myelination at 3 to 4 mm and 6 to 8 mm from the lesion site.37
Do NSAIDs Decrease Cystic Cavitation of the Spinal Cord?
Neuronal sprouting, functional recovery, amount of cell apoptosis, and cord edema all appear to show NSAIDs have a favorable role in injury mitigation, but it is less clear if they have a role in decreasing cystic cavitation. Three studies showed a decrease in the amount of tissue loss/cystic cavitation with only 2 studies being able to determine a significant difference. This is compared to 3 studies that found no significant difference in the degree of tissue loss. Only the article by Wang et al36 shows a significant decrease in tissue loss after administration of a Rho-A inhibiting NSAID. Hains et al33 also demonstrated a decrease in viable tissue loss after administration of a selective COX-2 inhibiting NSAID, but it has not been evaluated for Rho-A inhibiting properties. Simpson et al34 does not measure the amount of tissue loss, but instead quantifies the degree on a scale from mild to marked. The difference in the study was found to be a full grade. Other studies demonstrated no significant change in tissue loss at the site of injury.32,38,39
Does the Use of NSAIDs Decrease Cell Apoptosis After Injury?
All 3 studies evaluating cell apoptosis found decreased apoptosis after treatment with NSAIDs compared to control. The study by Campolo et al40 demonstrates both ATB-346 and naproxen significantly decrease proapoptotic Bax and increase antiapoptotic Bcl2 compared to controls. Additionally, the study found ATB-346 significantly increased Bcl2 compared to naproxen. ATB-346 also has fewer apoptotic cells after SCI compared to naproxen, while naproxen has fewer apoptotic cells compared to the control.40 Xing et al37 found ibuprofen and indomethacin significantly decrease the amount of apoptotic cells compared to control and naproxen. Naproxen had no significant difference compared to control. Nearly all apoptotic cells were in the white matter.37 Similar to the other studies, Hakan et al30 also demonstrated NSAIDs decrease apoptosis. In their study, meloxicam was found to have significantly less DNA fragmentation compared to control.
Is There Motor Improvement Compared to a Control Group After NSAID Administration?
A total of 10 studies evaluated the effectiveness of NSAIDs after SCI by assessing functional recovery (Table 3). Seven of the studies found significant improvement in functional recovery after NSAID administration compared to control, while 3 studies found no significant difference. None of previous questions evaluating NSAID effectiveness has as large of a difference in interstudy evaluation methods as functional recovery. For this reason, we will try and break down motor recovery into type of NSAID administered.
Table 3.
Compiled Data on Functional Evaluation Outcomes. Due to Study Heterogeneity, Studies Were Divided Into the Functional Evaluation Metric They Utilized During the Study.
| Study | Data |
|---|---|
| Basso-Beattie-Bresnahan (BBB) | |
| Pedram et al38 | Meloxicam: 8, control: 0 (P < .05) |
| Campolo et al40,a | Control: 2, nap: 4, ATB-346: 7. Naproxen and ATB-346 (P < .05 when nap compared to control and ATB-346 compared to nap). |
| Wang et al36,a | Ibu: 9.4, control: 6.8, nap: 7.7. Ibuprofen (P < .01) compared to naproxen and control. |
| Sharp et al35,a | Control: 11.9 versus ibu: 11.2 (P > .05) |
| Redondo-Castro and Navarro39,a | Ibu: 16 versus control: 17 (P > .05) |
| Fu et al31,a | BBB evaluated 42 days after injury. In hemisection injury group = ibu: 15, nap: 13, control: 13. In contusion model the control was 13.5, ibu: 16 (P < .05 for both BBB injury mechanisms). |
| Hains et al33 | BBB evaluated 2 days after injury = NS-398: 0.9 ± 0.7, control: 1.1 ± 0.4. 14 days after injury = NS-398: 12.6 ± 0.77, control 10.2 ± 0.69 (P > .05). Day 28 NS-398: 17.4 ± 1.25, control: 14.6 ± 0.72 (P < .05). |
| Gale | |
| Hakan et al30,a | Noninjured group: 6. SCI group: 2.5, SCI + mel: 3.0 (P > .05) |
| Tarlov | |
| Pantović et al41 | Control: 1.3, 0.1 mg/kg ind: 2.2 mg/kg, 0.3 mg/kg ind: 2.2, ind 1.0 mg/kg: 2.3, ind 3.0 mg/kg: 2.7. P < .05 for all treated groups in dose dependent manner. |
| Filament test/grid walk failure rate/stride length | |
| Sharp et al35,a | Von Frey filament sensory testing showed no significant difference 9.2 g ±5.4 in saline treated rats versus 8.5 ± 5.7 in IBU treated. Hindlimb walk and grid walk test resulted in failure of 26.75% for the saline group versus 25.57% for the ibuprofen-treated group. Mean stride length was 158.5 mm for saline treated group and 153.4 mm for the ibuprofen treated group; mean stride width was approximately 28 mm for both treatment groups (P > .05). |
| Fu et al31,a | Grid walk failure rate in the hemisection injured group for control: 62%, naproxen: 62%, ibu: 50% (P < .05). Contusion model failure rate = control: 65% and ibu: 45% (P < .05). Stride length in hemisection group: control: 125 mm, nap: 130 mm, ibu: 145 mm (P < .05), stride width = control: 60 mm, nap: 50 mm, ibu: 40 mm (P < .05). |
| Coordination test/max speed | |
| Redondo-Castro and Navarro39,a | Fine motor coordination foot beam test for ibu group: 1.75 steps versus saline: 1.25 steps. Maximum speed on treadmill for control group: 37 versus ibu group: 34 (P > .05 for both tests). |
| Locomotor score | |
| Simpson et al34 | Locomotor score based on ability to ascend inclined plane. Noninjured group at day 1 and at 6 weeks: 69.9 ± 3.2 and 67.4 ± 2.8, SCI-vehicle group: 41.8 ± 12 and 56.9 ± 13.2, sham-ind group: 58.4 ± 5.2 and 64.9 ± 5.2, SCI-ind group: 37.4 and 67.4 ± 4.4 locomotor score for SCI-ind compared to SCI-vehicle (P < .05). Motor score based on 4-point scale from normal to no movement at day 1 and 6 weeks after injury. Noninjured group: 4.0 and 4.0, SCI-vehicle group: 2.7 ± 1.1 and 3.3 ± 1.2, sham-ind group: 4.0 and 4.0, SCI-ind group: 3.1 ± 0.6 and 4.0 ± 4.0, SCI-vehicle group: 1.2 ± 0.5 and 1.0 ± 0.0, sham-ind group: 1.9 ± 0.3 and 1.0 ± 0.0, SCI-ind group: 2.8 ± 0.6 and 1.0 ± 0.0 (P < .05 for ind vs control). |
Abbreviations: SCI, spinal cord injury; ind, indomethacin; ibu, ibuprofen; nap, naproxen; mel, meloxicam.
a Data estimated from figures in the original article.
The study by Pedram et al38 evaluated meloxicam after an acute compression injury to the spinal cord. Using the Basso-Beattie-Bresnahan (BBB) score, they found acute administration of meloxicam after SCI leads to a large and significant difference in motor recovery. Hakan et al30 also administered meloxicam after a blunt impaction spinal cord injury. They used the Gale behavioral score and found no significant difference in recovery.
Campolo et al40 evaluated naproxen and ATB-346 using an acute compression injury mechanism. Naproxen significantly improved the BBB score compared to control, and ATB-346 significantly increased the BBB score compared to both control and naproxen. In contrast, Campolo et al,40 Wang et al,36 and Fu et al31 found naproxen to have no significant difference in motor recovery. Unlike Campolo et al,40 Wang et al36 and Fu et al31 used a mechanism of blunt impaction to the rat spinal cord.
Wang et al36 and Fu et al31 additionally evaluated ibuprofen. Fu et al31 had a subset of rats that underwent a sharp mechanism of injury and another subset of rats who underwent blunt impaction. The mechanism did not alter ibuprofen effectiveness in functional recovery. While Wang et al36 only used the BBB score for motor evaluation, Fu et al31 found a significant motor improvement after ibuprofen administration in the BBB score, a lower failure rate in the grid walk test, and a longer stride distance and shorter stride width. As previously discussed, Sharp et al35 tried to exactly replicate the study by Fu et al.31 They found no significant difference in the BBB score, Von Frey filament sensory test, grid walk test, mean stride length, or mean stride width between ibuprofen and the control groups. Similar to the study by Sharp et al,35 the study by Redondo-Castro and Navarro39 used a blunt injury mechanism and found no difference in the BBB score between ibuprofen and control. Interestingly, they had a much higher BBB score in both the control and ibuprofen groups compared to all other studies. The study also evaluated fine motor coordination with a foot beam test and maximum speed on a treadmill and found no difference between ibuprofen versus control.
Pantović et al41 and Guth et al32 both evaluated motor recovery after indomethacin was given after a crush injury to the spinal cord. Pantović et al41 used the Tarlov functional scale and found a dose-dependent increase in function with indomethacin administration. Guth et al32 used a locomotor score based on a rat’s ability to ascend an inclined plane and motor score based on a 4-point scale from normal to no movement. The study found a significant increase in functional improvement in indomethacin-administered rats compared to control.
Hains et al33 evaluated motor recovery using the BBB scale and found a significant improvement in motor function with pre-injury administration with NS-398. They evaluate the injured rats at days 2, 14, and 28 with only day 28 showing significant improvement in function.
Discussion
Based on the current data available, we were able to accept the hypothesis that NSAIDs have a role in neuroprotection, improve neuroregenerative abilities, and do not appear to increase morbidity or mortality. The data compiled in this systematic review demonstrate that the majority of animal model studies show improved motor function, decreased cell apoptosis, and decreased spinal cord edema after use of NSAIDs for treatment of SCI. Most studies also show Rho-A-inhibiting NSAIDs can improve neuronal sprouting. Although the safety profile was only addressed in a couple of studies, there was no increase in mortality from NSAID administration.
Although different characteristics of SCI injury and recovery were evaluated in this systematic review, there were some consistencies noted that have importance for clinical application. First, naproxen did not consistently show beneficial effects compared to controls. This may be attributable to the fact that naproxen is not a Rho-A inhibitor. Second, ketorolac seems to be a relatively poor NSAID to use in the acute setting of SCI. Although it is readily available in intravenous formulation, the penetrance of ketorolac into the CSF is 0.1% of its free plasma availability.24 One study did show that ketorolac decreased inflammation and offered neuroprotective effects if administered intrathecally, but intrathecal administration creates a barrier to care at many hospitals.42 In addition, meloxicam has not been effectively studied as a neuroprotective NSAID and needs higher quality studies before it could be considered for human use. In contrast, ibuprofen and indomethacin were the most frequently studied NSAIDs. Across all studies, only indomethacin (7 studies) was more frequently evaluated than ibuprofen (5 studies). This is due in part because ibuprofen readily crosses into the CSF with concentrations higher in CSF compared to unbound plasma concentrations.43 It has the added benefit of being a Rho-A inhibitor and has been shown to readily promote axonal sprouting.36 Taken together, this systematic review suggested that ibuprofen and indomethacin appear to be promising neuroprotective and neuroregenerative agents in the acute management of SCI.
Many of the studies included in the systematic review are heterogeneous in their study design. The injury mechanism, time from injury to evaluation, time of drug administration, parameters evaluated, including cord edema or cell apoptosis, and the functional assessment scoring systems are inconsistent throughout the majority of the studies. This makes it difficult to extract meaningful data on the neuroprotective abilities of any NSAID. It also makes it difficult for interstudy comparison. The reason for the significant heterogeneity includes failure to reach consensus on the appropriate factors to evaluate in an animal model before translating it into human studies.44 Additionally, no motor functional assessment has been validated as superior to others. Functional assessment was the most commonly studied measure for NSAID effectiveness, but due to the variability in scoring system, it is impossible to combine the data to perform a substantial meta-analysis. The BBB score was the most commonly used evaluation of functional improvement, but the Tarlov score, Gale motor score, and hindlimb placing and stepping reflexes were also used.
Additional animal studies would improve our confidence of the neuroprotective and neuroregenerative abilities of NSAIDs. Unfortunately, a couple of studies that were considered did not meet our inclusion criteria. Hsieh et al45,46 and Siegal et al47,48 did not meet our inclusion criteria based on mechanism of spinal cord injury. The studies by Siegal et al47,48 evaluated subacute compression with a malignant fibrous histiocytoma. They found administration of indomethacin significantly decreased cord edema and significantly improved functional motor improvement. Results of indomethacin were comparable to the results of steroids.
Hsieh et al45,46 used balloon occlusion causing ischemia of the spinal cord. Their studies found 60 μg ketorolac caused a significant decrease in cystic cavitation compared to control. Additionally, rats treated with ketorolac had near normal hindlimb placing/stepping reflexes where 4/6 rats treated with control had severe paraplegia. The study by Hallenbeck et al49 and Zhang et al50 were excluded because they did not separately study an NSAID compared to control. Instead the NSAID was part of a combination treatment regimen.
Some of the major limitations of the study include the significant study heterogeneity. The timing of NSAID administration, mechanism of spinal cord injury, type of NSAID administered, and method of assessing functional improvement resulted in large interstudy differences making it difficult to draw concrete conclusions from the data. Additionally, when study design was attempted to be replicated by a different group of investigators, results were conflicting. This may be due to some underlying bias in design and evaluation of the studies. As noted in Appendix A (available online), each study is at risk of bias with some studies explicitly stating the outcome assessors were not blinded. Due to the heterogeneity of the data, we were unable to perform a meta-analysis of the data. However, even if the design of studies allowed for a meta-analysis some studies likely would have been excluded. Furthermore, all studies evaluated in our systematic review were animal models. The pharmacokinetic profile of these drugs and resulting response to these drugs are often not perfectly mimicked across species. This could increase or decrease the efficacy of the neuroprotective and neuroregenerative properties of Rho-A inhibiting NSAIDs in the human population. Finally, systematic reviews rely on the compilation of data from multiple sources; any included study with low-quality data would inherently cause the systematic review to be flawed.
Conclusion
Based on preclinical studies using mostly rat models, there appears to be a benefit to administration of Rho-A inhibiting NSAIDs after SCI due to their neuroprotective and neuroregenerative properties. Furthermore, it appears there is a dose-response relationship with larger doses imparting improved neurologic outcomes. However, there are no previous retrospective or prospective data on NSAID administration after SCI in the adult patient population. For this reason, it is unclear if the potential benefits outweigh the risks of administration of NSAIDs after SCI. While animal studies exist, very few studies are easily comparable due to their significant heterogeneity in injury method and evaluation of SCI improvement. Furthermore, there is minimal preclinical data on appropriate NSAID doses, frequency of administration, and side effects at different doses. Based on this systematic review, there is a need for additional, well-designed, and reproducible animal studies to evaluate NSAIDs after spinal cord injury, ideally with a standardized animal injury model and motor function evaluation to address previous study heterogeneity.
Supplemental Material
Supplemental Material, Appendix_A for Nonsteroidal Anti-Inflammatory Drugs and Their Neuroprotective Role After an Acute Spinal Cord Injury: A Systematic Review of Animal Models by Mark J. Lambrechts and James L. Cook in Global Spine Journal
Supplemental Material, Appendix_B for Nonsteroidal Anti-Inflammatory Drugs and Their Neuroprotective Role After an Acute Spinal Cord Injury: A Systematic Review of Animal Models by Mark J. Lambrechts and James L. Cook in Global Spine Journal
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: James L. Cook, DVM, PhD  https://orcid.org/0000-0002-0862-995X
https://orcid.org/0000-0002-0862-995X
Supplemental Material: The supplemental material is available in the online version of the article.
References
- 1. Kumar R, Lim J, Mekary RA, et al. Traumatic spinal injury: global epidemiology and worldwide volume. World Neurosurg. 2018;113:e345–e363. [DOI] [PubMed] [Google Scholar]
- 2. Dukes EM, Kirshblum S, Aimetti AA, Qin SS, Bornheimer RK, Oster G. Relationship of American Spinal Injury Association Impairment Scale grade to post-injury hospitalization and costs in thoracic spinal cord injury. Neurosurgery. 2018;83:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cao Y, Chen Y, DeVivo M. Lifetime direct costs after spinal cord injury. Top Spinal Cord Inj Rehabil. 2011;16:10–16. [Google Scholar]
- 4. Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury—repair and regeneration. Neurosurgery. 2017;80(3 suppl):S9–S22. [DOI] [PubMed] [Google Scholar]
- 5. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. [DOI] [PubMed] [Google Scholar]
- 6. Evaniew N, Noonan VK, Fallah N, et al. Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a Canadian multi-center spinal cord injury registry. J Neurotrauma. 2015;32:1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fehlings MG, Wilson JR, Harrop JS, et al. Efficacy and safety of methylprednisolone sodium succinate in acute spinal cord injury: a systematic review. Global Spine J. 2017;7(3 suppl):116S–137S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fehlings MG, Tetreault LA, Wilson JR, et al. A clinical practice guideline for the management of acute spinal cord injury: introduction, rationale, and scope. Global Spine J. 2017;7(3 suppl):84S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. [DOI] [PubMed] [Google Scholar]
- 10. Nishio Y, Koda M, Kitajo K, et al. Delayed treatment with Rho-kinase inhibitor does not enhance axonal regeneration or functional recovery after spinal cord injury in rats. Exp Neurol. 2006;200:392–397. [DOI] [PubMed] [Google Scholar]
- 11. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martinez MN. Factors influencing the use and interpretation of animal models in the development of parenteral drug delivery systems. AAPS J. 2011;13:632–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma HS, Olsson Y, Cervós-Navarro J. Early perifocal cell changes and edema in traumatic injury of the spinal cord are reduced by indomethacin, an inhibitor of prostaglandin synthesis. Experimental study in the rat. Acta Neuropathol. 1993;85:145–153. [DOI] [PubMed] [Google Scholar]
- 14. Sharma HS, Olsson Y, Nyberg F, Dey PK. Prostaglandins modulate alterations of microvascular permeability, blood flow, edema and serotonin levels following spinal cord injury: an experimental study in the rat. Neuroscience. 1993;57:443–449. [DOI] [PubMed] [Google Scholar]
- 15. Gallivan ST, Johnston SA, Broadstone RV, Jortner BS, Reimer M. The clinical, cerebrospinal fluid, and histopathologic effects of epidural ketorolac in dogs. Vet Surg. 2000;29:436–441. [DOI] [PubMed] [Google Scholar]
- 16. Yaksh TL, Horais KA, Tozier N, et al. Intrathecal ketorolac in dogs and rats. Toxicol Sci. 2004;80:322–334. [DOI] [PubMed] [Google Scholar]
- 17. Li Q, Zhang Z, Cai Z. High-dose ketorolac affects adult spinal fusion: a meta-analysis of the effect of perioperative nonsteroidal anti-inflammatory drugs on spinal fusion. Spine (Phila Pa 1976). 2011;36:E461–E468. [DOI] [PubMed] [Google Scholar]
- 18. Pradhan BB, Tatsumi RL, Gallina J, Kuhns CA, Wang JC, Dawson EG. Ketorolac and spinal fusion: does the perioperative use of ketorolac really inhibit spinal fusion? Spine (Phila Pa 1976). 2008;33:2079–2082. [DOI] [PubMed] [Google Scholar]
- 19. Sucato DJ, Lovejoy JF, Agrawal S, Elerson E, Nelson T, McClung A. Postoperative ketorolac does not predispose to pseudoarthrosis following posterior spinal fusion and instrumentation for adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2008;33:1119–1124. [DOI] [PubMed] [Google Scholar]
- 20. Reuben SS, Ablett D, Kaye R. High dose nonsteroidal anti-inflammatory drugs compromise spinal fusion. Can J Anaesth. 2005;52:506–512. [DOI] [PubMed] [Google Scholar]
- 21. Park SY, Moon SH, Park MS, Oh KS, Lee HM. The effects of ketorolac injected via patient controlled analgesia postoperatively on spinal fusion. Yonsei Med J. 2005;46:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glassman SD, Rose SM, Dimar JR, Puno RM, Campbell MJ, Johnson JR. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine (Phila Pa 1976). 1998;23:834–838. [DOI] [PubMed] [Google Scholar]
- 23. Dimar JR, 2nd, Ante WA, Zhang YP, Glassman SD. The effects of nonsteroidal anti-inflammatory drugs on posterior spinal fusions in the rat. Spine (Phila Pa 1976). 1996;21:1870–1876. [DOI] [PubMed] [Google Scholar]
- 24. Rice AS, Lloyd J, Bullingham RE, O’Sullivan G. Ketorolac penetration into the cerebrospinal fluid of humans. J Clin Anesth. 1993;5:459–462. [DOI] [PubMed] [Google Scholar]
- 25. Kumpulainen E, Kokki H, Laisalmi M, et al. How readily does ketorolac penetrate cerebrospinal fluid in children? J Clin Pharmacol. 2008;48:495–501. [DOI] [PubMed] [Google Scholar]
- 26. Mannila A, Kumpulainen E, Lehtonen M, et al. Plasma and cerebrospinal fluid concentrations of indomethacin in children after intravenous administration. J Clin Pharmacol. 2007;47:94–100. [DOI] [PubMed] [Google Scholar]
- 27. Taivainen T, Hiller A, Rosenberg PH, Neuvonen P. The effect of continuous intravenous indomethacin infusion on bleeding time and postoperative pain in patients undergoing emergency surgery of the lower extremities. Acta Anaesthesiol Scand. 1989;33:58–60. [DOI] [PubMed] [Google Scholar]
- 28. Slappendel R, Weber EWG, Benraad B, Dirksen R, Bugter MLT. Does ibuprofen increase perioperative blood loss during hip arthroplasty? Eur J Anaesthesiol. 2002;19:829–831. [DOI] [PubMed] [Google Scholar]
- 29. Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hakan T, Toklu HZ, Biber N, et al. Meloxicam exerts neuroprotection on spinal cord trauma in rats. Int J Neurosci. 2011;121:142–148. [DOI] [PubMed] [Google Scholar]
- 31. Fu Q, Hue J, Li S. Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci. 2007;27:4154–4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guth L, Zhang Z, DiProspero NA, Joubin K, Fitch MT. Spinal cord injury in the rat: treatment with bacterial lipopolysaccharide and indomethacin enhances cellular repair and locomotor function. Exp Neurol. 1994;126:76–87. [DOI] [PubMed] [Google Scholar]
- 33. Hains BC, Yucra JA, Hulsebosch CE. Reduction of pathological and behavioral deficits following spinal cord contusion injury with the selective cyclooxygenase-2 inhibitor ns-398. J Neurotrauma. 2001;18:409–423. [DOI] [PubMed] [Google Scholar]
- 34. Simpson RK, Baskin DS, Dudley AW, Bogue L, Rothenberg F. The influence of long-term nifedipine or indomethacin therapy on neurologic recovery from experimental spinal cord injury. J Spinal Disord. 1991;4:420–427. [DOI] [PubMed] [Google Scholar]
- 35. Sharp KG, Yee KM, Stiles TL, Aguilar RM, Steward O. A re-assessment of the effects of treatment with a non-steroidal anti-inflammatory (ibuprofen) on promoting axon regeneration via RhoA inhibition after spinal cord injury. Exp Neurol. 2013;248:321–337. [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Budel S, Baughman K, Gould G, Song KH, Strittmatter SM. Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J Neurotrauma. 2009;26:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xing B, Li H, Wang H, et al. RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp Neurol. 2011;231:247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pedram MS, Dehghan MM, Shojaee M, et al. Therapeutic effects of simultaneous photobiomodulation therapy (PBMT) and meloxicam administration on experimental acute spinal cord injury: rat animal model. J Photochem Photobiol B. 2018;189:49–54. [DOI] [PubMed] [Google Scholar]
- 39. Redondo-Castro E, Navarro X. Chronic ibuprofen administration reduces neuropathic pain but does not exert neuroprotection after spinal cord injury in adult rats. Exp Neurol. 2014;252:95–103. [DOI] [PubMed] [Google Scholar]
- 40. Campolo M, Esposito E, Ahmad A, Di Paola R, Wallace JL, Cuzzocrea S. A hydrogen sulfide-releasing cyclooxygenase inhibitor markedly accelerates recovery from experimental spinal cord injury. FASEB J. 2013;27:4489–4499. [DOI] [PubMed] [Google Scholar]
- 41. Pantović R, Draganić P, Eraković V, Blagović B, Milin C, Simonić A. Effect of indomethacin on motor activity and spinal cord free fatty acid content after experimental spinal cord injury in rabbits. Spinal Cord. 2005;43:519–526. [DOI] [PubMed] [Google Scholar]
- 42. Bagriyanik HA, Ozogul C, Alaygut E, et al. Neuroprotective effects of ketorolac tromethamine after spinal cord injury in rats: an ultrastructural study. Adv Ther. 2008;25:152–158. [DOI] [PubMed] [Google Scholar]
- 43. Kokki H, Kumpulainen E, Lehtonen M, et al. Cerebrospinal fluid distribution of ibuprofen after intravenous administration in children. Pediatrics. 2007;120:e1002–e1008. [DOI] [PubMed] [Google Scholar]
- 44. Kwon BK, Okon EB, Tsai E, et al. A grading system to evaluate objectively the strength of pre-clinical data of acute neuroprotective therapies for clinical translation in spinal cord injury. J Neurotrauma. 2011;28:1525–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hsieh YC, Cheng H, Chan KH, Chang WK, Liu TM, Wong CS. Protective effect of intrathecal ketorolac in spinal cord ischemia in rats: a microdialysis study. Acta Anaesthesiol Scand. 2007;51:410–414. [DOI] [PubMed] [Google Scholar]
- 46. Hsieh YC, Liang WY, Tsai SK, Wong CS. Intrathecal ketorolac pretreatment reduced spinal cord ischemic injury in rats. Anesth Analg. 2005;100:1134–1139. [DOI] [PubMed] [Google Scholar]
- 47. Siegal T, Siegal T, Shapira Y, Sandbank U, Catane R. Indomethacin and dexamethasone treatment in experimental neoplastic spinal cord compression: part 1. Effect on water content and specific gravity. Neurosurgery. 1988;22:328–333. [DOI] [PubMed] [Google Scholar]
- 48. Siegal T, Siegal T, Shohami E, Shapira Y. Comparison of soluble dexamethasone sodium phosphate with free dexamethasone and indomethacin in treatment of experimental neoplastic spinal cord compression. Spine (Phila Pa 1976). 1988;13:1171–1176. [DOI] [PubMed] [Google Scholar]
- 49. Hallenbeck JM, Jacobs TP, Faden AI. Combined PGI2, indomethacin, and heparin improves neurological recovery after spinal trauma in cats. J Neurosurg. 1983;58:749–754. [DOI] [PubMed] [Google Scholar]
- 50. Zhang Z, Krebs CJ, Guth L. Experimental analysis of progressive necrosis after spinal cord trauma in the rat: etiological role of the inflammatory response. Exp Neurol. 1997;143:141–152. [DOI] [PubMed] [Google Scholar]
- 51. Winkler T, Sharma HS, Stålberg E, Olsson Y. Indomethacin, an inhibitor of prostaglandin synthesis attenuates alteration in spinal cord evoked potentials and edema formation after trauma to the spinal cord: an experimental study in the rat. Neuroscience. 1993;52:1057–1067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Appendix_A for Nonsteroidal Anti-Inflammatory Drugs and Their Neuroprotective Role After an Acute Spinal Cord Injury: A Systematic Review of Animal Models by Mark J. Lambrechts and James L. Cook in Global Spine Journal
Supplemental Material, Appendix_B for Nonsteroidal Anti-Inflammatory Drugs and Their Neuroprotective Role After an Acute Spinal Cord Injury: A Systematic Review of Animal Models by Mark J. Lambrechts and James L. Cook in Global Spine Journal