Abstract
Tumor-infiltrating immune/inflammatory cells, the important components of the tumor microenvironment (TME), remarkably affect the progression of human cancers. To understand the actual conditions within the TME of colorectal cancer (CRC), the interrelationship among tumor-infiltrating neutrophils, M2 macrophages, and regulatory T-cells (Tregs) was systematically analyzed. The infiltration conditions of CD66b+ neutrophils, CD163+ M2 macrophages, and FOXP3+ Tregs in tissue microarrays including 1021 cases of CRC were determined by immunohistochemical analysis. The prediction power of these immune cells for CRC prognosis was evaluated by subgroup analysis of the CRC cohort. Results revealed the existence pattern of infiltrating neutrophils, and Tregs/M2 macrophages fulfilled a “X-low implies Y-high” Boolean relationship, indicative of a mutually exclusive correlation between neutrophils and M2 macrophages, and between neutrophils and Tregs in the TME of CRC. What’s more, the tumor-infiltrating M2 macrophages and Tregs were associated with adverse prognostic factors, whereas neutrophils were corelated with favorable factors. The high infiltration of neutrophils predicted longer survival and better chemotherapeutic response. Nonetheless, high infiltration of M2 macrophages and Tregs predicted poor prognosis. The combination of these tumor-infiltrating immune cells can serve as an effective predictor for the survival of CRC and for the chemotherapeutic outcomes of stage II–III patients.
Keywords: colorectal cancer, immune cell infiltration, macrophages, neutrophils, regulatory T-cells
Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer and the second prevalent cause of cancer-related mortality across the globe.1 Accurate assessment of the prognosis is considered essential for the selection of most appropriate and timely treatment of patients with CRC. Although the tumor–node–metastasis (TNM) staging system is currently one of the commonly used prognostic model by clinicians, it may not provide full prognostic information. Against this backdrop, the identification of new biomarkers is required for more precise classification of CRC and to better guide the treatment. It is quite obvious that cancer progression and metastasis do not depend solely on the autonomous defects of cancer cells, but are also regulated by the tumor microenvironment (TME).2 The components within TME interact with each other, recruit outside cells, and act on cancer cells via secretion of cytokines and chemokines and thus establish the unique tumor behavior in different cancers.3 Consistently, illustrating the conditions of TME components may prove helpful for the evaluation of disease progression and provide potential biomarkers for CRC prognosis.
The immune/inflammatory cells are considered as the essential components of TME and have received tremendous attention as the determinants of cancer progression.2,4 Defining the roles of immune/inflammatory cells in the TME of different cancers is important for the development and application of cancer therapy, especially immunotherapy.5 It is known that selective targeting of tumor-associated inflammatory cells improves antitumor immunity.6 Tumor-infiltrating immune/inflammatory cells are believed to be useful biomarkers for the evaluation of tumor prognosis and the selection of suitable treatment options. CRC has served as a paradigm for the connection between inflammation and cancer,7 and has shown great potential in the exploration of immune microenvironment.8 Cells of the innate immune system, which include but are not limited to neutrophils and macrophages, can be easily detected in colorectal tumors at a very early stage. Furthermore, the cells of the adaptive immune system such as T-cells are subsequently recruited into tumors, where they exhibit either protumorigenic or antitumorigenic roles.9 Thus, immune/inflammatory cells in the TME are considered as prospective predictors of CRC prognosis.10
Tumor-infiltrating neutrophils constitute a significant fraction of the inflammatory cells in the TME. Nonetheless, the contribution of these cells in the inhibition or promotion of tumor development seems to differ depending on the tumor context.11,12 Studies in murine models of cancer have revealed the ability of neutrophils to polarize into two functional antagonistic populations via the suppression or activation of transforming growth factor-beta, referred to as antitumoral (N1) neutrophils and protumoral (N2) neutrophils.13,14 Recently, the existence of functionally distinct phenotypes of tumor-infiltrating neutrophils with interferon-γ (IFN-γ) and granulocyte macrophage colony-stimulating factor (GM-CSF) as the requisite factors for the development of antitumoral phenotype has been demonstrated in human cancer.11 In CRC, tumor-associated neutrophils (TANs) tend to be antitumoral as revealed by previous studies.7,15 It has been reported that the neutrophils play a key role in orchestrating innate and adaptive immune responses in the TME.16 A recent study reported that infiltrating CD66b+ neutrophils prevalently colocalize with CD8+ T-cells in CRC tissues and that TANs stimulate CD8+ T-cells, ultimately causing tumor inhibition.13 Moreover, TANs also act on macrophages and regulatory T-cells (Tregs), but different subtypes of neutrophils seem to have different effects on macrophages and Tregs.17–19 Tumor-infiltrating macrophages have also been found in two phenotypically different populations: M1 and M2 macrophages that perform tumor-suppressive and tumor-supportive functions, respectively.20 Some studies have revealed that IFN-γ alone or together with microbial lipopolysaccharide or cytokines such as tumor necrosis factor and GM-CSF induces polarization of human M1 macrophages and even remodels M2 macrophages into M1.21,22 The M1 macrophages play a classic role in Th1 response and in mediating resistance against tumor cells. The M2 macrophages, specifically marked by CD163,23,24 can produce some chemokines involved in Tregs, Th2, eosinophil, and basophil recruitment.21 And Tregs, characterized by their expression of the forkhead box transcription factor (FOXP3), are a barrier to antitumor immunity. It is now well substantiated that the high frequency of tumor-infiltrating FOXP3+ Tregs predicts poor clinical prognosis in the majority of malignancies.22 Notwithstanding, the role of FOXP3+ T-cells in CRC has been the object of conflicting reports.25–27 Recently, a study showed that a small part of FOXP3+ T-cells expresses low level of FOXP3, which are non-Tregs, and contributes to better prognosis.26 Taken together, many studies point toward tumor-infiltrating neutrophils, M2 macrophages, and Tregs as potential candidates for research. Moreover, they seem to be interconnected by some factors (e.g., IFN-γ). As such, it is also important to systematically analyze the interactions among these cells to better understand the actual conditions within the TME. Unfortunately, very few studies have been carried out on CRC in this direction. Consistently, this study was designed to decipher the correlations among tumor-infiltrating neutrophils, macrophages, and Tregs and to evaluate their prognostic value in CRC. Herein, we examined the infiltrating status of neutrophils, Tregs, and macrophages by detecting immunohistochemical CD66b, FOXP3, and CD163 expression in 1021 CRC tissue microarray (TMA) specimens and systematically analyzed the correlations between their existence conditions and survival outcomes of CRC. The outline for this study is shown in Fig.1.
Figure 1.
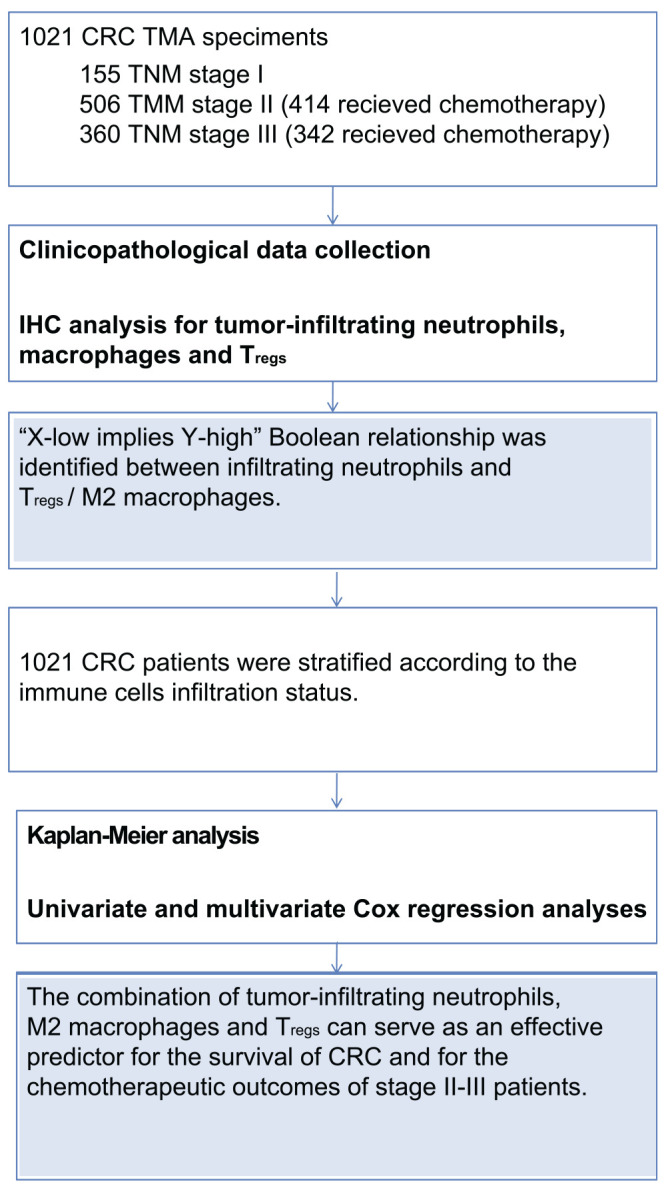
Outline diagram of this study. Abbreviations: CRC, colorectal cancer; TMA, tissue microarray; TNM, tumor–node–metastasis; IHC, immunohistochemistry.
Methods
Patients and Study Design
Pathologically proven formalin-fixed paraffin-embedded (FFPE) tissue specimens of 1021 CRC patients, who received curative surgery in Changhai Hospital of Naval Medical University (Shanghai, China) between September 2009 and December 2012, were enrolled in this study. Patients who received neoadjuvant chemoradiotherapy were not included in this cohort due to the possible influence of radiotherapy on local immune response. According to the National Comprehensive Cancer Network clinical practice guideline, all stage III CRC patients and a part of stage II CRC patients who were diagnosed with adverse prognostic factors, such as T3/T4, intestinal obstruction, or nerve invasion, received a standard postoperative chemotherapy (FOLFOX/CapeOX regimen). The follow-up examination of the patients was carried at our outpatient clinics or at outpatient clinics of local hospitals every 6–12 months. At the follow-up examination, the serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were measured. In addition, the chest computed tomography scans, abdominal ultrasonography, pelvic magnetic resonance imaging, and colonoscopy were performed for all patients. Telephone interview was conducted every 6–12 months to gain information about the condition of the patients. The median follow-up time was 58 months (interquartile range, 21–117 months). Written informed consent was obtained from the patients, and the study was approved by the Institutional Review Boards of Changhai Hospital of Second Military Medical University.
Immunohistochemistry
TMAs containing the FFPE tumor specimens were commercially constructed (Outdo Biotech; Shanghai, China). Primary antibodies against CD66b (clone: G10F5, no. 555723; dilution 1:400; BD Biosciences, San Jose, CA, USA), FOXP3 (clone: 236A/E7, ab20034, dilution 1:100; Abcam, Cambridge, UK), and CD163 (clone: 10D6, MAB-0206, ready-to-use; MXB Biotechnologies, Fuzhou, China) were used, respectively, for antibody incubation as per the manufacturers’ guidelines. Deparaffinage, epitope retrieval (EDTA for CD66b, citrate for FOXP3 and CD163), and immunostaining were carried out on the TMA sections by following the instructions of the pathological department.
Quantitative Evaluation of Immunostaining
Stained TMA slides were digitally scanned using Aperio AT2 (Leica Biosystems) at a resolution of 20× by bright-field microscopy. These images were then accessible using Spectrum (Leica Biosystems). Once slides were scanned, Aperio ImageScope (version 11.2.0.780) was used to view the images for their analysis. Images were examined for quality and were amended, as necessary. Tumor regions were identified and annotated to appropriately represent the heterogeneity of staining for image analysis. The quantitative evaluation of immunostaining was done separately by two independent investigators who were blinded to the clinicopathological characteristics and outcome of patients. Only intratumoral-infiltrating immune cells and not the peritumoral ones were counted in our analysis. Five non-contiguous microscopic fields that represent the densest immune cells were randomly selected from each sample to ensure representativeness and homogeneity. Positive-stained cells in the five random fields were counted manually, and the average count of each sample was recorded. The field area was 0.046 mm2 (100× magnification) for CD163 and 0.1836 mm2 (50× magnification) for CD66b and FOXP3. The average counts for each sample made by the two observers were then compared, and when the difference between their counts was <20% of the maximum value, the average of the two was used as the final count. However, if the difference exceeded 20%, disagreements were resolved by consensus.
Identification of Boolean Relationship
The Boolean implication (BooleanNet)28,29 was detected by evaluating whether the upper-right quadrant in the scatter plot of Fig. 2A is significantly sparsely populated with sample points compared with the other quadrants. When the false-discovery rate (FDR) of a sparsity test was <0.005 in the upper-right quadrant of a “X vs Y” two-axis plot, the Boolean relationship was identified. StepMiner algorithm28 was chosen to define the thresholds of the cell counts, which were used to classify the samples as low or high infiltrated ones. Briefly, for each of the immune cells, we sorted the positive-stained cell counts of all 1021 samples from low to high and fitted the ordered data with a rising step function. Next, we used the StepMiner algorithm to search the step of largest rising jump and identified the point as the threshold.
Figure 2.
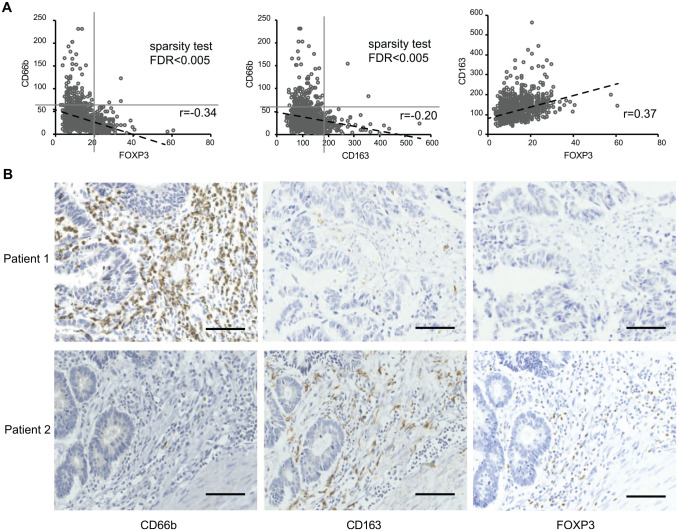
Relationships of the three tumor-infiltrating immune cells and their immunohistochemical staining in CRC specimens. (A) The existence pattern of infiltrating CD66b+ neutrophils and FOXP3+ Tregs, and that of CD66b+ neutrophils and CD163+ M2 macrophages fulfilled the “X-low implies Y-high” Boolean relationship. (B) IHC staining of CD66b, FOXP3, and CD163 in the CRC samples from the same patient: patient 1 with CD66b-high, FOXP3-low, and CD163-low and patient 2 with CD66b-low, FOXP3-high, and CD163-high. Scale bars = 100 µm in length. Abbreviations: CRC, colorectal cancer; IHC, immunohistochemistry; FDR, false-discovery rate.
Statistics
All analyses were performed using SPSS 19.0 for Windows (SPSS; Chicago, IL). Pearson’s correlation methods were performed to identify correlations for quantitative variables with normal distributions. Chi-square test, Fisher’s exact test, Student’s t-test, and Mann–Whitney U-test were used to determine the associations between clinicopathological variables and the infiltrating condition (high or low) of each sample. Kaplan–Meier analysis with log-rank test was performed to estimate disease-free survival (DFS) and overall survival (OS). Univariate and multivariate Cox regression analyses were used to identify independent prognostic factors. The results were considered statistically significant at p<0.05.
Results
Relevance of Tumor-infiltrating Neutrophils, M2 Macrophages, and Tregs in CRC Tissues
To gain insights on the infiltration of neutrophils, M2 macrophages, and Tregs in CRC tissues, immunohistochemical analysis was performed to detect the protein expression of classical markers of the immune cells, CD66b, CD163, and FOXP330–33 The counts of CD66b+ neutrophils, CD163+ macrophages, and FOXP3+ Tregs within tumor beds represented the infiltration conditions of these immune cells.
In the scatter plots of Fig. 2A, it was found that the existence pattern of infiltrating CD66b+ neutrophils and FOXP3+ Tregs, and that of CD66b+ neutrophils and CD163+ M2 macrophages fulfilled the “X-low implies Y-high” Boolean relationship, suggesting their mutual exclusivity. FOXP3+ Tregs and CD163+ M2 macrophages always were found to be either less or absent in tumors with more CD66b+ neutrophil infiltration. However, CD66b+ neutrophils always presented less in tumors with more infiltration of either FOXP3+ Tregs or CD163+ M2 macrophages. This mutual exclusivity between neutrophils and Tregs/M2 macrophages, to some extent, reflects that TANs may play a role in the exclusion of M2 macrophages and Tregs in CRC.
The thresholds were identified as 60 for CD66b, 20 for FOXP3, and 181 for CD163 by StepMiner algorithm. According to these thresholds, the population of 1021 patients was stratified into CD66b-high (136/1021) and CD66b-low (885/1021) subgroups, FOXP3-high (178/1021) and FOXP3-low (843/1021) subgroups, and CD163-high (105/1021) and CD163-low (916/1021) subgroups. Among the 1021 patients, only 3 showed CD66bhiFOXP3hi and 2 showed CD66bhiCD163hi, which were considered as discrete values. The IHC staining of CD66b, FOXP3, and CD163 in the tumor samples from the same patient is shown in Fig. 2B.
Associations Between Tumor-infiltrating Immune Cells and Clinicopathological Characteristics of CRC Patients
The clinicopathological characteristics of the patients with different immune cell infiltration were analyzed as depicted in Table 1. It was found that more tumor-infiltrating FOXP3+ Tregs were significantly associated with low differentiation grade (p<0.001), high TNM stage (p<0.05), high serum CEA (p<0.05), and high serum CA199 (p<0.05). In addition, more tumor-infiltrating CD163+ macrophages were significantly associated with low differentiation grade (p<0.001), high serum CEA (p<0.05), and high serum CA199 (p<0.05), whereas low infiltration of CD66b+ neutrophils was associated with high serum CEA (p<0.001) and high serum CA199 (p<0.05). Taken together, the results point toward a close correlation between the presence of these tumor-infiltrating immune cells and the prognosis of CRC patients.
Table 1.
Characteristics of CRC Patients With Different Immune Cell Infiltration Conditions.
| Characteristics | CD163-Low | CD163-High | P Value | Foxp3-Low | Foxp3-High | P Value | CD66b-Low | CD66b-High | P Value |
|---|---|---|---|---|---|---|---|---|---|
| n = 916 | n = 105 | n = 843 | n = 178 | n = 885 | n = 136 | ||||
| Age, mean ± SD | 60.65 ± 12.28 | 62.29 ± 14.44 | 0.268 | 60.64 ± 12.48 | 61.70 ± 12.68 | 0.305 | 60.79 ± 12.49 | 61.03 ± 12.72 | 0.836a |
| Sex, n (%) | |||||||||
| Male | 535 (58.4) | 71 (67.6) | 521 (59.9) | 102 (57.0) | 538 (59.2) | 85 (60.7) | |||
| female | 381 (41.6) | 34 (32.4) | 0.054 | 349 (40.1) | 77 (43.0) | 0.504 | 371 (40.8) | 55 (39.3) | 0.782b |
| Tumor location, n (%) | |||||||||
| Colon | 518 (55.3) | 58 (51.8) | 505 (59.9) | 87 (56.7) | 525 (59.3) | 81 (59.6) | |||
| Rectum | 419 (44.7) | 54 (48.2) | 0.075 | 338 (40.1) | 92 (43.3) | 0.450 | 360 (40.7) | 55 (40.4) | 1.000b |
| Differential grade, n (%) | |||||||||
| Poor | 35 (3.8) | 11 (10.5) | 30 (3.6) | 16 (9.0) | 42 (4.7) | 4 (2.9) | |||
| Moderate | 792 (86.5) | 73 (69.5) | 713 (84.6) | 152 (85.4) | 747 (84.5) | 118 (86.8) | |||
| Well | 89 (9.7) | 21 (20) | 0.000 | 100 (11.8) | 10 (5.6) | 0.000 | 96 (10.8) | 14 (10.3) | 0.618c |
| TNM stage, n (%) | |||||||||
| I | 145 (15.8) | 10 (9.5) | 130 (15.4) | 25 (14.0) | 133 (15.0) | 22 (16.2) | |||
| II | 447 (48.8) | 59 (56.2) | 431 (51.1) | 75 (42.2) | 437 (49.4) | 69 (50.7) | |||
| III | 324 (35.4) | 36 (34.3) | 0.171 | 282 (33.5) | 78 (43.8) | 0.029 | 315 (35.6) | 45 (33.1) | 0.837c |
| Adjuvant chemotherapy, n (%) | |||||||||
| Yes | 677 (73.9) | 79 (75.2) | 630 (74.7) | 126 (70.8) | 658 (74.4) | 98 (72.1) | |||
| No | 239 (26.1) | 26 (24.8) | 0.815 | 213 (25.3) | 52 (29.2) | 0.301 | 227 (25.6) | 38 (27.9) | 0.600b |
| Serum CEA, n (%) | |||||||||
| <5 ng/ml | 584 (63.8) | 54 (51.4) | 541 (64.2) | 97 (54.5) | 532 (60.1) | 106 (77.9) | |||
| ≥5 ng/ml | 332 (36.2) | 51 (48.6) | 0.015 | 302 (35.8) | 81 (45.5) | 0.017 | 353 (39.9) | 30 (22.1) | 0.000b |
| Serum CA199, n (%) | |||||||||
| <37 U/ml | 790 (86.2) | 81 (77.1) | 729 (86.5) | 142 (79.8) | 747 (84.4) | 124 (91.2) | |||
| ≥37 U/ml | 126 (13.8) | 24 (22.9) | 0.019 | 114 (13.5) | 36 (20.2) | 0.027 | 138 (15.6) | 12 (8.8) | 0.037b |
Abbreviations: CRC, colorectal cancer; TNM, tumor–node–metastasis; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199.
Student’s t-test.
Chi-square test or Fisher’s exact test.
Mann–Whitney U-test (non-parametric).
Prognostic Impact of the Individual Tumor-infiltrating Immune Cells
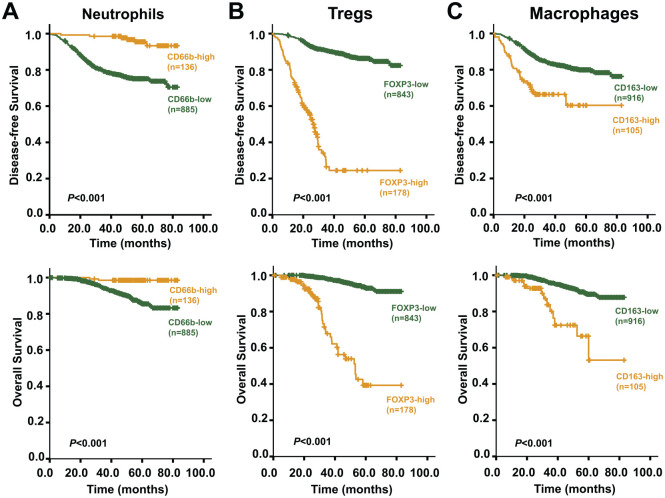
Next, the survival analysis was used to assess the impact of CD66b+ neutrophils, FOXP3+ Tregs, and CD163+ macrophages, individually, on CRC prognosis. Kaplan–Meier curves and log-rank tests revealed that higher numbers of tumor-infiltrating CD66b+ neutrophils were significantly (p<0.001) associated with both longer DFS and OS for CRC patients (Fig. 3A), whereas more marked infiltration of CD163+ M2 macrophages or FOXP3+ Tregs into tumor tissues was significantly (p<0.001) associated with both shorter DFS and OS (Fig. 3B and C).
Figure 3.
Kaplan–Meier survival curves showing comparison of DFS and OS between high and low infiltration of immune/inflammatory cells. (A) Comparisons of DFS and OS between high and low infiltration of neutrophils (CD66b+); (B) comparisons of DFS and OS between high and low infiltration of Tregs (FOXP3+); (C) comparisons of DFS and OS comparison between high and low infiltration of M2 macrophages (CD163+). Abbreviations: DFS, disease-free survival; OS, overall survival.
Moreover, as each of the parameters of tumor-infiltrating immune cells was subjected to multivariate Cox regression analysis, taking other significant factors (tumor grade, TNM stage, serum CEA, serum CA199) which had been screened by univariate analysis as covariates, all tumor-infiltrating immune cells were found to be independent predictors of DFS and OS for CRC patients. Fewer CD66b+ neutrophil infiltration was associated with high risks of recurrence and death, and more CD163+ M2 macrophage or FOXP3+ Tregs infiltration was associated with high rate of recurrence and death (Table 2).
Table 2.
Cox Regression Analysis of Infiltrating Immune Cells and Clinicopathological Covariates.
| Characteristics | Disease-free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CD66b-low vs CD66b-high | 6.672 (2.958–15.05) | <0.001 | 3.969 (1.739–9.061) | <0.001 | 9.378 (2.304–38.174) | <0.05 | 5.393 (1.307–22.243) | <0.05 |
| CD163-high vs CD163-low | 2.781 (1.914–4.042) | <0.001 | 1.707 (1.159–2.516) | <0.05 | 4.597 (2.587–8.166) | <0.001 | 2.101 (1.156–3.818) | <0.05 |
| FOXP3-high vs FOXP3-low | 10.25 (7.618–13.792) | <0.001 | 8.009 (5.862–10.942) | <0.001 | 14.177 (9.021–22.28) | <0.001 | 10.039 (6.176–16.318) | <0.001 |
| Age (>60 vs ≤60) | 1.051 (0.799–1.383) | 0.721 | 1.009 (0.656–1.551) | 0.969 | ||||
| Gender (male vs female) | 1.052 (0.795–1.391) | 0.724 | 1.095 (0.705–1.699) | 0.687 | ||||
| Location (rectal vs colon) | 0.891 (0.676–1.174) | 0.411 | 0.878 (0.569–1.355) | 0.557 | ||||
| TNM (per increase in stage) | 1.938 (1.551–2.421) | <0.001 | 1.832 (1.46–2.298) | <0.001 | 1.271 (0.914–1.767) | 0.155 | 1.036 (0.733–1.462) | 0.843 |
| Grade (well vs moderate vs poor) | 0.287 (0.197–0.419) | <0.001 | 0.339 (0.236–0.488) | <0.001 | 0.266 (0.148–0.479) | <0.001 | 0.311 (0.173–0.558) | <0.001 |
| Chemo (no vs yes) | 1.786 (1.235–2.581) | <0.05 | 1.146 (0.686–1.913) | 0.603 | ||||
| Serum CEA (<5 ng/ml vs ≥5 ng/ml) | 0.604 (0.459–0.795) | <0.001 | 0.758 (0.567–1.013) | 0.061 | 0.613 (0.398–0.944) | <0.05 | 0.557 (0.353–0.879) | <0.05 |
| Serum CA199 (<37 U/ml vs ≥37 U/ml) | 0.517 (0.372–0.719) | <0.001 | 0.762 (0.537–1.08) | 0.126 | 0.679 (0.388–1.188) | 0.175 | 0.975 (0.543–1.753) | 0.934 |
Abbreviations: HR, hazard ratio; CI, confidence interval; TNM, tumor–node–metastasis; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199.
Prognostic Impacts of the Combinations of Tumor-infiltrating Immune Cells
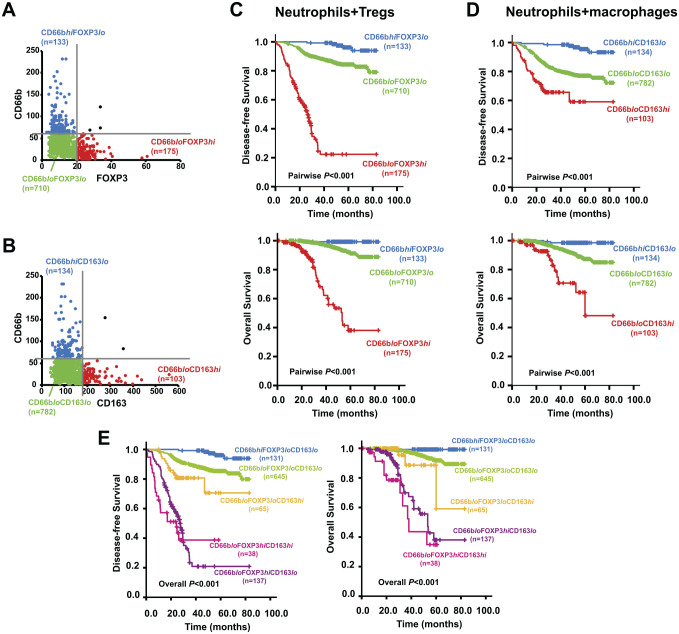
The combined existence patterns of the infiltrating immune cells demonstrated the emergence of several subgroups: CD66bhiFOXP3lo (133/1021), CD66bloFOXP3lo (710/1021) and CD66bloFOXP3hi (175/1021) (Fig. 4A); CD66bhiCD163lo (134/1021), CD66bloCD163lo (782/1021) and CD66bloCD163hi (103/1021) (Fig. 4B); CD66bhiFOXP3loCD163lo (131/1021), CD66bloFOXP3loCD163lo (645/1021), CD66bloFOXP3loCD163hi (65/1021), CD66bloFOXP3hiCD163lo (137/1021), and CD66bloFOXP3hiCD163hi (38/1021). To further clarify the role of tumor-infiltrating immune cells in CRC prognosis, Kaplan–Meier analysis and multivariate Cox regression analysis were carried out to evaluate the association of patient survival outcomes with various combinations of tumor-infiltrating cells.
Figure 4.
Classification of different subgroups based on the combinations of immune/inflammatory cell infiltration and the survival curves of these subgroups showing comparison of DFS and OS. (A) Subgroups classified by the combination of infiltrating CD66b+ neutrophils and FOXP3+ Tregs; (B) subgroups classified by the combination of infiltrating CD66b+ neutrophils and CD163+ M2 macrophages; (C) comparison of DFS and OS among different subgroups classified by the combination of infiltrating CD66b+ neutrophils and FOXP3+ Tregs; (D) comparison of DFS and OS among different subgroups classified by the combination of infiltrating CD66b+ neutrophils and CD163+ M2 macrophages; (E) DFS and OS comparison among subgroups classified by the three-marker classifier. Abbreviations: DFS, disease-free survival; OS, overall survival.
When tumor-infiltrating CD66b+ neutrophils and FOXP3+ Tregs were combined as a two-marker classifier, the 5-year DFS and OS rate was the lowest for patients with CD66bloFOXP3hi infiltration, higher for those with CD66bloFOXP3lo infiltration, and the highest for those with CD66bhiFOXP3lo infiltration (DFS: 22.3% vs 84.4% vs 96.1%, p<0.01; OS: 38.1% vs 91.2% vs 99.2%, p<0.01). Patients with more FOXP3+ Tregs infiltration and concurrently less CD66b+ neutrophil infiltration had shorter DFS and OS (Fig. 4C). Multivariate Cox regression analysis showed that this two-marker classifier was an independent predictor for DFS and OS and that CD66bloFOXP3hi infiltration was associated with the highest hazard ratio (HR) of 38.848 (95% confidence interval [CI], 15.532–97.164; p<0.001) for recurrence and 108.311 (95% CI, 15.532–97.164; P < 0.001) for death, together with other significant covariates (Table 3).
Table 3.
Multivariate Cox Regression Analysis of Two-marker Infiltrating Immune Cells and Clinicopathological Covariates.
| Character | Disease-free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CD66b(A) and FOXP3(B) | ||||
| AhiBlo | 1 | 1 | ||
| AloBlo | 4.601 (1.869–11.329) | <0.001 | 10.038 (1.379–73.089) | <0.05 |
| AloBhi | 38.848 (15.532–97.164) | <0.001 | 108.311 (14.726–796.638) | <0.001 |
| TNM (per increase in stage) | 1.825 (1.457–2.287) | <0.001 | 1.087 (0.769–1.536) | 0.637 |
| Grade (poor vs moderate vs well) | 0.325 (0.225–0.47) | <0.001 | 0.324 (0.18–0.584) | <0.001 |
| CEA (<5 ng/ml vs ≥5 ng/ml) | 0.731 (0.549–0.975) | <0.05 | 0.58 (0.367–0.915) | <0.05 |
| CA199 (<37 U/ml vs ≥37 U/ml) | 0.783 (0.553–1.108) | 0.167 | 0.955 (0.53–1.72) | 0.877 |
| CD66b(A) and CD163(B) | ||||
| AhiBlo | 1 | 1 | ||
| AloBlo | 5.613 (2.478–12.713) | <0.001 | 7.665 (1.874–31.358) | <0.05 |
| AloBhi | 14.68 (6.076–35.468) | <0.001 | 34.614 (7.768–154.238) | <0.001 |
| TNM (per increase in stage) | 1.976 (1.576–2.478) | <0.001 | 1.356 (0.971–1.895) | 0.074 |
| Grade (poor vs moderate vs well) | 0.277 (0.195–0.395) | <0.001 | 0.232 (0.132–0.41) | <0.001 |
| CEA (<5 ng/ml vs ≥5 ng/ml) | 0.809 (0.603–1.085) | 0.157 | 0.675 (0.429–1.063) | 0.09 |
| CA199(<37 U/ml vs ≥37 U/ml) | 0.715 (0.504–1.013) | 0.059 | 0.96 (0.535–1.725) | 0.893 |
Abbreviations: HR, hazard ratio; CI, confidence interval; TNM, tumor–node–metastasis; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199.
Similar observations were also made in the combination of infiltrating CD66b+and CD163+ cells. Patients with CD66bloCD163hi had the shortest DFS and OS among all three subgroups, whereas patients with CD66bhiCD163lo had the longest DFS and OS (Fig. 4D). The 5-year DFS and OS rate was 59.1% vs 77.1% vs 95.4% (p<0.01) and 48.2% vs 87.3% vs 93.5% (p<0.01), respectively, among CD66bloCD163hi, CD66bloCD163lo, and CD66bhiCD163lo subgroups. When the CD66bhiCD163lo subgroup was used as a reference, the combination of infiltrating CD66b+ neutrophils and CD163+ macrophages could independently predict the prognosis of CRC with gradually increased HR values (Table 3).
Combining the infiltration of three immune cells together as a three-marker classifier, the survival outcomes among CRC patients could be further discriminated. The most favorable DFS and OS were shown in patients with CD66bhiFOXP3loCD163lo immune cell infiltration, followed by CD66bloFOXP3loCD163lo, CD66bloFOXP3loCD163hi, and CD66bloFOXP3hiCD163lo in sequence, and patients with CD66bloFOXP3hiCD163hi infiltration showed the poorest DFS and OS (Fig. 4E). Multivariate Cox regression analysis also revealed this three-marker classifier as an independent predictor of DFS and OS and that the risks of recurrence and death gradually increased when the CD66bhiCD163loFOXP3lo subgroup was used as a reference, followed by CD66bloCD163loFOXP3lo (HR = 4.243 for DFS, HR = 9.516 for OS), CD66bloCD163loFOXP3hi (HR = 12.156 for DFS, HR =22.805 for OS), CD66bloCD163hiFOXP3lo (HR = 38.257 for DFS, HR = 91.951 for OS), and CD66bloCD163hiFOXP3hi (HR = 50.604 for DFS, HR = 190.152 for OS), all ps<0.05 (Table 4). These results above indicate that the survival outcomes of CRC patients could be more effectively and specifically predicted according to the infiltration pattern of the three immune cells.
Table 4.
Multivariate Cox Regression Analysis of Three-marker Infiltrating Immune Cells and Clinicopathological Covariates.
| Character | Disease-free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| CD66b(A), FOXP3(B), and CD163(C) | ||||
| AhiBloClo | 1 | 1 | ||
| AloBloClo | 4.243 (1.718–10.478) | <0.05 | 9.516 (1.305–69.41) | <0.05 |
| AloBloChi | 12.156 (4.261–34.678) | <0.001 | 22.805 (2.335–222.679) | <0.05 |
| AloBhiClo | 38.257 (15.194–96.327) | <0.001 | 91.951 (12.377–683.107) | <0.001 |
| AloBhiChi | 50.604 (18.634–137.426) | <0.001 | 190.152 (24.364–1484.058) | <0.001 |
| TNM (per increase in stage) | 1.876 (1.493–2.357) | <0.001 | 1.078 (0.759–1.529) | 0.676 |
| Grade (poor vs moderate vs well) | 0.33 (0.23–0.473) | <0.001 | 0.305 (0.17–0.55) | <0.001 |
| CEA (<5 ng/ml vs ≥5 ng/ml) | 0.761 (0.569–1.017) | 0.065 | 0.558 (0.352–0.884) | <0.05 |
| CA199 (<37 U/ml vs ≥37 U/ml) | 0.781 (0.551–1.108) | 0.166 | 0.986 (0.549–1.774) | 0.964 |
Abbreviations: HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis; CEA, carcinoembryonic antigen; CA199, carbohydrate antigen 199.
Immune Cell Infiltration and the Prognosis of CRC Patients Who Received Adjuvant Chemotherapy
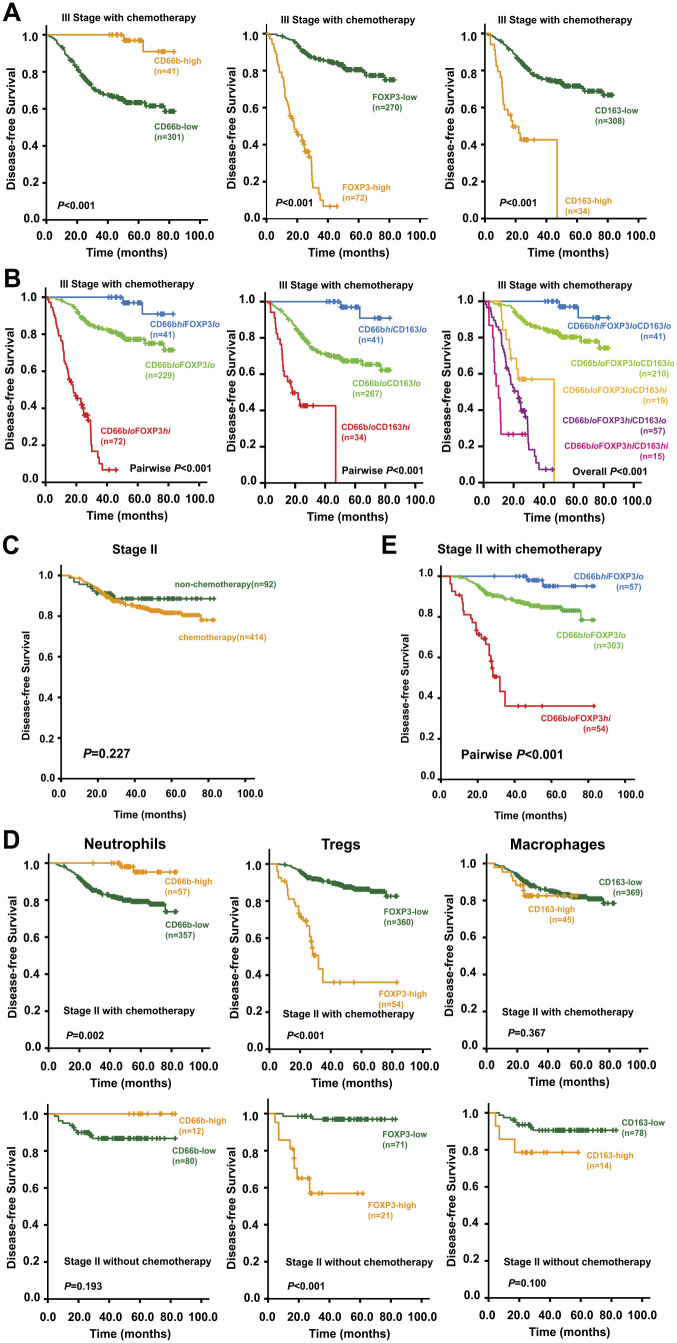
Next, the association between immune cell infiltration status and DFS was examined among patients who either received or did not receive the adjuvant chemotherapy. In our cohort, almost all patients with stage III CRC (342/360) received postoperative chemotherapy. Stage III CRC patients with high infiltration of CD66b+ neutrophils or low infiltration of FOXP3+ Tregs/CD163+ M2 obtained better outcomes after receiving adjuvant chemotherapy (Fig. 5A). Furthermore, combining the infiltration status of CD66b+ neutrophils with that of FOXP3+ Tregs or CD163+ M2 could further discriminate the prognosis after adjuvant chemotherapy (Fig. 5B). The survival curves showed that the immune cell infiltration discriminatorily predicted the prognosis of stage III CRC patients who received adjuvant chemotherapy. And as expected, the patients gained the optimum survival outcomes when the favorable infiltration status of the three immune cells was combined. The results reveal that the infiltration status of the three immune cells could be a predictor for chemosensitivity of CRC.
Figure 5. (continued).
Kaplan–Meier survival curves of stage II and stage III CRC patients with or without chemotherapy. (A) Kaplan–Meier survival curves for comparison of DFS between high and low infiltration of individual immune/inflammatory cells among stage III CRC patients with chemotherapy. (B) Kaplan–Meier survival curves of stage III CRC patients with chemotherapy showing the comparison of DFS among the subgroups classified by different combinations. (C) Kaplan–Meier survival curves showing the comparison of DFS between stage II patients with and without chemotherapy. (D) Kaplan–Meier survival curves showing the comparison of DFS between high and low infiltration of individual immune/inflammatory cells among stage II CRC patients with or without chemotherapy. (E) Kaplan–Meier survival curves showing the comparison of DFS among different subgroups classified by the combination of CD66b+ neutrophils and FOXP3+ Tregs among stage II CRC patients with chemotherapy. Abbreviations: CRC, colorectal cancer; DFS, disease-free survival.
Among the 506 patients with stage II CRC, 414 patients were diagnosed with adverse prognostic factors and received postoperative chemotherapy, and rest of the 92 did not. Treatment with adjuvant chemotherapy was not associated with a higher 5-year DFS rate for stage II CRC in our cohort (Fig. 5C). Considering the clinicopathological differences between patients who received chemotherapy and those who did not, the stage II CRC patients were divided into chemo-group and non-chemo-group, and subsequently the contribution of immune cell infiltration to stage II CRC survival and chemotherapy outcomes was evaluated. Survival analyses showed that high infiltration of CD66b+ neutrophils was associated with significantly better DFS in stage II CRC patients treated with adjuvant chemotherapy. However, the association was not significant for those who were not treated. The high density of FOXP3+ Tregs infiltration indicated poor DFS for patients either treated or not treated with adjuvant chemotherapy. However, the infiltrating status of CD163+ M2 failed to predict survival outcomes of stage II CRC patients with or without adjuvant chemotherapy (Fig. 5D). Taken together, combining CD66b+ neutrophils and FOXP3+ Tregs as a two-marker classifier could clearly distinguish the DFS of stage II CRC patients with adjuvant chemotherapy (Fig. 5E). This indicates that the infiltrating condition of CD66b+ neutrophils combined with FOXP3+ Tregs may predict the chemotherapeutic response of stage II CRC patients, which could be used as an assessment factor for the selection of postoperative treatment of stage II CRC patients.
Discussion
Immunophenotyping (type, density, and location of immune cells) of the tumor samples has been found to have superior value for the prognosis of CRC. Some researchers even consider it as a better predictor than the traditional staging method.8,34 This study started with the analysis of prognostic roles of tumor-infiltrating neutrophils, M2 macrophages, and Tregs in a large cohort of human CRC. The correlations between these cells in CRC tissues and their combined prognostic value were examined by immunohistochemistry.
The findings of this study showed that the infiltration of M2 macrophages and Tregs was associated with adverse prognostic factors, such as poor differentiation, high TNM stage, and high levels of tumor markers. In contrary, the infiltration with neutrophils was related to favorable factors (Table 1). As expected, the results of survival analysis suggested the unfavorable prognostic roles of tumor-infiltrating M2 macrophages and Tregs, and the favorable prognostic role of tumor-infiltrating neutrophils (Fig. 3), with all of them as independent predictors of DFS and OS for CRC (Table 2). Although the neutrophil infiltration has been associated with poor prognosis in diverse human tumors,35–38 our results demonstrate that tumor-infiltrating neutrophils in CRC present the antitumoral phenotype, which is consistent with several recent studies on CRC.7,15 The M2 macrophages known to promote tumor growth and angiogenesis, and suppress adaptive immunity, are associated with poor prognosis in numerous cancers including CRC.30,39–43 Such results were further validated in our cohort. Tumor-infiltrating FOXP3+ T-cells have functionally distinct subpopulations which contribute in opposing ways to determine the prognosis, but no better marker has been identified to define them.25,26,44 As cells with high levels of FOXP3 are considered as Tregs and CRCs are commonly infiltrated by these FOXP3hi Tregs,26 FOXP3 was used as the biomarker of Tregs and only strongly stained cells were counted in IHC analysis. Our results showed that infiltrating Tregs predict poor prognosis in CRC as they do in other cancers.
It is well established that immune cells interact and communicate with each other closely.18,45 In CRC, there is a positive correlation between the infiltration of CD66b+ neutrophils and CD8+ T-cells. The CD66b+ neutrophils enhance the responsiveness of CD8+ T-cells to T-cell receptor triggering.15 In this study, the existence of a mutually exclusive correlation between tumor-infiltrating neutrophils and M2 macrophages and between neutrophils and Tregs was reported. The M2 macrophages and Tregs always showed less in tumors with more neutrophil infiltration, and neutrophils always showed less in tumors with more infiltration of either M2 macrophages or Tregs. This study for the first time revealed the spatial correlations of neutrophils, M2 macrophages, and Tregs in more than 1000 cases of human CRC tumors, which is indicative of a repulsive interaction between these immune cells. In some human cancers, the protumoral TANs can recruit M2 macrophages and Tregs.17 However, if antitumoral neutrophils have the contrary action of excluding M2 macrophages, Tregs remain largely unknown. The findings of this study provide important insights and prospectus for future research. These immune cells should be considered as a whole presenting the immunophenotyping of CRC, due to the close relationships and potential interactions between them. However, most of the previous studies focused on only one of them, and the results could be biased. This study clarified that the combination of tumor-infiltrating neutrophils, M2 macrophages, and Tregs is a better predictor for patient survival, compared with any single immune cell infiltration condition. And the infiltration of these immune cells also relates to the effects of chemotherapy. These results of the significant roles of immune cells in CRC are in agreement with previous studies.10
In the transition from benign lesion to malignant invasive cancer, the TME is flooded with chemokines, cytokines, and growth factors.21 Under this context, immune cells and inflammatory cells are regulated by the TME of recruitment, viability, polarization, and distribution,44,45 and they also interact with each other through paracrine pathway. It has been known that IFN-γ induces the antitumoral phenotype in human neutrophils. Such neutrophils are capable of cross-presenting antigens and triggering and augmenting T-cell responses.11,14 Furthermore, immunosuppressive M2 macrophages could be “re-programmed” by some immunological stimuli, such as IFN-γ or interferon-α, into immunostimulatory M1 macrophages.21,22,46 A recent study has reported that IFN-γ could drive Treg fragility to promote antitumor immunity.47 It seems that IFN-γ plays a dominant role in shaping immune environment. Moreover, IFN-γ-dominant immune profiles signified an improved prognosis.48 It has been reported that genetic variations in IFN-γ and its receptor are closely associated with the risk of CRC and survival after diagnosis49 and that the deficiency of IFN-γ or its receptor promotes the development of CRC. Above reports just explain the interrelationship among the three immune cells that we observed and exhibit consistency with our results.
With a certain threshold level of IFN-γ in the tumor environment, tumor-infiltrating neutrophils tend to be induced into an antitumoral phenotype, macrophages are reprogrammed from immunosuppressive M2 into immunostimulatory M1, and resident Tregs are functionally fragile. In this condition, Treg recruitment is restricted, and antitumor immunity is promoted. However, we failed to find a clear correlation between the mRNA expression of IFN-γ and the expression of immune cell markers in TCGA data sets. Insufficient sample size and the deviation between mRNA expression and protein expression could be the reasons. As TME is multifactorial, dynamic, and tumor-specific, IFN-γ may be just one of the candidates. More research endeavors are required to elucidate the function of IFN-γ on these immune cells in the TME of CRC. If possible, in vivo experiments using neutrophil-recruiting chemokine-overexpressing mouse cell lines could be performed to study on the mechanism. More potential factors need to be unveiled, and inducing those factors into tumors could become novel treatment options for CRC through optimizing TME and regulating antitumor immunity.
In conclusion, the combination of tumor-infiltrating neutrophils, M2 macrophages, and Tregs can serve as an effective predictor for the survival of CRC and for the chemotherapeutic outcomes of stage II–III patients. There exists a mutually exclusive correlation between tumor-infiltrating neutrophils and M2 macrophages, and between tumor-infiltrating neutrophils and Tregs. Additional studies are required to validate the interaction among tumor-infiltrating neutrophils, M2 macrophages, and Tregs.
Acknowledgments
The authors acknowledge Dr. Hanlin Tang, Department of microbiology, Second Military Medical University, Shanghai, for providing bioinformatics assistance and the doctors of Department of Pathology, Changhai Hospital, Second Military Medical University, Shanghai, for their technical support. The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: XX and GY contributed equally to this paper. GY, JM and XX was in charge of IHC staining and the quantitative evaluation of immunostaining. XX and FC analyzed the whole data independently. QQ was responsible for the follow-up of CRC patients. WZ and GY was involved in the pathological diagnosis and recruitment of patients in the hospital. XX and JM were responsible for statistical analysis. XX, WZ, and FC designed and organized the study and wrote the manuscript. All authors read and approved the final version of the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the grant from the National Natural Science Foundation of China (81402005 to Fuao Cao) and sponsored by Shanghai Sailing Program (20YF1450100 to Xiaowen Xu).
Contributor Information
Xiaowen Xu, Department of Digestive Endoscopy, Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China.
Jun Ma, Department of Colorectal Surgery, Changhai Hospital, Naval Medical University, Shanghai, China.
Guanyu Yu, Department of Colorectal Surgery, Changhai Hospital, Naval Medical University, Shanghai, China.
Qun Qiu, Department of Colorectal Surgery, Changhai Hospital, Naval Medical University, Shanghai, China.
Wei Zhang, Department of Colorectal Surgery, Changhai Hospital, Naval Medical University, Shanghai, China.
Fuao Cao, Department of Colorectal Surgery, Changhai Hospital, Naval Medical University, Shanghai, China.
Literature Cited
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. 2018. Nov; 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013. Nov; 19(11):1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hui LL, Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015. Nov 1;368(1):7–13. [DOI] [PubMed] [Google Scholar]
- 4. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016. Aug 23;16(9):582–98. [DOI] [PubMed] [Google Scholar]
- 5. Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Brit J Cancer. 2013. Mar 5;108(4): 914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, Jacobs RC, Ye J, Patel AA, Gillanders WE, Fields RC, DeNardo DG, Hawkins WG, Goedegebuure P, Linehan DC. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018. Jun;67(6):1112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S, Polentarutti N, Malesci A, Marone G, Roncalli M, Laghi L, Garlanda C, Mantovani A, Jaillon S. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016. Jul 15;139(2):446–56. [DOI] [PubMed] [Google Scholar]
- 8. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006. Sep 29;313(5795):1960–4. [DOI] [PubMed] [Google Scholar]
- 9. Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010. Jun;138(6):2101–14. [DOI] [PubMed] [Google Scholar]
- 10. Ye LL, Zhang TM, Kang ZC, Guo GQ, Sun YJ, Lin KM, Huang QJ, Shi XY, Ni ZL, Ding N, Zhao KN, Chang WJ, Wang JJ, Lin F, Xue XY. Tumor-infiltrating immune cells act as a marker for prognosis in colorectal cancer. Front Immunol. 2019. Oct 17;10:2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singhal S, Bhojnagarwala PS, O’Brien S, Moon EK, Garfall AL, Rao AS, Quatromoni JG, Stephen TL, Litzky L, Deshpande C, Feldman MD, Hancock WW, Conejo-Garcia JR, Albelda SM, Eruslanov EB. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016. Jul 11;30(1):120–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saha S, Biswas SK. Tumor-associated neutrophils show phenotypic and functional divergence in human lung cancer. Cancer Cell. 2016. Jul 11;30(1):11–3. [DOI] [PubMed] [Google Scholar]
- 13. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng GJ, Ling LN, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009. Sep 8;16(3):183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017. Jan 17;46(1):15–28. [DOI] [PubMed] [Google Scholar]
- 15. Governa V, Trella E, Mele V, Tornillo LG, Amicarella F, Cremonesi E, Muraro MG, Xu H, Droeser R, Daster SR, Bolli M, Rosso R, Oertli D, Eppenberger-Castori S, Terracciano LM, Iezzi G, Spagnoli GC. The interplay between neutrophils and CD8(+) T cells improves survival in human colorectal cancer. Clin Cancer Res. 2017. Jul 15;23(14):3847–58. [DOI] [PubMed] [Google Scholar]
- 16. Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2016. Jan;37(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016. Jun;150(7):1646–58. [DOI] [PubMed] [Google Scholar]
- 18. Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z, Fridlender ZG. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17-A new mechanism of impaired antitumor immunity. Int J Cancer. 2014. Sep 1;135(5):1178–86. [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat Inflamm. 2016;2016:6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou WC, Ke SQ, Huang Z, Flavahan W, Fang XG, Paul J, Wu L, Sloan AE, McLendon RE, Li XX, Rich JN, Bao SD. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015. Feb;17(2):170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013. Nov;218(11):1402–10. [DOI] [PubMed] [Google Scholar]
- 22. Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer. 2009. Jul 15;125(2):367–73. [DOI] [PubMed] [Google Scholar]
- 23. Huang XP, Pan YM, Ma J, Kang ZC, Xu XW, Zhu Y, Chen JK, Zhang W, Chang WJ, Zhu JB. Prognostic significance of the infiltration of CD163(+) macrophages combined with CD66b(+) neutrophils in gastric cancer. Cancer Med. 2018. May;7(5):1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TRDJ, Reedquist KA, Tak PP, Baeten DLP. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012. Jan 31;375(1–2):196–206. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017. Jan;27(1):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016. Jun;22(6):679–84. [DOI] [PubMed] [Google Scholar]
- 27. Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010. Jun 1;126(11):2635–43. [DOI] [PubMed] [Google Scholar]
- 28. Sahoo D, Dill DL, Gentles AJ, Tibshirani R, Plevritis SK. Boolean implication networks derived from large scale, whole genome microarray datasets. Genome Biol. 2008. Oct 30;9(10):R157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dalerba P, Sahoo D, Paik S, Guo XQ, Yothers G, Song N, Wilcox-Fogel N, Forgo E, Rajendran PS, Miranda SP, Hisamori S, Hutchison J, Kalisky T, Qian DL, Wolmark N, Fisher GA, van de Rijn M, Clarke MF. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. New Engl J Med. 2016. Jan 21;374(3):211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Herrera M, Herrera A, Dominguez G, Silva J, Garcia V, Garcia JM, Gomez I, Soldevilla B, Munoz C, Provencio M, Campos-Martin Y, de Herreros AG, Casal I, Bonilla F, Pena C. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 2013. Apr;104(4):437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009. Apr;9(4):239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dominguez-Soto A, Sierra-Filardi E, Puig-Kroger A, Perez-Maceda B, Gomez-Aguado F, Corcuera MT, Sanchez-Mateos P, Corbi AL. Dendritic cell-specific ICAM-3-grabbing nonintegrin expression on M2-polarized and tumor-associated macrophages is macrophage-CSF dependent and enhanced by tumor-derived IL-6 and IL-10. J Immunol. 2011. Feb 15;186(4):2192–200. [DOI] [PubMed] [Google Scholar]
- 33. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003. Feb 14;299(5609):1057–61. [DOI] [PubMed] [Google Scholar]
- 34. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013. Oct;14(10):1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trellakis S, Farjah H, Bruderek K, Dumitru CA, Hoffmann TK, Lang S, Brandau S. Peripheral blood neutrophil granulocytes from patients with head and neck squamous cell carcinoma functionally differ from their counterparts in healthy donors. Int J Immunopath Ph. 2011. Jul–Sep;24(3):683–93. [DOI] [PubMed] [Google Scholar]
- 36. Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011. May;54(5):948–55. [DOI] [PubMed] [Google Scholar]
- 37. Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009. Oct 1;27(28):4709–17. [DOI] [PubMed] [Google Scholar]
- 38. Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, Antoine M, Soler P, Cadranel J. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003. Mar 15;63(6):1405–12. [PubMed] [Google Scholar]
- 39. Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I, Munesue S, Harashima A, Harada S, Yamamoto H, Ohta T. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016. Oct;19(4):1052–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008. Sep;216(1):15–24. [DOI] [PubMed] [Google Scholar]
- 41. Niino D, Komohara Y, Murayama T, Aoki R, Kimura Y, Hashikawa K, Kiyasu J, Takeuchi M, Suefuji N, Sugita Y, Takeya M, Ohshima K. Ratio of M2 macrophage expression is closely associated with poor prognosis for Angioimmunoblastic T-cell lymphoma (AITL). Pathol Int. 2010. Apr;60(4):278–83. [DOI] [PubMed] [Google Scholar]
- 42. Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, Beppu T, Baba H, Takeya M. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010. Aug;101(8):1913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M, Takeya M. Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci. 2011. Jul;102(7):1424–31. [DOI] [PubMed] [Google Scholar]
- 44. Masugi Y, Nishihara R, Yang J, Mima K, da Silva A, Shi Y, Inamura K, Cao Y, Song MY, Nowak JA, Liao XY, Nosho K, Chan AT, Giannakis M, Bass AJ, Hodi FS, Freeman GJ, Rodig S, Fuchs CS, Qian ZR, Ogino S. Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut. 2017. Aug;66(8):1463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015. Apr 3;348(6230):74–80. [DOI] [PubMed] [Google Scholar]
- 46. De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S, Falini A, Guiclotti LG, Galli R, Naldini L. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008. Oct 7;14(4):299–311. [DOI] [PubMed] [Google Scholar]
- 47. Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali DAA. Interferon-gamma drives Treg fragility to promote anti-tumor immunity. Cell. 2017. Jun 1;169(6):1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan HN, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015. Jan;5(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Slattery ML, Lundgreen A, Bondurant KL, Wolff RK. Interferon-signaling pathway: associations with colon and rectal cancer risk and subsequent survival. Carcinogenesis. 2011. Nov;32(11):1660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]