Abstract
Background:
Deep sternal wound infections are a financially costly complication of cardiac surgery with serious implications for patient morbidity and mortality. Prophylactic antimicrobials have been shown to reduce the incidence of infection significantly. In 2018, the European Association for CardioThoracic Surgery (EACTS) provided clear guidance advising that third-generation cephalosporins are the first-line prophylactic antimicrobial of choice for cardiac surgery via median sternotomy as a result of their broad spectrum of activity and association with reduced postoperative mortality. Despite this guidance, it was believed that UK practice differed from this as a consequence of national concerns surrounding cephalosporins use and Clostridioides difficile infection.
Methods:
A survey was developed and distributed to all UK and Republic of Ireland (ROI) cardiac surgery centres in January 2019 to quantify this variation.
Results:
Of the 38 centres, 34 responded. Variation existed between the antimicrobial agent used, as well as the dosage, frequency and duration of suggested regimens even among centres using the same antimicrobial agent. The most common antimicrobial prophylaxis prescribed was a combination of flucloxacillin and gentamicin (16, 47%). Followed by cefuroxime (6, 17.6%) and cefuroxime combined with a glycopeptide (4, 11.7%). In patients colonised with methicillin-resistant Staphylococcus aureus or those with penicillin allergy gentamicin combined with teicoplanin was most common (42% and 50%, respectively).
Discussion:
This variation in antimicrobial agents and regimens may well contribute to the varying incidence of surgical site infection seen across the UK and ROI.
Keywords: Cardiac surgery, surgical site infection, deep sternal wound infection, antimicrobial prophylaxis, cephalosporins, Clostridioides difficile, Clostridium difficile, adult, infection prevention
Background
Surgical site infection (SSI) remains a costly complication of cardiac surgery (Findeisen et al., 2018; Graf et al., 2010), with an incidence in the range of 1.3%–7.9% (Figuerola-Tejerina et al., 2016; Mannien et al., 2011; Pan et al., 2017). It has been demonstrated to increase the hospital length of stay by up to two weeks (Graf et al., 2010; Joshi et al., 2016), readmission rates sixfold (Joshi et al., 2016) and the risk of mortality up to tenfold (Andrade et al., 2019). Additionally, it often results in a requirement for extended outpatient follow-up, prolonged antimicrobial therapy and even reoperation (Findeisen et al., 2018). These factors can almost treble the cost of cardiac surgery from around €13,000 to over €36,000 per procedure (Graf et al., 2010) and does not touch upon the psychological impact on patients, families and carers. SSI surveillance is mandatory within UK NHS hospitals, across all surgical specialties (Cooper et al., 2019) and guidelines have been implemented to reduce the incidence (National Institute for Health and Care Excellence [NICE], 2019). There is, however, significant heterogeneity in how this is applied in adult cardiac surgery (Cardiothoracic Interdisciplinary Research Network, 2020). Antimicrobial prophylaxis is a major component of the NICE guidelines as it helps reduce SSIs following many surgical procedures (Andrade et al., 2019; Bratzler et al., 2004; Mangram, 2001) and has long been the standard of care in cardiac surgery (Kreter and Woods, 1992). Despite this, within cardiac surgery, controversy remains on the antimicrobial of choice, dosing regimen and duration of treatment even in light of recent European guidance (Table 1) (Sousa-Uva et al., 2017). No guidance currently exists for the UK and ROI. A recent GRIFT report highlighted a regional variation in the incidence of SSI after cardiac surgery (1%–8%) within the UK and ROI (Richens, 2018). This variation may have resulted from regional differences in the antimicrobial prophylaxis regimens prescribed for adult cardiac surgery patients.
Table 1.
European Guidelines 2018 (Sousa-Uva et al., 2018).
| First line | Cefazolin or cefuroxime (Class Ia) |
|---|---|
| High-risk cases (MRSA +ve) | Vancomycin (Class IIab) |
| Penicillin allergy | Clindamycin or vancomycin (Class Ib) |
| First dose | < 60 min before skin incision (Class Ib) Vancomycin or fluoroquinolones < 120 min before incision (Class IIab) |
| Optimal duration | 24 h but should not exceed 48 h (Class IIab) |
MRSA, methicillin-resistant Staphylococcus aureus.
The suggested use of cefalozin or cefuroxime is due to a reduction in postoperative hospital-acquired pneumonia and all-cause mortality (Lador et al., 2012). The numbers needed to treat with a second- or third-generation cephalosporin to prevent one pneumonia was 74 and to prevent one death was 88. This finding is important as postoperative pneumonia or ventilation-associated pneumonia are significant predictors of death after cardiac surgery and antimicrobial prophylaxis targeted to reduce rates of pneumonia in critical care settings have been shown to reduce mortality (Liberati et al., 2009; Riera et al., 2010). This was supported by a meta-analysis involving 57 trials that reviewed SSI after cardiac surgery (Lador et al., 2012). There was no significant difference in rates of deep sternal wound infection (DSWI) or other SSIs in patients receiving beta-lactam antimicrobial prophylaxis with gram-negative cover in comparison to gram-positive prophylaxis alone. However, the use of beta-lactams did lead to a reduction in postoperative lower respiratory tract infections and all-cause mortality.
Antimicrobial prophylaxis is administered to cover the most frequently encountered pathogenic organisms causing SSI (Moinipoor et al., 2013). Cardiac surgery complicates this through the added risk of microbial transposition as a result of the frequent use of autologous venous and arterial grafts from the limbs and the potential for seeding of microorganisms. Moreover, the use of synthetic material in aortic or valvular surgery is another pertinent risk factor. The Public Health England (PHE) annual report of surveillance of SSIs in NHS hospitals in England (Cooper et al., 2019) demonstrated that enterobacterales were the most prevalent causative organism in 2018–2019, the three most common of which include Escherichia coli, coliforms and Proteus morabilus. Many of the SSIs were also attributed to the Staphylococcus species, the most common of which include coagulase-negative staphylococci and methicillin-sensitive S. aureus (MSSA) (Cooper et al., 2019). In addition, it is not unusual for SSIs to be polymicrobial. The complexity of antimicrobial prophylaxis in adult cardiac surgery is enhanced further still when considered across the wider scope of antimicrobial stewardship and antimicrobial resistance (NICE, 2016). This survey was conducted to determine the current practice of antimicrobial prophylaxis in adult cardiac surgery throughout the UK and ROI.
Methods
All centres registered as performing adult cardiac surgery in the UK (n = 35) and ROI (n = 3) on the Society of Cardiothoracic Surgery of Great Britain and Ireland (SCTS) database were contacted directly. Requests were made via email for local cardiac surgery antimicrobial prophylaxis guidelines, for primary cardiac procedures via median sternotomy. Centres that did not reply to the initial email were followed up with at least one further email. After this, all centres were individually contacted by telephone, and if cardiothoracic surgery departments were not accessible, attempts were made through local microbiology services on at least two occasions.
Adult cardiac surgery antimicrobial guidelines were requested and subsequently scrutinised for the following:
choice of antimicrobial
timing of administration
dose
frequency of administration
duration of course
In addition, information was collected on antimicrobial prophylaxis in relation to penicillin allergy, Methicillin-resistant Staphylococcus aureus (MRSA) and Methicillin-sensitive Staphylococcus aureus (MSSA) carriage. According to the NHS Health Research Authority, this survey is not considered research as defined by the UK Policy Framework for Health and Social Care Research and therefore, ethical committee approval was not required. Local approval from either service line manager or clinical lead was requested from all involved centres.
Results
Of the 38 centres, 34 (89%) responded with details of their local antimicrobial prophylaxis guidelines.
Antimicrobial regimen
Nine different prophylactic antimicrobial regimens are used across 34 centres in the UK and ROI (Table 2 and Figure 1) in adult cardiac surgery. Antimicrobials given in the event of reoperation for bleeding and or tamponade were excluded. The most common prophylactic regimen was flucloxacillin and gentamicin in 16 of 34 centres (47%), although these regimens varied both in dose administered and duration of treatment. Cefuroxime was used in six centres and another six centres used cefuroxime in combination with another antimicrobial: glycopeptide antimicrobials vancomycin (n = 2) and teicoplanin (n = 2) in four centres and gentamicin in two. Three centres used co-amoxiclav alone, another centre combined this with gentamicin and another used a combination of ciprofloxacin and flucloxacillin. One centre used flucloxacillin alone.
Table 2.
Antimicrobial prophylaxis regimen.
| Flucloxacillin and gentamicin | 16 |
| Flucloxacillin | 1 |
| Flucloxacillin + ciprofloxacin | 1 |
| Cefuroxime | 6 |
| Cefuroxime + vancomycin | 2 |
| Cefuroxime + teicoplanin | 2 |
| Cefuroxime + gentamicin | 2 |
| Co-amoxiclav | 3 |
| Co-amoxiclav + gentamicin | 1 |
| Total | 34 |
Figure 1.
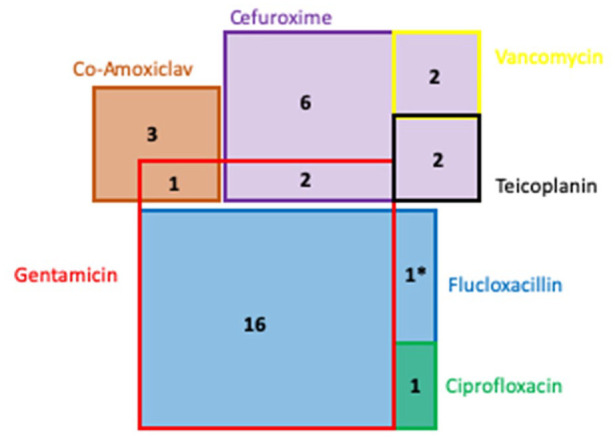
Illustration of cardiac antimicrobial prophylaxis at 34 Trusts across the UK and ROI. Area relative to use of antimicrobial agent, with overlap indicating combined regimes. *Flucloxacillin use in a single centre is not a proportional representation.
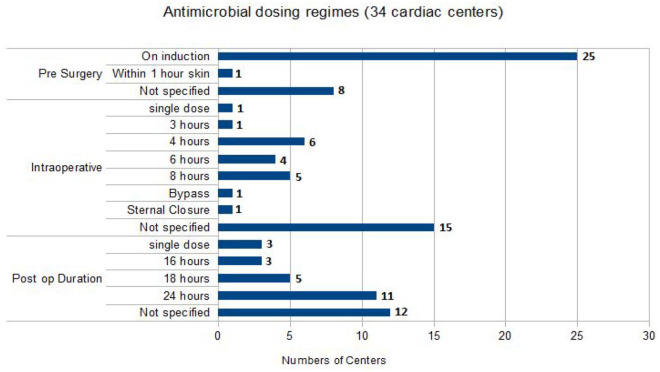
Timing of administration and antimicrobial dose
All guidelines specified that the first dose of antimicrobial(s) should be administered at induction of anaesthesia with the approximated skin incision expected 30–60 min later. Flucloxacillin was administered in doses of either 1 g (n = 13) or 2 g (n = 4), and one trust dosed according to weight. Further doses were administered at a variety of time points between 3 h and 8 h intraoperatively and postoperatively. Cefuroxime dosing varied considerably; at induction, either a dose of 750 mg (n = 1) or 1.5 g (n = 10) was given. This was followed by an additional two or three doses of either 750 mg of 1.5 g at 8-h time points. Centres using co-amoxiclav administered either two (n = 1) or three (n = 2) doses of 1.2 g at 8-h time points from induction. Gentamicin was most commonly given as a single dose based on patient weight and renal function; doses were in the range of 1.5–7 mg/Kg. The glycopeptides, vancomycin and teicoplanin were given in doses of 1.5 g or 400–800 mg with up to two repeated doses, respectively. Figure 2 summarises the different antimicrobial dosing regimens used in different centres.
Figure 2.
Illustration of different dosing regimens used in adult cardiac antimicrobial prophylaxis.
Antimicrobial duration
Of the 34 respondent centres, 25 (64%) detailed the duration of antimicrobial prophylaxis regimens in their guidelines. Eleven centres stopped within the first 24 h of surgery and 11 stopped at 24 h postoperatively. A single trust continued for 48 h postoperatively, another implemented single doses of gentamicin and flucloxacillin, one centre gave two postoperative doses and another centre’s regimen varied depending on the specific procedure being performed but generally stopped within 12 h of surgery extending for valvular procedures.
Antimicrobial choice in those with a penicillin allergy
Of the 34 respondents, 26 (76.4%) provided guidance on their antimicrobial regimen of choice in penicillin allergic patients (Table 3). The most common regimen was teicoplanin and gentamicin in 13 centres (50%). The remaining centres used a combination of a glycopeptide and another antimicrobial, most commonly ciprofloxacin. Two centres used teicoplanin alone, one centre used a combination of teicoplanin and clarithromycin, and one centre used clindamycin alone.
Table 3.
Antimicrobial prophylaxis regimen in penicillin allergy.
| Teicoplanin + gentamicin | 13 |
| Vancomycin or gentamicin | 3 |
| Ciprofloxacin + vancomycin | 3 |
| Teicoplanin + ciprofloxacin | 3 |
| Teicoplanin | 2 |
| Clindamycin | 1 |
| Clarithromycin + gentamicin | 1 |
| Total | 26 |
Antimicrobial of choice in those colonised with MRSA
Of the 34 centres, 19 (55.8%) included details in their antimicrobial prophylaxis for patients colonised with MRSA (Table 4). All centres used a glycopeptide in combination with another antimicrobial, most commonly gentamicin (n = 11, 58%).
Table 4.
Antimicrobial prophylaxis regimens in MRSA-positive patients.
| Teicoplanin + gentamicin | 8 |
| Teicoplanin | 4 |
| Vancomycin + gentamicin | 3 |
| Vancomycin + co-amoxiclav | 1 |
| Teicoplanin + ciprofloxacin | 1 |
| Cefuroxime + vancomycin | 1 |
| Cefuroxime + teicoplanin | 1 |
| Total | 19 |
MRSA, methicillin-resistant Staphylococcus aureus.
Discussion
Antimicrobial prophylaxis is one of the most important measures to reduce the incidence of SSI (Hamouda et al., 2015; Kreter and Woods, 1992; Luo et al., 2015; NICE, 2019; White et al., 2013) alongside non-pharmacological measures such as preoperative decontamination therapy, aseptic surgical technique and glucose control (Cardiothoracic Interdisciplinary Research Network et al., 2020). However, the evidence base underpinning these interventions is limited (Cardiothoracic Interdisciplinary Research Network et al., 2019) and a national survey indicates significant variation in non-pharmacological practices to prevent SSI after cardiac surgery (Cardiothoracic Interdisciplinary Research Network et al., 2020). This survey focused exclusively on antimicrobial prophylaxis, where controversy remains on the most appropriate antimicrobial and duration of treatment despite guidelines suggesting the use of cephalosporins administered on induction and continued for 24–48 h postoperatively (Hamouda et al., 2015; Mertz et al., 2011). Current UK practice differs somewhat from European guidance, a likely consequence of the increased Clostridioides difficile infections seen during the 1990s with cephalosporin antimicrobials (Lee et al., 2019; Slimings and Riley, 2014). This resulted in a national drive coordinated by NICE to reduce the prescription of second-, third- and fourth-generation cephalosporins (NICE, 2015). Over the following decade, this increased vigilance led to an overhaul of local antimicrobial practice away from the prescription of broad-spectrum antimicrobials, especially cephalosporin and quinolone antimicrobials (Lee et al., 2019). This is particularly pertinent in cardiac surgery because of the diverse range of organisms that are associated with SSIs (Chaudhuri et al., 2012; Cooper et al., 2019; Ma and An, 2018; Moinipoor et al., 2013; Pan et al., 2017). Ma and An (2018) reviewed deep sternal wound infections in their centre between 2011 and 2015, identifying 170 patients that had clinical DSWIs. Microbiological diagnosis was available in 77 patients; of these, 54% were caused by gram-negative bacilli. Pseudomonas aeruginosa occurred most frequently (22.5%) followed by Acinetobacter baumannii (15.9%). MSSA accounted for 20.4% of infections and MRSA, 5.7%. Polymicrobial infection was detected in 11.7% of patients. P. aeruginosa was found to be 100% resistant to cefalozin and cefuroxime.
In a single-centre study conducted in Poland between 1 January 2016 and 31 May 2017, swabs were taken from 164 patients after cardiac surgery via median sternotomy complicated by prolonged wound healing (Kotnis-Gaska et al., 2018). In 114 cases, patients developed a sternal wound infection with a positive culture. The most common pathogens included Staphylococcus epidermidis, Enterococcus faecium, S. aureus, Klebsiella pneumoniae and P. aeruginosa. In most cases, S. epidermidis was methicillin-resistant (43.5%). Polymicrobial infections were detected in 48 patients.
This highlights the importance of ensuring broad-spectrum antimicrobial prophylaxis in cardiac surgery includes both gram-negative and gram-positive activity (Lador et al., 2012). It is however likely that the specific organisms causing SSI is likely to change between centres within the UK and ROI and certainly across the globe (Kotnis-Gaska et al., 2018; Lin et al., 2003; Ma and An, 2018; Moinipoor et al., 2013; Pan et al., 2017). It is therefore essential that antimicrobial regimens are tailored to local microbial epidemiology (NICE, 2019).
Timing of administration
Adherence to a strict timing of the initial antimicrobial dose is difficult due to practical considerations, such as anaesthetic induction time and duration of operation. A study in 1992 demonstrated that preoperative administration of cefuroxime within 120 min of incision resulted in an SSI rate of 0.6% in comparison to 1.4% in those patients treated intraoperatively and 3.3% in those treated postoperatively (Classen et al., 1992). In addition, Koch et al. (2012) evaluated the timings of antimicrobial prophylaxis (cefuroxime + vancomycin) administration in 28,250 cardiac surgery operations via median sternotomy and demonstrated that the lowest rate of SSI (1.8%) was identified in the group in which cefuroxime was administered 15 min before incision. The risk of SSI increased when > 15 minutes passed between antimicrobial administration and skin incision. This was 2.2% and 2.8% at 45 min and 60 min, respectively. The highest rate of infection (3.7%) was noted when cefuroxime was administered 75 min before skin incision. Unsurprisingly, the optimal timing of administration for vancomycin was different; with the lowest SSI rate (1.8%) noted when given 32 min before surgical incision. This increased to 2.2% when administered 45 min before skin incision, 3.2% and 4.6% at 60 and 75 min respectively. Post-incision administration demonstrated an SSI rate of 3.3%.
These studies highlight the failure of generic guidelines to appropriately define the timing of antimicrobial prophylaxis administration (Sousa-Uva et al., 2017). To ensure maximal antimicrobial activity the timing of administration should to be tailored to the specific pharmacokinetics of the agent being administered (Hamouda et al., 2015; Lador et al., 2012). Broadly speaking, this means antimicrobials with short half-lives such as cefuroxime and beta-lactams are administered closer to the time of skin incision.
Duration
Historically, prophylactic antimicrobials in cardiac surgery were continued until all drains and lines had been removed (Krieger et al., 1983); the presumption being that antimicrobials would protect the exposed wound edges from infection as a consequence of the external opening to the environment. Contemporary evidence has refuted this presumption and demonstrated that antimicrobials may in fact facilitate the colonisation of foreign devices with resistant organisms (Harbarth et al., 2000; Terpstra et al., 1999).
In a four-year observational study between 1993 and 1997, Harbarth et al. (2000) showed there was no difference in the incidence of sternal wound infection in patients that had short (48 h) or long (> 48 h) duration of prophylaxis with a cephalosporin or glycopeptide antimicrobial. The > 48 hour group was, however, associated with increased rates of acquired antimicrobial resistance. More recent studies have validated this finding (Gupta et al., 2010; Hamouda et al., 2015). Tamayo et al. (2008) reported a single dose of cefazolin resulted in a greater rate of SSI (8.3%) in comparison to a 24 hour postoperative regimen (3.6%). These studies suggest that patients undergoing cardiac surgery should not receive > 48 hours of antimicrobial prophylaxis postoperatively, but the duration should be greater than a single dose at induction (Gupta et al., 2010; Hamouda et al., 2015; Mertz et al., 2011; Scottish Intercollegiate Guidelines Network, 2008).
Clostridioides difficile infection and antimicrobial prophylaxis
Clostridioides difficile infection is a potentially fatal infection, known to cause pseudomembranous colitis and up to 25% of antimicrobial associated diarrhoea (Bartlett and Gerding, 2008; Lee et al., 2019). It occurs more frequently in the elderly and vulnerable patient groups, where it is particularly lethal, and is associated with a 30-day mortality as high as 25% in some UK hospitals (Karas et al., 2010). Consequently, reducing Clostridioides difficile infection has been an NHS Improvement objective for 2019 and remains so in 2020 (NHS Improvement, 2019). This influences the antimicrobial guidance issued across UK hospitals (Lee et al., 2019; NICE, 2015). The risk of Clostridioides difficile infection is increased by the use of broad-spectrum antimicrobials, particularly those administered for three days or more (Brown et al., 2013; Slimings and Riley, 2014). In a meta-analysis of antimicrobials and hospital-acquired Clostridioides difficile, the strongest associations were third-generation cephalosporins (odds ratio [OR] = 3.20, 95% confidence interval [CI] = 1.80–5.71; n = 6 studies), clindamycin (OR = 2.86, 95% CI = 2.04–4.02; n = 6) and second-generation cephalosporins (OR = 2.23, 95% CI = 1.47–3.37; n = 6) (Slimings and Riley, 2014).
The most common antimicrobial prophylactic regimen used in the UK was flucloxacillin and a single dose of gentamicin before skin incision. This probably reflects an attempt made by many UK and ROI cardiac surgery centres to reduce the of risk of Clostridioides difficile infection while also achieving good gram-negative cover and potent affects against staphylococcus species, in accordance with their local microbiology epidemiology (Lador et al., 2012; White et al., 2013). White et al. (2013) compared prophylactic cefuroxime to a combination of flucloxacillin and gentamicin and found there were fewer SSIs associated with the gentamicin and flucloxacillin group (2.7% vs. 3.2% for the cefuroxime group). Although these results were not statistically significant, it appears to suggest the flucloxacillin and gentamicin regimen may be at least as effective, if not more effective for SSI prevention while ensuring a reduction in Clostridioides difficile infections (0.058% vs. 0.52%; P = 0.02).
Limitations
This survey had a number of limitations, most notably its observational nature and reflection of practice only at the specific time the survey was completed. The response rate was not complete (89%), although for an observational survey this was better than anticipated. Furthermore, the four centres that declined to take part are arguably less likely to be following evidence-based practice and therefore their omission is only likely to have limited the variation demonstrated.
Conclusion
This survey demonstrates that antimicrobial prophylaxis in the UK and Ireland varies between centres and, in most cases, deviates from EACTS guidance for antimicrobial prophylaxis in cardiac surgery. In conjunction with non-pharmacological measures (Cardiothoracic Interdisciplinary Research Network et al., 2019), this is likely to be a contributing factor to the regional variation in the incidence of SSI following cardiac surgery (1%–8%) highlighted in the recent GIRFT report (Richens, 2018). The most common regimen, flucloxacillin and gentamicin, appears to be at least as effective as third-generation cephalosporins in preventing SSI without the added risk of increasing Clostridium difficile infection (White et al., 2013). The national focus on minimising Clostridioides difficile infections is the likely driver of regional antimicrobial prophylaxis variation. More clarity in the form of national guidelines are warranted and may help to reduce regional variations in SSIs.
Acknowledgments
Many thanks to Dr Lewis Jones, Consultant Microbiologist at University Plymouth Hospitals, who provided key assistance with researching and structuring this report.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Peer review statement: Not commissioned; blind peer-reviewed.
ORCID iDs: James Kofi Ackah  https://orcid.org/0000-0001-6918-0300
https://orcid.org/0000-0001-6918-0300
Pedram Panahi  https://orcid.org/0000-0002-1601-4920
https://orcid.org/0000-0002-1601-4920
References
- Andrade LS, Siliprandi EMO, Karsburg LL, Berlesi FP, Carvalho O, Rosa DSD, Santos RPD. (2019) Surgical site infection prevention bundle in cardiac surgery. Arquivos Brasileiros de Cardiologia 112: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JG, Gerding DN. (2008) Clinical Recognition and Diagnosis of Clostridium difficile Infection. Clinical Infectious Diseases 46: S12–S18. [DOI] [PubMed] [Google Scholar]
- Bratzler DW, Houck PM, Surgical Infection Prevention Guidelines Writers Workgroup, American Academy of Orthopaedic Surgeons, American Association of Critical Care Nurses, American Association of Nurse Anesthetists, American College of Surgeons, American College of Osteopathic Surgeons, American Geriatrics Society, American Society of Anesthesiologists, American Society of Colon and Rectal Surgeons, American Society of Health-System Pharmacists, American Society of Perianesthesia Nurses, Ascension Health, Association of Perioperative Registered Nurses, Association for Professionals in Infection Control and Epidemiology, Infectious Diseases Society of America, Medical Letter, Premier, Society for Healthcare Epidemiology of America, Society of Thoracic Surgeons and Surgical Infection Society. (2004) Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clinical Infectious Diseases 38: 1706–1715. [DOI] [PubMed] [Google Scholar]
- Brown KA, Khanafer N, Daneman N, Fisman DN. (2013) Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrobial Agents and Chemotherapy 57: 2326–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiothoracic Interdisciplinary Research Network, Rogers L, Vaja R, Bleetman D, Ali JM, Rochon M, Sanders J, Tanner J, Lamagni TL, Talukder S, Quijano-Campos JC, Lai F, Loubani M, Murphy G. (2019) Interventions to prevent surgical site infection in adults undergoing cardiac surgery. Cochrane Database of Systematic Reviews 2019: CD013332. [Google Scholar]
- Cardiothoracic Interdisciplinary Research Network., National Cardiac Benchmarking Collaborative, Public Health England. (2020) National Survey of Variations in Practice in the Prevention of Surgical Site Infections in Adult Cardiac Surgery, United Kingdom & Republic of Ireland. London: Public Health England. Available at: https://www.ncbc-nhs.org/ (accessed 25 July 2020). [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Shekar K, Coulter C. (2012) Post-operative deep sternal wound infections: making an early microbiological diagnosis. European Journal of Cardio-Thoracic Surgery 41: 1304–1308. [DOI] [PubMed] [Google Scholar]
- Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. (1992) The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. New England Journal of Medicine 326: 281–286. [DOI] [PubMed] [Google Scholar]
- Cooper K, Lamagni T, Harrington P, Wloch C, Hopkins S. (2019) Surveillance of surgical site infections in NHS hospitals in England, April 2018 to March 2019. London: PHE Publications. Available at: www.gov.uk/phe (accessed 19 April 2020). [Google Scholar]
- Figuerola-Tejerina A, Rodriguez-Caravaca G, Bustamante-Munguira J, Maria San Roman-Montero J, Duran-Poveda M. (2016) Epidemiological Surveillance of Surgical Site Infection and its Risk Factors in Cardiac Surgery: A Prospective Cohort Study. Revista Espanola de Cardiologia (English Edition) 69: 842–848. [DOI] [PubMed] [Google Scholar]
- Findeisen A, Arefian H, Doenst T, Hagel S, Pletz MW, Hartmann M, Maschmann J. (2018) Economic burden of surgical site infections in patients undergoing cardiac surgery. European Journal of Cardio-Thoracic Surgery 55: 494–500. [DOI] [PubMed] [Google Scholar]
- Graf K, Ott E, Vonberg RP, Kuehn C, Haverich A, Chaberny IF. (2010) Economic aspects of deep sternal wound infections. European Journal of Cardio-Thoracic Surgery 37: 893–896. [DOI] [PubMed] [Google Scholar]
- Gupta A, Hote MP, Choudhury M, Kapil A, Bisoi AK. (2010) Comparison of 48 h and 72 h of prophylactic antibiotic therapy in adult cardiac surgery: a randomized double blind controlled trial. Journal of Antimicrobial Chemotherapy 65: 1036–1041. [DOI] [PubMed] [Google Scholar]
- Hamouda K, Oezkur M, Sinha B, Hain J, Menkel H, Leistner M, Leyh R, Schimmer C. (2015) Different duration strategies of perioperative antibiotic prophylaxis in adult patients undergoing cardiac surgery: an observational study. Journal of Cardiothoracic Surgery 10: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. (2000) Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 101: 2916–2921. [DOI] [PubMed] [Google Scholar]
- Joshi V, Vaja R, Richens D. (2016) Cost analysis of gentamicin-impregnated collagen sponges in preventing sternal wound infection post cardiac surgery. Journal of Wound Care 25: 22–25. [DOI] [PubMed] [Google Scholar]
- Karas JA, Bradshaw S, Mahmud W, Enoch DA. (2010) Mortality in hospitalized older adults associated with Clostridium difficile infection at a district hospital. Infectious Disease Reports 2: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CG, Nowicki ER, Rajeswaran J, Gordon SM, Sabik JF, 3rd, Blackstone EH. (2012) When the timing is right: Antibiotic timing and infection after cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 144: 931–937.e4. [DOI] [PubMed] [Google Scholar]
- Kotnis-Gaska A, Mazur P, Olechowska-Jarzab A, Stanisz A, Bulanda M, Undas A. (2018) Sternal wound infections following cardiac surgery and their management: a single-centre study from the years 2016-2017. Kardiochirurgia i Torakochirurgia Polska 15: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreter B, Woods M. (1992) Antibiotic prophylaxis for cardiothoracic operations. Meta-analysis of thirty years of clinical trials. Journal of Thoracic and Cardiovascular Surgery 104: 590–599. [PubMed] [Google Scholar]
- Krieger JN, Kaiser DL, Wenzel RP. (1983) Nosocomial urinary tract infections cause wound infections postoperatively in surgical patients. Surgery, Gynecology & Obstetrics 156: 313–318. [PubMed] [Google Scholar]
- Lador A, Nasir H, Mansur N, Sharoni E, Biderman P, Leibovici L, Paul M. (2012) Antibiotic prophylaxis in cardiac surgery: systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy 67: 541–550. [DOI] [PubMed] [Google Scholar]
- Lee HY, Hsiao HL, Chia CY, Cheng CW, Tsai TC, Deng ST, Chen CL, Chiu CH. (2019) Risk factors and outcomes of Clostridium difficile infection in hospitalized patients. Biomedical Journal 42: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. (2009) Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database of Systematic Reviews 2009; CD000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Hsu RB, Chang SC, Lin FY, Chu SH. (2003) Poststernotomy mediastinitis due to methicillin-resistant Staphylococcus aureus endemic in a hospital. Clinical Infectious Diseases 37: 679–684. [DOI] [PubMed] [Google Scholar]
- Luo S, Lai Y, Liu C, Chen Y, Qiao X. (2015) Prophylactic use of gentamicin/flucloxacillin versus cefuroxime in surgery: a meta analysis of clinical studies. International Journal of Clinical and Experimental Medicine 8: 17856–17867. [PMC free article] [PubMed] [Google Scholar]
- Ma JG, An JX. (2018) Deep sternal wound infection after cardiac surgery: a comparison of three different wound infection types and an analysis of antibiotic resistance. Journal of Thoracic Disease 10: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangram AJ. (2001) A brief overview of the 1999 CDC Guideline for the Prevention of Surgical Site Infection. Centers for Disease Control and Prevention. Journal of Chemotherapy 13 (Spec No 1): 35–39. [DOI] [PubMed] [Google Scholar]
- Mannien J, Wille JC, Kloek JJ, Van Benthem BH. (2011) Surveillance and epidemiology of surgical site infections after cardiothoracic surgery in The Netherlands, 2002–2007. Journal of Thoracic and Cardiovascular Surgery 141: 899–904. [DOI] [PubMed] [Google Scholar]
- Mertz D, Johnstone J, Loeb M. (2011) Does duration of perioperative antibiotic prophylaxis matter in cardiac surgery? A systematic review and meta-analysis. Annals of Surgery 254: 48–54. [DOI] [PubMed] [Google Scholar]
- Moinipoor AA, Abbasi M, Amouzeshi A, Esfahanizadeh J, Amini S. (2013) Deep sternal wound infection following cardiac surgery Epidemiology and causative germs. Journal of Surgery and Trauma 1: 21–25. [Google Scholar]
- National Institute for Health and Care Excellence. (2015) Clostridium difficile infection: risk with broad Clostridium difficile infection: risk with broadspectrum antibiotics spectrum antibiotics. London: NICE. Available at: https://nice.org.uk/guidance/esmpb1 (accessed 19 April 2020). [Google Scholar]
- National Institute for Health and Care Excellence. (2016) Antimicrobial stewardship. London: NICE. Available: https://www.nice.org.uk/guidance/qs121/resources/antimicrobial-stewardship-pdf-75545353537477 (accessed 25 June 2020). [Google Scholar]
- National Institute for Health and Care Excellence. (2019) Surgical site infections: prevention and treatment. London: NICE. Available: https://www.nice.org.uk/guidance/ng125/resources/surgical-site-infections-prevention-and-treatment-pdf-66141660564421 (accessed 11 April 2019). [PubMed] [Google Scholar]
- NHS Improvement. (2019) Clostridium difficile infection objectives for NHS organisations in 2019/20 and guidance on the intention to review financial sanctions and sampling rates from 2020/21. London: NHS Improvement. Available at: https://improvement.nhs.uk/documents/808/CDI_objectives_for_NHS_organisations_in_2019_12March.pdf (accessed 18 July 2020). [Google Scholar]
- Pan L, Mo R, Zhou Q, Wang D. (2017) Deep sternal wound infection after cardiac surgery in the Chinese population: a single-centre 15-year retrospective study. Journal of Thoracic Disease 9: 3031–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richens D. (2018) Cardiothoracic Surgery: GIRFT Programme National Speciality Report. England: NHS. Available at: https://gettingitrightfirsttime.co.uk/wp-content/uploads/2018/04/GIRFT-Cardiothoracic-Report-1.pdf (accessed 19 April 2020). [Google Scholar]
- Riera M, Ibanez J, Herrero J, Ignacio Saez De, Ibarra J, Enriquez F, Campillo C, Bonnin O. (2010) Respiratory tract infections after cardiac surgery: impact on hospital morbidity and mortality. Journal of Cardiovascular Surgery 51: 907–914. [PubMed] [Google Scholar]
- Scottish Intercollegiate Guidelines Network. (2008) Antibiotic Prophylaxis in Surgery: A National Clinical Guideline. Edinburgh: SIGN. [Google Scholar]
- Slimings C, Riley TV. (2014) Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy 69: 881–891. [DOI] [PubMed] [Google Scholar]
- Sousa-Uva M, Head SJ, Milojevic M, Collet J-P, Landoni G, Castella M, Dunning J, Gudbjartsson T, Linker NJ, Sandoval E, Thielmann M, Jeppsson A, Landmesser U. (2017) 2017. EACTS Guidelines on perioperative medication in adult cardiac surgery. European Journal of Cardio-Thoracic Surgery 53: 5–33. [DOI] [PubMed] [Google Scholar]
- Tamayo E, Gualis J, Flórez S, Castrodeza J, Eiros Bouza JM, Álvarez FJ. (2008) Comparative study of single-dose and 24-hour multiple-dose antibiotic prophylaxis for cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 136: 1522–1527. [DOI] [PubMed] [Google Scholar]
- Terpstra S, Noordhoek GT, Voesten HG, Hendriks B, Degener JE. (1999) Rapid emergence of resistant coagulase-negative staphylococci on the skin after antibiotic prophylaxis. Journal of Hospital Infection 43: 195–202. [DOI] [PubMed] [Google Scholar]
- White RW, West R, Howard P, Sandoe J. (2013) Antimicrobial regime for cardiac surgery: the safety and effectiveness of short-course flucloxacillin (or teicoplanin) and gentamicin-based prophylaxis. Journal of Cardiac Surgery 28: 512–516. [DOI] [PubMed] [Google Scholar]