Abstract
The Co‐HCW study is a prospective cohort study among hospital staff, including healthcare workers (HCWs) and administration staff, at the Jena University Hospital (JUH), Germany. The objectives of this study were to assess SARS‐CoV‐2 IgG seroprevalence, individual exposure risk factors and compliance of HCWs to wear personal protective equipment (PPE). After the first nosocomial COVID‐19 outbreak at JUH, mandatory masking was implemented on 20th March 2020. We evaluated point seroprevalence using two IgG detecting immunoassays and issued a questionnaire to assess COVID‐19 exposure, clinical symptoms and compliance to wear PPE. Antibody retesting was offered to participants with a divergent result of both immunoassays 5–10 weeks after the first test. Between 19th May and 19th June 2020, we analysed 660 participants [out of 3,228; 20.4%]. Among them, 212 participants (32.1%) had received a previous COVID‐19 test. Four of them (1.9%) reported a positive test result. After recruitment, 18 participants (2.7%) had SARS‐CoV‐2 antibodies in at least one immunoassay. Overall, 21 participants (3.2%) had any evidence of a past or current SARS‐CoV‐2 infection. Among them, 13 (61.9%) were not aware of direct COVID‐19 exposure and 9 (42.9%) did not report any clinical symptoms. COVID‐19 exposure at home (adjusted OR (aOR) with 95% CI: 47.82 (5.49, 416.62)) was associated with SARS‐CoV‐2 seroprevalence. We observed no evidence for an association between seroprevalence and exposure at work (aOR 0.48 (0.13, 1.70)) or with COVID‐19 risk area according to the working place (aOR for intermediate‐risk vs. high‐risk: 1.97 (0.42, 9.22), aOR for low‐risk versus high‐risk: 2.10 (0.40, 11.06); p = .655). Reported compliance of HCWs to wear PPE differed (p < .001) between working in high‐risk (98.3%) and in intermediate‐risk areas (69.8%). In conclusion, compared to administration staff, we observed no additional risk to acquire SARS‐CoV‐2 infections by patient care, probably due to high compliance to wear PPE.
Keywords: healthcare workers, nosocomial transmission, SARS‐CoV‐2, seroepidemiologic studies, universal masking
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a novel beta coronavirus that was first identified in December 2019 in Wuhan, China (Huang et al., 2020). As of the beginning of 2020, the outbreak progressed and has been characterized as a pandemic in March 2020 (Abebe et al., 2020; Whitworth, 2020). The clinical presentation of the disease caused by SARS‐CoV‐2, corona virus disease 2019 (COVID‐19) (Abebe et al., 2020), varies significantly and ranges from asymptomatic and mild to critical courses (Chen et al., 2020; Guan et al., 2020; Pergolizzi et al., 2020). As asymptomatic or pre‐symptomatic patients can spread the virus (Furukawa et al., 2020; He et al., 2020; Long et al., 2020; Slifka & Gao, 2020), it is challenging to timely identify and isolate respective cases.
SARS‐CoV‐2 is highly transmissible from human to human mainly via inhalation of infectious respiratory droplets but also via close personal contact (shaking hands) and via touching contaminated surfaces (Patel et al., 2020). As a consequence, nosocomial transmission of insufficiently protected healthcare workers (HCWs) can occur during aerosol generating procedures (Patel et al., 2020; Reychler et al., 2020), in the regular patient contact particularly when exposed to patients with a delayed diagnosis of COVID‐19 and also in close contact with asymptomatic but virus carrying colleagues (Baker et al., 2020; Taylor et al., 2020; Treibel et al., 2020; Zhao et al., 2020). A recent analysis of more than 2 million community members and nearly 100,000 frontline HCWs in the United States and the UK found an increased risk of having a positive SARS‐CoV‐2 test result among HCWs (adjusted hazard ratio 3.40, 95% confidence interval 3.37–3.43) (Nguyen et al., 2020). According to a recent meta‐analysis including 127,480 HCWs, the estimated overall seroprevalence of SARS‐CoV‐2 antibodies was 8.7% (range: 0.0%–45.3%) and varied among continents (12.7% in North America, 8.5% in Europe, 8.2% in Africa, 4.0% in Asia) (Galanis et al., 2021). However, in the literature reported seroprevalence rates among HCWs show a high variability even within the countries: 1.6%–15.1% in Germany (Finkenzeller et al., 2020; Korth et al., 2020), 4.0%–11.0% in Spain (Dacosta‐Urbieta et al., 2020; Garcia‐Basteiro et al., 2020), 24.4%–31.6% in UK (Shields et al., 2020; Grant et al., 2020), 6.0%–27.0% in United States (Self et al., 2020; Venugopal et al., 2021) and 0.0%–11.1% in India (Kumar, Sathyapalan et al., 2020; Kumar, Bhartiya et al., 2020). Although only moderate seroprevalence rates among HCWs were reported from China (1.3%–3.8%) (Xu et al., 2020), a rapid review and meta‐analysis found that the proportion of nosocomial infections among confirmed COVID‐19 cases was 44% in China during the early outbreak and that 33% of COVID‐19 patients infected in hospitals were medical staff (Zhou et al., 2020).
According to the COVID‐19‐Dashboard of the Robert Koch Institute (https://experience.arcgis.com/experience/478220a4c454480e823b17327b2bf1d4), the first COVID‐19 cases were detected in the city of Jena, Germany, on 11th March 2020. Only five days later, a HCW returning from skiing in Austria caused the first nosocomial outbreak at the Jena University Hospital (JUH) that involved further 31 newly confirmed SARS‐CoV‐2 infections until 19th March. Hence, it is of great importance to implement infection prevention programmes and provide HCWs with sufficient personal protective equipment (PPE) in order to reduce nosocomial transmissions (Chou et al., 2020).
The primary objective of this study was to assess SARS‐CoV‐2 IgG seroprevalence among hospital staff of JUH, including HCWs (with patient contact) and administration staff (without patient contact). Secondary objectives were to determine individual exposure risk factors, to compare seroprevalence rates between hospital staff working at different COVID‐19 risk areas according to working place and to provide insight into the effectiveness of and compliance of HCWs with the use of PPE.
2. MATERIALS AND METHODS
2.1. Study design and setting
The Co‐HCW study (SARS‐CoV‐2 seroprevalence and infection status in hospital staff members at JUH) is a prospective, single‐centre observational cohort study conducted at JUH, a 1,400‐bed academic hospital in Germany. The city of Jena is the second largest town in the federal state Thuringia, located in Central Germany, and has nearly 109,000 inhabitants. JUH is the only university hospital in Thuringia. It is the largest employer and the only hospital in the city of Jena. Since 16th March 2020, 50 SARS‐CoV‐2 positive patients were hospitalized and additional 73 SARS‐CoV‐2 positive outpatients were seen at the emergency department (valid at 19th June 2020). Due to the increasing number of SARS‐CoV‐2 cases (in Jena and at JUH), mandatory masking for all staff members at JUH was implemented on 20th March 2020.
Research was conducted in accordance with the Declaration of Helsinki and national and institutional standards. The study protocol was approved by the local ethics committee of the Friedrich‐Schiller‐University Jena (approval no. 2020–1774), and the study was registered at the German Clinical Trials Register (DRKS00022432). This manuscript only covers the first of three study visits.
2.2. Enrolment and data management
Being a contracted staff member of JUH, working in a predefined hospital area and willing to sign a written informed consent were the only inclusion criteria. Predefined areas were as follows: Department of Medicine IV, Department of Anesthesiology, Emergency Medicine, Occupational Health, Hospital Entrance, Department of Neurology, Department of Child and Adolescent Psychiatry, Department of Psychiatry and Psychotherapy, Institute of Infectious Diseases and Infection Control and Hospital and administration area without patient contact (as provided in Table S1). Individuals working outside these pre‐categorized risk areas (namely laboratory personal where at least a proportion deals with COVID‐19 related clinical specimens but has no patient contact), participating outside the planned study period or who did not provide a blood sample were excluded.
Participants were recruited between 19th May 2020 and 19th June 2020. All eligible staff members were informed by a prior email. Enrolment was conducted by on‐site visit, and additionally, individual appointments were made possible for those who were out off duty during the on‐site visit but willing to participate in the study. Participation was voluntary.
After pseudonymization at the study centre, blood samples were sent to the Department of Clinical Chemistry and Laboratory Medicine (JUH) and the Institute of Medical Microbiology (JUH) for testing of IgG antibodies against SARS‐CoV‐2 by two different immunoassays (see below).
Pseudonymized questionnaires were digitalized with support of data management of the Institute of General Practice and Family Medicine (JUH). After digitalization, the whole data set was checked for plausibility and for missing data. In case of not plausible or missing data, original data included in the paper questionnaire were rechecked and manually added to the electronic data set (e.g. age missing or age <18 years or >65 years).
2.3. Questionnaire
The questionnaire included questions on demographics, working area, individual exposure to confirmed COVID‐19 cases, return from COVID‐19 risk areas since February 2020, results of previous polymerase chain reaction (PCR) or serology test for COVID‐19, clinical symptoms within the last two months such as cold‐like symptoms, diarrhoea, taste disturbances and smell disorders. The maximum severity of cold‐like symptoms, included in the Wisconsin Upper Respiratory Symptom Survey (WURSS‐24), was asked. WURSS‐24 is identical to the WURSS‐21 (Barrett et al., 2005), except for the addition of the items assessing headache, body ache and fever. Response options range from 0 to 7 (0 = do not have, 1 = very mildly, 3 = mildly, 5 = moderately, 7 = severely).
To evaluate the risk for nosocomial transmissions, HCWs with an individual face‐to‐face contact within 1 metre with a confirmed COVID‐19 patient or at least with its surroundings received an extended questionnaire that also included questions on the compliance concerning use of personal protective equipment (PPE) as recently published by the WHO (https://apps.who.int/iris/bitstream/handle/10665/331496/WHO‐2019‐nCov‐HCW_risk_assessment‐2020.2‐eng.pdf last accessed at 2nd February 2021).
2.4. SARS‐CoV‐2 antibody testing
Presence of SARS‐CoV‐2 antibodies was investigated once by two different commercially available IgG detecting immunoassays: an enzyme‐linked immunosorbent assay EDI Novel Coronavirus SARS‐CoV‐2 IgG ELISA (Epitope Diagnostics Inc.) and a chemiluminescence‐based immunoassay Elecsys Anti‐SARS‐CoV‐2 (Roche). Both assays target recombinant nucleocapsid protein and were carried out according to the manufacturers’ instructions. Sensitivities and specificities as provided by the manufacturers are high for both tests (≥98%). In case of two corresponding negative test results by both immunoassays, the participant was regarded as SARS‐CoV‐2 seronegative. Volunteers with at least one positive test result were regarded as SARS‐CoV‐2 seropositive. In case of a ‘borderline’ test result for EDI IgG ELISA and a negative Elecsys Roche test, the test persons were neither classified as SARS‐CoV‐2 seropositive nor seronegative. Retesting was offered to all participants with a divergent result of both immunoassays five to ten weeks after the first test.
2.5. Outcomes and further definitions
The primary outcome of the study was to assess the seroprevalence of SARS‐CoV‐2 antibodies in hospital staff of JUH using two IgG detecting immunoassays. Secondary outcomes were (a) seroprevalence rates stratified by their risk of COVID‐19 exposure during work (see below for definition of COVID‐19 risk areas), (b) potential risk factors and clinical symptoms for seropositive employees and (c) compliance of HCWs in high‐risk and intermediate‐risk areas to wear PPE in case of an individual reported contact with a confirmed COVID‐19 positive patient or its surroundings.
We classified hospital staff in three groups according to their risk of a contact with COVID‐19 patients: low, intermediate and high risk. The low‐risk group included staff members working in the administration without patient contact. The intermediate‐risk group were HCWs that had regular patient contact but did not routinely treat patients with confirmed or suspected SARS‐CoV‐2 infections. The high‐risk group included HCWs working at areas with confirmed COVID‐19 patients and areas that deal with a high number of suspected COVID‐19 cases (see Table S1). Participants presented any evidence of past or current COVID19 infection if they were seropositive for SARS‐CoV‐2 IgG antibodies by at least one immunoassay after recruitment and/or reported evidence of a positive SARS‐CoV‐2 test (PCR or serology) prior recruitment.
2.6. Sample size considerations
As previous data on SARS‐CoV‐2 IgG seroprevalence rates of HCWs in Germany were sparse (Schwierzeck et al., 2020), our intention was to conduct an exploratory study focussing on the precision of the prevalence estimate in the defined exposure groups (i.e. the group comparisons by hypothesis test was not the primary objective). Thus, we assumed a true prevalence of 5%. One‐hundred and fifty participants per group should be targeted to get 95% confidence intervals (for the proportion) with a precision (half width of confidence interval) of about 3.5%.
2.7. Statistical analysis
Characteristics of participants are summarized (overall, stratified by test result) as absolute and relative frequencies or as median together with first and third quartile (Q1, Q3). Point seroprevalence of SARS‐CoV‐2 in hospital staff is described with absolute and relative frequencies together with 95% Clopper–Pearson confidence intervals (CIs). To compare seroprevalence rates between participants working at different COVID‐19 risk areas, to analyse clinical symptoms and to identify potential risk factors for seropositive compared to seronegative participants, we apply uni‐ and multivariable logistic regression modelling with the seropositivity as dependent variable and the investigated factor as independent variable. In the multivariable models, we first adjusted for age and sex and, then, additionally for returning from a COVID‐19 risk area since February 2020 (see Tables 1 and S4). As sensitivity analysis, we repeated the logistic regression modelling with any evidence of a past or current SARS‐CoV‐2 infection as dependent variable (see Tables S3 and S5). We provide (adjusted) odds ratios (OR) together with 95% CI and p‐value. Compliance of HCWs (in high and intermediate‐risk areas) to wear PPE is assessed with Fisher's exact test. We compare those HCWs who stated to always or mostly wear PPE to those who stated not to wear PPE or did not provide information on this issue.
TABLE 1.
Characteristics of the study population—overall and stratified by antibody test result (SARS‐CoV‐2 IgG)
| Variable | Overall (n = 660) | SARS‐CoV−2 IgG | Logistic regression | ||||
|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | ||||||
| Detectable (n = 18) | Not detectable (n = 627) | OR (95% CI) | p‐value | adjusted OR (95% CI) | p‐value | ||
| Demographics | |||||||
| Age, in years | 40.5 (32.0, 49.0) | 43.0 (35.3, 52.3) | 40.0 (32.0, 49.0) | 1.02 (0.98, 1.07) | .310 | 1.02 (0.98, 1.07) | .322 |
| Male sex | 174 (26.4%) | 5 (27.8%) | 169 (27.0%) | 1.04 (0.37, 2.97) | .938 | 1.10 (0.38, 3.14) | .862 |
| Profession a | |||||||
| Medical doctor | 103 (15.6%) | 5 (27.8%) | 94 (15.0%) | ref. | .413 | ref. | .379 |
| Nurse or care worker | 215 (32.6%) | 5 (27.8%) | 206 (32.9%) | 0.46 (0.13, 1.61) | .224 | 0.38 (0.10, 1.34) | .143 |
| Cleaner | 6 (0.9%) | 1 (5.6%) | 5 (0.8%) | 3.76 (0.37, 38.56) | .265 | 2.75 (0.25, 29.93) | .406 |
| Reception staff | 19 (2.9%) | 1 (5.6%) | 18 (2.9%) | 1.04 (0.12, 9.48) | .969 | 0.73 (0.07, 7.32) | .787 |
| Administration staff | 180 (27.3%) | 6 (33.3%) | 169 (27.0%) | 0.67 (0.20, 2.25) | .514 | 0.51 (0.13, 1.93) | .319 |
| Other profession | 130 (19.7%) | 0 (0.0%) | 128 (20.4%) | ‐ | ‐ | ‐ | ‐ |
| COVID−19 risk group according to working place | |||||||
| High‐risk | 137 (20.8%) | 2 (11.1%) | 133 (21.2%) | ref. | .574 | ref. | .655 |
| Intermediate‐risk | 343 (52.0%) | 10 (55.6%) | 325 (51.8%) | 2.05 (0.44, 9.46) | .360 | 1.97 (0.42, 9.22) | .389 |
| Low‐risk | 180 (27.3%) | 6 (33.3%) | 169 (27.0%) | 2.36 (0.47, 11.89) | .298 | 2.10 (0.40, 11.06) | .382 |
| Returning from risk areas since February 2020 b | |||||||
| Yes | 85 (12.9%) | 1 (5.6%) | 83 (13.2%) | 0.39 (0.05, 2.93) | .357 | 0.40 (0.05, 3.01) | .370 |
| Reported COVID−19 exposure | |||||||
| Reported exposure | 206 (31.2%) | 5 (27.8%) | 199 (31.7%) | 0.83 (0.29, 2.35) | .722 | 0.89 (0.31, 2.59) | .832 |
| Place of reported exposure c , d | |||||||
| At work | 198 (30.0%) | 3 (16.7%) | 193 (30.8%) | 0.45 (0.13, 1.57) | .211 | 0.48 (0.13, 1.70) | .255 |
| At home | 4 (0.6%) | 2 (11.1%) | 2 (0.3%) | 39.06 (5.17, 295.00) | <.001 | 47.82 (5.49, 416.62) | <.001 |
| Other place | 16 (2.4%) | 0 (0.0%) | 16 (8.0%) | ‐ | ‐ | ‐ | ‐ |
| Clinical symptoms within the last 2 months c | |||||||
| Any clinical symptom | 272 (41.2%) | 9 (50.0%) | 254 (40.5%) | 1.47 (0.58, 3.75) | .422 | 1.54 (0.60, 3.96) | .368 |
| Cold‐like symptoms | 249 (37.7%) | 9 (50.0%) | 232 (37.0%) | 1.70 (0.67, 4.35) | .266 | 1.80 (0.70, 4.64) | .220 |
| Diarrhoea | 72 (10.9%) | 2 (11.1%) | 68 (10.8%) | 1.03 (0.23, 4.57) | .971 | 0.99 (0.22, 4.43) | .994 |
| Taste disturbance | 9 (1.4%) | 2 (11.1%) | 6 (1.0%) | 12.94 (2.42, 69.10) | .003 | 14.91 (2.67, 83.41) | .002 |
| Smell disorders | 16 (2.4%) | 2 (11.1%) | 13 (2.1%) | 5.90 (1.23, 28.36) | .027 | 6.31 (1.28, 31.03) | .024 |
The number of participants (n) is provided. Median together with first and third quartile or absolute and relative frequencies are provided. Furthermore, results from uni‐ and multivariable logistic regression modelling (odds ratio (OR) and adjusted OR with 95% confidence interval (CI) and p‐value) comparing participants with detectable SARS‐CoV‐2 IgG antibodies by at least one immunoassay and participants without detectable SARS‐CoV‐2 by both immunoassays are given. The reference category (ref.) is provided, if necessary. The adjusted OR was calculated adjusting for age, sex and returning from a COVID‐19 risk area. The complete results are provided in Table S4. Participants with a borderline test result by EDI ELISA IgG and a negative Elecsys Roche test (n = 15) are included in the overall characterization but were neither classified as seronegative nor as seropositive. For detailed results of this subgroup, see Table S2.
Abbreviations: ‐, not applicable.
Information on profession is missing for 7 participants. ‘other profession’ excluded from logistic regression analysis due to sample size issues in the two groups.
Information missing on one participant.
Multiple answers possible.
‘Other place’ excluded from logistic regression analysis due to sample size issues in the two groups.
We applied a two‐sided significance level of 0.05 and did not correct for multiple testing as all analyses were considered exploratory. Clopper–Pearson CIs were calculated with Microsoft Excel 2016. All other analyses were done with SPSS Statistics version 25.0 for Windows (IBM Corp., Armonk, NY, USA).
3. RESULTS
3.1. Characteristics of the study population
We identified 3,228 hospital staff members who were eligible for study inclusion. Among them, 721 participants (22.3%) were included, and 660 of 721 participants (91.5%) could be analysed (see Figure 1). Of the 660 analysed participants, 174 (26.4%) were males and 486 (73.6%) were females. The median age of the participants was 40.5 (Q1‐Q3: 32.0–49.0) years. The most common professions involved included nurses (n = 215, 32.6%), followed by administration staff (n = 180, 27.3%), medical doctors (n = 103, 15.6%), nursing assistants (n = 18; 2.7%), psychologists (n = 18; 2.7%) and ergo therapists (n = 17; 2.6%). Two‐hundred six participants (31.2%) reported direct exposure to a confirmed COVID‐19 case. Among 198 staff members with direct COVID‐19 contact at work, 12 participants (6.1%) reported contact to a SARS‐CoV‐2 positive colleague. Direct COVID‐19 contact outside the JUH included household contacts (n = 4), patient contacts at other health care facilities (n = 6), in the ambulance (n = 5) or at a home visit (n = 1), direct contact in town (n = 2), at vacation (n = 1) or with a former employee (n = 1). Further details on the participants are provided in Table 1.
FIGURE 1.
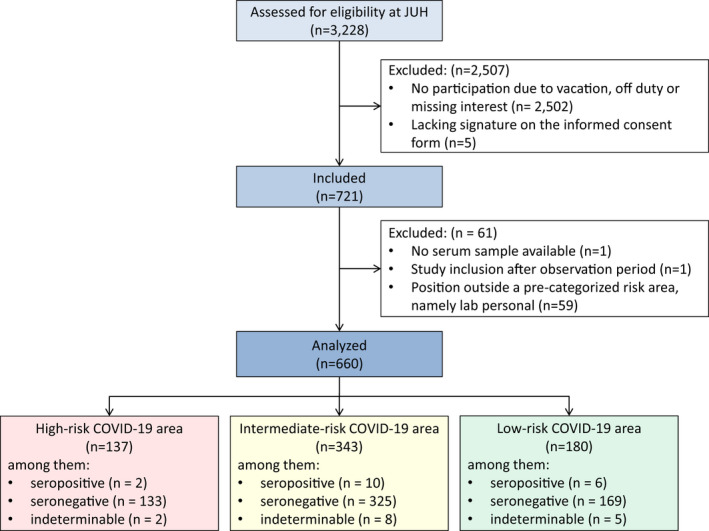
Flow chart of the Co‐HCW study. The number of hospital staff members (n) is provided. Reasons for exclusions are given. Hospital staff members, including healthcare workers and administration staff, working at predefined areas at Jena University Hospital (JUH) were eligible for study inclusion. Working areas were classified into three categories according to the risk to deal with COVID‐19 positive patients (see Table S1 for the definition). Note that we decided not to assign laboratory personal to a pre‐categorized risk area because a proportion dealt with COVID‐19 related clinical specimens but there was no patient contact
3.2. Point seroprevalence, infection status and previous testing for COVID‐19
Among the 660 participants, 627 (95.0%) were tested negative for SARS‐CoV‐2 IgG antibodies by both immunoassays. Two participants (0.3%) were tested positive by Elecsys Roche and EDI IgG ELISA, and 16 participants (2.4%) were only tested positive by EDI IgG ELISA. Fifteen participants (2.3%) had a ‘borderline’ result for EDI IgG ELISA. Hence, 18 staff members (2.7%, 95% CI 1.6%‐4.3%), 12 HCWs (2.5% within the group HCWs, 95% CI 1.3%‐4.3%) and 6 administration staff members (3.3% within the group administration staff, 95% CI 1.2%‐7.1%) had detectable SARS‐CoV‐2 IgG antibodies in at least one immunoassay. When considering also previously reported PCR and serology results, cumulative SARS‐CoV‐2 infection rate in our participants was 3.2% (95% CI 2.0%‐4.8%). Among the 18 participants with detectable SARS‐CoV‐2 IgG antibodies, only 9 (50.0%) reported clinical symptoms within the last two months (see Table 2).
TABLE 2.
Current and reported test results and clinical symptoms for COVID‐19 in hospital staff members of Jena University Hospital stratified by (a) detectable antibodies after recruitment, (b) history of past positive SARS‐CoV‐2 polymerase chain reaction (PCR) or serology, and (c) any evidence of a COVID‐19 infection
| Participants seropositive for SARS‐CoV−2 IgG antibodies after recruitment | Participants with reported evidence of a positive SARS‐CoV−2 test prior recruitment | Participants with any evidence of past/current SARS‐CoV−2 infection | |
|---|---|---|---|
| Overall | |||
| Number of hospital staff members | 18 out of 660 tested | 4 out of 212 tested | 21 out of 660 tested |
| Proportion (95% CI) | 2.7% (1.6% to 4.3%) | 1.9% (0.5% to 4.8%) | 3.2% (2.0% to 4.8%) |
| Among respective hospital staff members | |||
| Not previously diagnosed as COVID−19 by PCR or serology | 17 (94.4%, 72.7% to 99.9%) | ‐ | 17 (81.0%, 58.1% to 94.6%) |
| COVID−19 symptoms reported | 9 (50.0%, 26.0% to 74.0%) | 4 (100%, 38.8% to 100.0%) | 12 (57.1%, 34.0% to 78.2%) |
| Maximum severity of cold‐like symptoms within the last two months | 0.5 (0.0, 4.0) | 3.0 (1.3, 4.0) | 1.0 (0.0, 4.0) |
Absolute and relative frequencies together with 95% Clopper–Pearson confidence intervals (CI) or median together with first and third quartile are reported. Severity of illness (cold‐like symptoms) is defined according to the Wisconsin Upper Respiratory Symptom Survey (0 = no illness, 1 = very mild, 3 = mild, 5 = moderate, 7 = severe). Abbreviations: ‐, not applicable.
3.3. Follow‐up of discrepant immunoassay results
Thirteen of 15 participants (86.7%) with a ‘borderline’ test result for EDI IgG ELISA were retested after 5.4 to 9.4 weeks (median: 6.5 weeks), and 13/13 (100%) became negative in the retest. For clinical symptoms in this group, we refer to Table S2. Twelve of 16 participants (75.0%) with a positive result for EDI IgG ELISA but negative Elecsys Roche test were retested after 5.1 to 8.3 weeks (median: 6.4 weeks). Three (25.0%) of them had unchanged test results, whereas 9 of 12 participants (75.0%) became negative in the retest. All 3 participants (2 HCWs and 1 administration employee) with a persistent positive test result for EDI IgG ELISA but negative Elecsys Roche test did not report any clinical symptoms.
3.4. Potential risk factors and clinical symptoms for antibody positivity of staff members
As shown in Table 1, we did not observe evidence for an association of antibody positivity with the demographics, the professions or COVID‐19 risk area (all p‐values from logistic regression >0.05). The two persons who were tested positive by both immunoassays were administration staff members. The only parameters that were associated with SARS‐CoV‐2 seropositivity in staff members included close COVID‐19 contact at home (adjusted OR 47.82, 95% CI 5.49 to 416.62), taste disturbances (adjusted OR 14.91, 95% CI 2.67 to 83.41) and smell disorders (adjusted OR 6.31, 95% CI 1.28 to 31.03). These results are similar to our sensitivity analysis results for any evidence of a past or current SARS‐CoV‐2 infection (see Tables S3 and S5).
3.5. Compliance to wear PPE in case of an individual contact with a COVID‐19 positive patient and/or its surroundings
Reported compliance to wear PPE was associated with the COVID‐19 risk area according to the working place (p < .001). Compliance of HCWs working in COVID‐19 high‐risk was 98.3% (yes: n = 114; no: n = 2) and in intermediate‐risk areas 69.8% (yes: n = 62, most time: n = 5, no: n = 21, no answer given: n = 8). Detailed information on compliance to wear different items of PPE and different circumstances is given in Figure 2.
FIGURE 2.
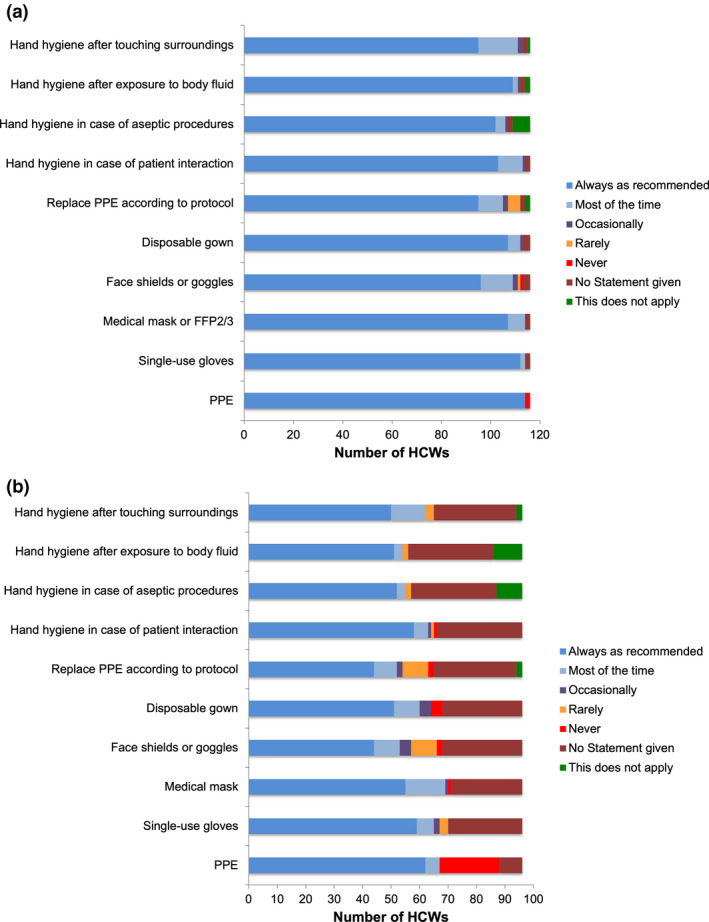
Compliance to wear personal protective equipment (PPE) in case of an individual reported contact with a confirmed COVID‐19 positive patient or its surroundings in healthcare workers (HCWs) from (a) high‐risk COVID‐19 areas (n = 116 HCWs) versus (b) intermediate‐risk COVID‐19 areas (n = 96 HCWs). The definitions of risk areas are provided in Table S1
A single HCW reported a puncture/sharp accident with material contaminated with biological fluid/respiratory secretions during a healthcare interaction with a COVID‐19 patient. This HCW remained seronegative.
4. DISCUSSION
The main findings of our study were as follows: Altogether, (a) seroprevalence rate of SARS‐CoV‐2 IgG and cumulative SARS‐CoV‐2 infection rate among hospital staff were low (2.7% and 3.2%), (b) we did not detect an association between seroprevalence rate and risk area according to the hospital workplace, but (c) participants with a SARS‐CoV‐2 IgG seroprevalence reported more frequently individual exposures to a SARS‐CoV‐2 positive household contact and the clinical symptoms taste disturbance as well as smell disorders and (d) HCWs in the high‐risk group reported a remarkable compliance of 98.2% regarding PPE administration whereas compliance in the intermediate‐risk group was significantly lower with 69.8%.
Even additionally considering previously reported PCR and serology results, the evidence of a past or current SARS‐CoV‐2 infection among hospital staff remained below the expected 5% derived from reports of a similar hospital in Münster, Germany (Schwierzeck et al., 2020). An explanation might be a low SARS‐CoV‐2 seroprevalence among the Jena population. So far, the only seroprevalence data available from Jena region are from an unselected sample of 180 pregnant women showing a seroconversion of 0.6% between 6th April and 13th May 2020 (Zöllkau et al., 2020). In contrast, in a population‐based cohort study in the Thuringian community Neustadt am Rennsteig, Germany, we detected a seroprevalence of 8.4% between 22nd March and 5th April 2020 (Weis et al., 2020). For Germany, confirmed COVID‐19 cases are available for all regions from the official site of the Robert Koch Institute (https://experience.arcgis.com/experience/478220a4c454480e823b17327b2bf1d4 last accessed at 2nd February 2021). During the observation period of the present study, the cumulative number of COVID‐19 cases in the city of Jena was 158 (19th May 2020) to 160 (19th June 2020), corresponding to a confirmed infection rate of only 0.14% of the Jena population.
Whereas only a limited number of surveillance studies among HCWs report similar low seroprevalence rates (Korth et al., 2020; Kumar, Sathyapalan et al., 2020; Dacosta‐Urbieta et al., 2020; Xu et al., 2020), the majority of studies in HCWs found SARS‐CoV‐2 seroprevalence rates above 5% (Finkenzeller et al., 2020; Garcia‐Basteiro et al., 2020; Grant et al., 2020; Iversen et al., 2020; Kumar, Bhartiya et al., 2020; Self et al., 2020; Shields et al., 2020; Venugopal et al., 2021). Moreover, some of those studies with higher seroprevalence rates also found an increased risk for a higher seroprevalence in HCWs working in a COVID‐19 unit (Grant et al., 2020; Iversen et al., 2020;), whereas other studies including ours did not find this association (Garcia‐Basteiro et al., 2020; Korth et al., 2020). Contrary, in the present study, HCWs working at COVID‐19 high‐risk areas had the numerically lowest (1.5%) and administration staff had the numerically highest seroprevalence rates (3.3%). However, we found an association between seroprevalence or evidence of a current or past SARS‐CoV‐2 infection and a reported individual exposure to a SARS‐CoV‐2 positive household contact. Similarly, in another study from Belgium with 3,056 participants (including clinical and non‐clinical hospital staff as well as volunteers), a household contact with suspected or confirmed COVID‐19 was associated with antibody positivity (13.7% seroprevalence with household contacts versus 4.8% seroprevalence without household exposure, p < .001) (Steensels et al., 2020). Additionally, the authors Luo et al. who evaluated the risk for transmission in 3,410 close contacts of COVID‐19 patients identified household contact as the main setting for transmission of SARS‐CoV‐2 (10.3%) (Luo et al., 2020). Community acquisition is hence a major aspect that needs to be considered.
Another finding of the present study was a remarkable compliance of HCWs in the high‐risk group regarding PPE administration. We assume that the awareness regarding personal protection was higher in those HCWs who are repeatedly exposed to COVID‐19 patients. An increased awareness might lead to better adherence to other hygienic measurements as well (Houghton et al., 2020). In addition, a daily routine in PPE use improves correct donning and doffing and, thus, reduces the risk of contamination. The results of the study support the importance of adequate PPE use to prevent transmission from patient to HCW. Additionally, mandatory masking might have reduced nosocomial transmissions also in employees without patient contact. However, compliance to mandatory masking during working hours was not evaluated in our study. According to Wang et al., implementation of mandatory masking of HCWs and patients can be effective to reduce SARS‐CoV‐2 infection rates (Wang et al., 2020). In a recently published report by Self et al. that included 3,248 frontline HCWs, seroprevalence of SARS‐CoV‐2 antibodies was lower among HCWs who reported always wearing a face covering while caring for patients compared to those who did not (6% vs. 9%) (Self et al., 2020).
It is known, that pre‐ and asymptomatic COVID‐19 infected persons can be contagious despite absence of any subjective feeling of illness. In a population‐based study by Gudbjartsson et al. (2020) including 30,576 people from Iceland, nearly one third of the SARS‐CoV‐2 infections were asymptomatic and durability of SARS‐CoV‐2 antibody levels was over 4 months. In our study population, only 50% of those tested positive reported any clinical symptoms, but the presence of taste disturbances or smell disorders were both associated with seropositivity of SARS‐CoV‐2 IgG. Similarly, the authors Iversen et al. (2020) identified loss of smell or taste as the symptom that was most strongly associated with seropositivity in HCWs in Denmark.
The validity regarding sensitivity and specificity of SARS‐CoV‐2 serology testing has not yet been investigated entirely (Deeks et al., 2020). According to the recently published IDSA guidelines on the diagnosis of COVID‐19, there will be false positive and false negative tests, but the most reliable spot of measuring SARS‐CoV‐2 antibodies is 3–4 weeks after exposure to the virus/onset of clinical symptoms (Hanson et al., 2020). In the present study, we used two different immunoassays, the Elecsys Anti‐SARS‐CoV‐2 Roche Diagnostics and the EDI Novel Coronavirus SARS‐CoV‐2 IgG ELISA. A recent head‐to‐head comparison of both immunoassays found acceptable agreement between both tests (Egger et al., 2020). In our study, the Roche Elecsys assay did not identify asymptomatic COVID‐19 cases, which was already observed in the CoNAN study mentioned above (Weis et al., 2020). This assay was only positive in 2 participants (0.3%) who were tested at beginning of June 2020 and had developed a symptomatic COVID‐19 disease in the second half of March 2020. In contrast, the EDI IgG ELISA was positive in 2.7% of participants (9 asymptomatic and 9 symptomatic cases) including the two persons with the positive Roche Elecsys assay. It is still a matter of debate to what extend seroprevalence of SARS‐CoV‐2 IgG can really reflect immunity and status after infection. Nonetheless, serological examination is a method, which is easily available and highly cost‐effective compared to PCR (Alter & Seder, 2020).
This study has the following limitations: Despite the high number of participants, the recruitment rate was below 10% of the total JUH staff and results of previous COVID‐19 testing and compliance using PPE were only recorded by self‐reports. We determined antibody titres repeatedly only in those with discrepant results. As SARS‐CoV‐2 infection generates two waves of antibodies, the provided data do not reflect long‐lived immunity (Alter & Seder, 2020).
In our study, reported contact with a COVID‐19 patient was not found to be a risk factor for seroprevalence of SARS‐CoV‐2 antibodies, whereas contacts with infected family members were highly predictive. In line, we found a high awareness and compliance with PPE and no evidence for higher seroprevalence in HCWs caring for COVID‐19 patients, whereas administration employees with no patient contacts had numerically higher seroprevalence rates. We conclude that for HCWs, community transmission may play a larger role for COVID‐19 infection than professional exposure when using appropriate PPE.
CONFLICT OF INTERESTS
None to declare.
AUTHOR CONTRIBUTIONS
CB, AK and MWP had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SW, MWP, ASch, JM, BL, MKi, ASta, ASte, MBau, WB, FZ and MW involved in study concept and design. CB, AK, SH and JA involved in acquisition of data. CR, MKi, MBai and BL involved in performing of seroprevalence testing. CB and MKe involved in statistical analyses. CB, AK, MKe and MWP drafted the manuscript. All authors involved in critical revision of the manuscript and additional important intellectual content, data interpretation. MWP and CB supervised the study.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received by the local ethics committee of the Friedrich‐Schiller‐University Jena (ethical approval number 2020‐1774). The research was conducted in accordance with the Declaration of Helsinki and national and institutional standards.
Supporting information
Table S1‐S5
ACKNOWLEDGEMENTS
We thank Stefanie Beier, Jana Schmidt, Stefanie Kolanos and Monique Philippe for excellent technical support.
Bahrs C, Kimmig A, Weis S, et al. Prospective surveillance study in a 1,400‐bed university hospital: COVID‐19 exposure at home was the main risk factor for SARS‐CoV‐2 point seroprevalence among hospital staff. Transbound Emerg Dis.2022;69:720–730. 10.1111/tbed.14041
Bahrs and Kimmig contributed equally.
Funding information
This study was partly supported by the local ethics committee of Friedrich‐Schiller‐University Jena and financed by internal funding.
DATA AVAILABILITY STATEMENT
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- Abebe, E. C. , Dejenie, T. A. , Shiferaw, M. Y. , & Malik, T. (2020). The newly emerged COVID‐19 disease: A systemic review. Virology Journal, 17, 96. 10.1186/s12985-020-01363-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter, G. , & Seder, R. (2020). The Power of Antibody‐Based Surveillance. The New England Journal of Medicine, 383, 1782–1784. 10.1056/NEJMe2028079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, M. A. , Rhee, C. , Fiumara, K. , Bennett‐Rizzo, C. , Tucker, R. , Williams, S. A. , Wickner, P. , Beloff, J. , McGrath, C. , Poulton, A. , & Klompas, M. (2020). COVID‐19 infections among HCWs exposed to a patient with a delayed diagnosis of COVID‐19. Infection Control & Hospital Epidemiology, 27, 1–2. 10.1017/ice.2020.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, B. , Brown, R. , Mundt, M. , Safdar, N. , Dye, L. , Maberry, R. , & Alt, J. (2005). The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. Journal of Clinical Epidemiology, 58, 609–617. 10.1016/j.jclinepi.2004.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , Qiu, Y. , Wang, J. , Liu, Y. , Wei, Y. , Xia, J. , Yu, T. , Zhang, X. , & Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet, 395, 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, R. , Dana, T. , Buckley, D. I. , Selph, S. , Fu, R. , & Totten, A. M. (2020). Epidemiology of and risk factors for coronavirus infection in health care workers: A living rapid review. Annals of Internal Medicine, 173, 120–136. 10.7326/M20-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacosta‐Urbieta, A. , Rivero‐Calle, I. , Pardo‐Seco, J. , Redondo‐Collazo, L. , Salas, A. , Gómez‐Rial, J. , & Martinón‐Torres, F. (2020). Seroprevalence of SARS‐CoV‐2 Among Pediatric Healthcare Workers in Spain. Frontiers in Pediatris, 8, 547. 10.3389/fped.2020.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks, J. J. , Dinnes, J. , Takwoingi, Y. , Davenport, C. , Spijker, R. , Taylor‐Phillips, S. , Adriano, A. , Beese, S. , Dretzke, J. , Ferrante di Ruffano, L. , Harris, I. M. , Price, M. J. , Dittrich, S. , Emperador, D. , Hooft, L. , Leeflang, M. M. G. , & Van den Bruel, A. (2020). Antibody tests for identification of current and past infection with SARS‐CoV‐2. Cochrane Database of Systematic Reviews, 10.1002/14651858.CD013652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger, M. , Bundschuh, C. , Wiesinger, K. , Gabriel, C. , Clodi, M. , Mueller, T. , & Dieplinger, B. (2020). Comparison of the Elecsys(R) Anti‐SARS‐CoV‐2 immunoassay with the EDI enzyme linked immunosorbent assays for the detection of SARS‐CoV‐2 antibodies in human plasma. Clinica Chimica Acta, 509, 18–21. 10.1016/j.cca.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkenzeller, T. , Faltlhauser, A. , Dietl, K. H. , Paetzel, C. , Szczypien, N. , Klawonn, F. , Bodmann, K. F. , & von Meyer, A. (2020). SARS‐CoV‐2‐Antikörper bei Intensiv‐ und Klinikpersonal. Medizinische Klinik Intensivmedizin Und Notfallmedizin, 115(Suppl 3), S139–S145. 10.1007/s00063-020-00761-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa, N. W. , Brooks, J. T. , & Sobel, J. (2020). Evidence supporting transmission of Severe Acute Respiratory Syndrome Coronavirus 2 while presymptomatic or asymptomatic. Emerging Infectious Diseases, 26, e201595. 10.3201/eid2607.201595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis, P. , Vraka, I. , Fragkou, D. , Bilali, A. , & Kaitelidou, D. (2021). Seroprevalence of SARS‐CoV‐2 antibodies and associated factors in health care workers: A systematic review and meta‐analysis. Journal of Hospital Infection, 108, 120–134. 10.1016/j.jhin.2020.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Basteiro, A. L. , Moncunill, G. , Tortajada, M. , Vidal, M. , Guinovart, C. , Jiménez, A. , Santano, R. , Sanz, S. , Méndez, S. , Llupià, A. , Aguilar, R. , Alonso, S. , Barrios, D. , Carolis, C. , Cisteró, P. , Chóliz, E. , Cruz, A. , Fochs, S. , Jairoce, C. , … Dobaño, C. (2020). Seroprevalence of antibodies against SARS‐CoV‐2 among health care workers in a large Spanish reference hospital. Nature Communications, 11, 3500. 10.1038/s41467-020-17318-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, J. J. , Wilmore, S. M. S. , McCann, N. S. , Donnelly, O. , Lai, R. W. L. , Kinsella, M. J. , Rochford, H. L. , Patel, T. , Kelsey, M. C. , & Andrews, J. A. (2021). Seroprevalence of SARS‐CoV‐2 antibodies in healthcare workers at a London NHS Trust. Infection Control & Hospital Epidemiology, 42(2), 212–214. 10.1017/ice.2020.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W.‐J. , Ni, Z.‐Y. , Hu, Y. , Liang, W.‐H. , Ou, C.‐Q. , He, J.‐X. , Liu, L. , Shan, H. , Lei, C.‐L. , Hui, D. S. C. , Du, B. , Li, L.‐J. , Zeng, G. , Yuen, K.‐Y. , Chen, R.‐C. , Tang, C.‐L. , Wang, T. , Chen, P.‐Y. , Xiang, J. , … Zhong, N.‐S. (2020). Clinical characteristics of coronavirus disease 2019 in China. The New England Journal of Medicine, 382, 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson, D. F. , Norddahl, G. L. , Melsted, P. , Gunnarsdottir, K. , Holm, H. , Eythorsson, E. , Arnthorsson, A. O. , Helgason, D. , Bjarnadottir, K. , Ingvarsson, R. F. , Thorsteinsdottir, B. , Kristjansdottir, S. , Birgisdottir, K. , Kristinsdottir, A. M. , Sigurdsson, M. I. , Arnadottir, G. A. , Ivarsdottir, E. V. , Andresdottir, M. , & Jonsson, F. … Stefansson, K. Humoral immune response to SARS‐CoV‐2 in Iceland. New England Journal of Medicine, 383(18), 1724–1734. 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, K. E. , Caliendo, A. M. , Arias, C. A. , Englund, J. A. , Hayden, M. K. , Lee, M. J. , Loeb, M. , Patel, R. , Altayar, O. , El Alayli, A. , Sultan, S. , Falck‐Ytter, Y. , Lavergne, V. , Morgan, R. L. , Murad, M. H. , Bhimraj, A. , & Mustafa, R. A. (2020). Infectious Diseases Society of America Guidelines on the Diagnosis of COVID‐19: Serologic testing. Clinical Infectious Diseases, ciaa1343. 10.1093/cid/ciaa1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, X. , Lau, E. H. Y. , Wu, P. , Deng, X. , Wang, J. , Hao, X. , Lau, Y. C. , Wong, J. Y. , Guan, Y. , Tan, X. , Mo, X. , Chen, Y. , Liao, B. , Chen, W. , Hu, F. , Zhang, Q. , Zhong, M. , Wu, Y. , Zhao, L. , … Leung, G. M. (2020). Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nature Medicine, 26, 672–675. 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- Houghton, C. , Meskell, P. , Delaney, H. , Smalle, M. , Glenton, C. , Booth, A. , Chan, X. H. S. , Devane, D. , & Biesty, L. M. (2020). Barriers and facilitators to healthcare workers' adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: A rapid qualitative evidence synthesis. Cochrane Database of Systematic Reviews, 4, CD013582. 10.1002/14651858.CD013582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , Zhang, L. , Fan, G. , Xu, J. , Gu, X. , Cheng, Z. , Yu, T. , Xia, J. , Wei, Y. , Wu, W. , Xie, X. , Yin, W. , Li, H. , Liu, M. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395, 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen, K. , Bundgaard, H. , Hasselbalch, R. B. , Kristensen, J. H. , Nielsen, P. B. , Pries‐Heje, M. , Knudsen, A. D. , Christensen, C. E. , Fogh, K. , Norsk, J. B. , Andersen, O. , Fischer, T. K. , Jensen, C. A. J. , Larsen, M. , Torp‐Pedersen, C. , Rungby, J. , Ditlev, S. B. , Hageman, I. , Møgelvang, R. , … Ullum, H. (2020). Risk of COVID‐19 in health‐care workers in Denmark: An observational cohort study. The Lancet Infectious Diseases, S1473–3099(20), 30589–30592. 10.1016/S1473-3099(20)30589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth, J. , Wilde, B. , Dolff, S. , Anastasiou, O. E. , Krawczyk, A. , Jahn, M. , Cordes, S. , Ross, B. , Esser, S. , Lindemann, M. , Kribben, A. , Dittmer, U. , Witzke, O. , & Herrmann, A. (2020). SARS‐CoV‐2‐specific antibody detection in healthcare workers in Germany with direct contact to COVID‐19 patients. Journal of Clinical Virology, 128, 104437. 10.1016/j.jcv.2020.104437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. , Sathyapalan, D. , Ramachandran, A. , Subhash, K. , Biswas, L. , & Beena, K. V. (2020). SARS‐CoV‐2 antibodies in healthcare workers in a large university hospital, Kerala, India. Clinical Microbiology and Infection, S1198‐743X(20)30562‐0. 10.1016/j.cmi.2020.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, N. , Bhartiya, S. , Desai, S. , Mutha, A. , Beldar, A. , & Singh, T. (2020). Seroprevalence of Antibodies Against SARS‐CoV‐2 Among Health Care Workers in Mumbai, India. Asia Pacific Journal of Public Health, 1010539520977307. 10.1177/1010539520977307 [DOI] [PubMed] [Google Scholar]
- Long, Q. X. , Tang, X. J. , Shi, Q. L. , Li, Q. , Deng, H. J. , Yuan, J. , Hu, J. L. , Xu, W. , Zhang, Y. , Lv, F. J. , Su, K. , Zhang, F. , Gong, J. , Wu, B. , Liu, X. M. , Li, J. J. , Qiu, J. F. , Chen, J. , & Huang, A. L. (2020). Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nature Medicine, 26, 1200–1204. 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- Luo, L. , Liu, D. , Liao, X. , Wu, X. , Jing, Q. , Zheng, J. , Liu, F. , Yang, S. , Bi, H. , Li, Z. , Liu, J. , Song, W. , Zhu, W. , Wang, Z. , Zhang, X. , Huang, Q. , Chen, P. , Liu, H. , Cheng, X. , … Mao, C. (2020). Contact settings and risk for transmission in 3410 close contacts of patients With COVID‐19 in Guangzhou, China : A prospective cohort study. Annals of Internal Medicine, 173, 879–887. 10.7326/M20-2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, L. H. , Drew, D. A. , Graham, M. S. , Joshi, A. D. , Guo, C.‐G. , Ma, W. , Mehta, R. S. , Warner, E. T. , Sikavi, D. R. , Lo, C.‐H. , Kwon, S. , Song, M. , Mucci, L. A. , Stampfer, M. J. , Willett, W. C. , Eliassen, A. H. , Hart, J. E. , Chavarro, J. E. , Rich‐Edwards, J. W. , … Zhang, F. (2020). Risk of COVID‐19 among front‐line health‐care workers and the general community: A prospective cohort study. The Lancet Public Health, 5, e475–e483. 10.1016/S2468-2667(20)30164-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, K. P. , Vunnam, S. R. , Patel, P. A. , Krill, K. L. , Korbitz, P. M. , Gallagher, J. P. , Suh, J. E. , & Vunnam, R. R. (2020). Transmission of SARS‐CoV‐2: An update of current literature. European Journal of Clinical Microbiology & Infectious Diseases, 39, 2005–2011. 10.1007/s10096-020-03961-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergolizzi, J. V. Jr , Magnusson, P. , LeQuang, J. A. , Breve, F. , Paladini, A. , Rekatsina, M. , Yeam, C. T. , Imani, F. , Saltelli, G. , Taylor, R. Jr , Wollmuth, C. , & Varrassi, G. (2020). The current clinically relevant findings on COVID‐19 pandemic. Anesthesiology and Pain Medicine, 10, e103819. 10.5812/aapm.103819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reychler, G. , Vecellio, L. , & Dubus, J. C. & Group Aerosoltherapy GAT of the French Language Respiratory Society Société de Pneumologie de Langue Française SPLF . (2020). Nebulization: A potential source of SARS‐CoV‐2 transmission. Respiratory Medicine and Research, 78, 100778. 10.1016/j.resmer.2020.100778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwierzeck, V. , Correa‐Martinez, C. L. , Schneider, K. N. , Mellmann, A. , Hennies, M. T. , Hafezi, W. , Czeschinski, P. , & Kampmeier, S. (2020). SARS‐CoV‐2 in the Employees of a Large University Hospital. Deutsches Ärzteblatt International, 117, 344–345. 10.3238/arztebl.2020.0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self, W. H. , Tenforde, M. W. , Stubblefield, W. B. , Feldstein, L. R. , Steingrub, J. S. , Shapiro, N. I. , Ginde, A. A. , Prekker, M. E. , Brown, S. M. , Peltan, I. D. , Gong, M. N. , Aboodi, M. S. , Khan, A. , Exline, M. C. , Files, D. C. Gibbs, K. W. , Lindsell, C. J. , Rice, T. W. , Jones, I. D. , … Zellner, B. (2020). Seroprevalence of SARS‐CoV‐2 among frontline health care personnel in a multistate hospital network ‐ 13 Academic Medical Centers, April‐June 2020. Morbidity and Mortality Weekly Report, 69, 1221–1226. 10.15585/mmwr.mm6935e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, A. , Faustini, S. E. , Perez‐Toledo, M. , Jossi, S. , Aldera, E. , Allen, J. D. , Al‐Taei, S. , Backhouse, C. , Bosworth, A. , Dunbar, L. A. , Ebanks, D. , Emmanuel, B. , Garvey, M. , Gray, J. , Kidd, I. M. , McGinnell, G. , McLoughlin, D. E. , Morley, G. , O'Neill, J. , … Richter, A. G. (2020). SARS‐CoV‐2 seroprevalence and asymptomatic viral carriage in healthcare workers: A cross‐sectional study. Thorax, 75, 1089–1094. 10.1136/thoraxjnl-2020-215414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka, M. K. , & Gao, L. (2020). Is presymptomatic spread a major contributor to COVID‐19 transmission? Nature Medicine, 26, 1531–1533. 10.1038/s41591-020-1046-6 [DOI] [PubMed] [Google Scholar]
- Steensels, D. , Oris, E. , Coninx, L. , Nuyens, D. , Delforge, M. L. , Vermeersch, P. , & Heylen, L. (2020). Hospital‐Wide SARS‐CoV‐2 Antibody Screening in 3056 Staff in a Tertiary Center in Belgium. JAMA, 324, 195–197. 10.1001/jama.2020.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, J. , Rangaiah, J. , Narasimhan, S. , Clark, J. , Alexander, Z. , Manuel, R. , & Balasegaram, S. (2020). Nosocomial Coronavirus Disease 2019 (COVID‐19): Experience from a large Acute NHS Trust in South‐West London. Journal of Hospital Infection, 106, 621–625. 10.1016/j.jhin.2020.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treibel, T. A. , Manisty, C. , Burton, M. , McKnight, Á. , Lambourne, J. , Augusto, J. B. , Couto‐Parada, X. , Cutino‐Moguel, T. , Noursadeghi, M. , & Moon, J. C. (2020). COVID‐19: PCR screening of asymptomatic health‐care workers at London hospital. The Lancet, 395, 1608–1610. 10.1016/S0140-6736(20)31100-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal, U. , Jilani, N. , Rabah, S. , Shariff, M. A. , Jawed, M. , Mendez Batres, A. , Abubacker, M. , Menon, S. , Pillai, A. , Shabarek, N. , Kasubhai, M. , Dimitrov, V. , & Menon, V. (2021). SARS‐CoV‐2 seroprevalence among health care workers in a New York City hospital: A cross‐sectional analysis during the COVID‐19 pandemic. International Journal of Infectious Diseases, 102, 63–69. 10.1016/j.ijid.2020.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Ferro, E. G. , Zhou, G. , Hashimoto, D. , & Bhatt, D. L. (2020). Association Between Universal Masking in a Health Care System and SARS‐CoV‐2 Positivity Among Health Care Workers. Journal of the American Medical Association, 324, 703–704. 10.1001/jama.2020.12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis, S. , Scherag, A. , Baier, M. , Kiehntopf, M. , Kamradt, T. , Kolanos, S. , Ankert, J. , Glöckner, S. , Makarewicz, O. , Hagel, S. , Bahrs, C. , Kimmig, A. , Proquitté, H. , Guerra, J. , Rimek, D. , Löffler, B. , Pletz, M. W. , Enders, P. , … Kuhn, S. (2020). Antibody response using six different serological assays in a completely PCR‐tested community after a coronavirus disease 2019 outbreak—the CoNAN study. Clinical Microbiology and Infection, 10.1016/j.cmi.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth, J. (2020). COVID‐19: A fast evolving pandemic. Transactions of the Royal Society of Tropical Medicine & Hygiene, 114, 241–248. 10.1093/trstmh/traa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Sun, J. , Nie, S. , Li, H. , Kong, Y. , Liang, M. , Hou, J. , Huang, X. , Li, D. , Ma, T. , Peng, J. , Gao, S. , Shao, Y. , Zhu, H. , Lau, J. Y. , Wang, G. , Xie, C. , Jiang, L. , Huang, A. , … Hou, F. F. (2020). Seroprevalence of immunoglobulin M and G antibodies against SARS‐CoV‐2 in China. Nature Medicine, 26, 1193–1195. 10.1038/s41591-020-0949-6 [DOI] [PubMed] [Google Scholar]
- Zhao, D. , Wang, M. , Wang, M. , Zhao, Y. , Zheng, Z. , Li, X. , Zhang, Y. , Wang, T. , Zeng, S. , Hu, W. , Yu, W. , & Hu, K. (2020). Asymptomatic infection by SARS‐CoV‐2 in healthcare workers: A study in a large teaching hospital in Wuhan, China. International Journal of Infectious Diseases, 99, 219–225. 10.1016/j.ijid.2020.07.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q. , Gao, Y. , Wang, X. , Liu, R. , Du, P. , Wang, X. , Zhang, X. , Lu, S. , Wang, Z. , Shi, Q. , Li, W. , Ma, Y. , Luo, X. , Fukuoka, T. , Ahn, H. S. , Lee, M. S. , Liu, E. , Chen, Y. , Luo, Z. , & Yang, K. (2020). Nosocomial infections among patients with COVID‐19, SARS and MERS: A rapid review and meta‐analysis. Annals of Translational Medicine, 8, 629. 10.21037/atm-20-3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllkau, J. , Baier, M. , Scherag, A. , Schleußner, E. , & Groten, T. (2020). Period Prevalence of SARS‐CoV‐2 in an Unselected Sample of Pregnant Women in Jena, Thuringia. Zeitschrift Für Geburtshilfe Und Neonatologie, 224, 194–198. 10.1055/a-1206-1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S5
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.


