ABSTRACT
BACKGROUND AND PURPOSE
The ongoing Coronavirus Disease 2019 (COVID‐19) pandemic is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). COVID‐19 is occasionally associated with manifold diseases of the central nervous system (CNS). We sought to present the neuroimaging features of such CNS involvement. In addition, we sought to identify typical neuroimaging patterns that could indicate possible COVID‐19‐associated neurological manifestations.
METHODS
In this systematic literature review, typical neuroimaging features of cerebrovascular diseases and inflammatory processes associated with COVID‐19 were analyzed. Reports presenting individual patient data were included in further quantitative analysis with descriptive statistics.
RESULTS
We identified 115 studies reporting a total of 954 COVID‐19 patients with associated neurological manifestations and neuroimaging alterations. A total of 95 (82.6%) of the identified studies were single case reports or case series, whereas 660 (69.2%) of the reported cases included individual information and were thus included in descriptive statistical analysis. Ischemia with neuroimaging patterns of large vessel occlusion event was revealed in 59.9% of ischemic stroke patients, whereas 69.2% of patients with intracerebral hemorrhage exhibited bleeding in a location that was not associated with hypertension. Callosal and/or juxtacortical location was identified in 58.7% of cerebral microbleed positive images. Features of hemorrhagic necrotizing encephalitis were detected in 28.8% of patients with meningo‐/encephalitis.
CONCLUSIONS
Manifold CNS involvement is increasingly reported in COVID‐19 patients. Typical and atypical neuroimaging features have been observed in some disease entities, so that familiarity with these imaging patterns appears reasonable and may assist clinicians in the differential diagnosis of COVID‐19 CNS manifestations.
Keywords: COVID‐19, CT, neuroimaging, MRI, SARS‐CoV‐2
Introduction
About 9 months have passed since the outbreak of the Coronavirus Disease 2019 (COVID‐19) pandemic, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Being initially reported as a pure respiratory infection, COVID‐19 is increasingly considered as a multi‐organ disease, giving rise to cardiovascular, renal, gastrointestinal, hepatic, hematological, metabolic as well as neurological disorders. 1 , 2 , 3 In this context, manifold neurological manifestations involving the central as well as the peripheral nervous system have been increasingly described. 4 , 5 In particular, diverse cerebrovascular diseases are frequently observed in association with SARS‐CoV‐2 infection. 6 Beyond, inflammatory and immune‐mediated processes such as encephalitis, myelitis, and demyelination have been occasionally described in some cases, an observation, which might indicate a possible neurotropism of the virus. 7 However, apart from the direct involvement of the central nervous system (CNS), many of the observed neurological manifestations might be related to secondary parainfectious or postinfectious pathophysiological mechanisms, because immune‐mediated vulnerability of the CNS is frequently being observed in association to several viral infections 8 as well as to prolonged critical hospitalization. 9 Worth emphasizing, CNS involvement was also reported in the prior two coronavirus epidemics in the past, severe acute respiratory syndrome coronavirus 1 (SARS‐CoV‐1) and Middle East respiratory syndrome coronavirus (MERS‐CoV) epidemic in 2002‐2003 and 2012, respectively. 10 , 11
Although the prevalence of neurological involvement among COVID‐19 patients is relatively low considering the number of people infected from SARS‐CoV‐2, the absolute number has exponentially increased due to the ongoing pandemic. As many of the neurological symptoms and signs are nonspecific, for example, confusion, agitation, headache, generalized muscle weakness, delirium, and disorders of consciousness, it is a challenging task for the clinician to differentiate between direct involvement of the nervous system and neurologic manifestations of systematic causes such as metabolic derangement and hypoxia.
In view of the former considerations, neuroimaging examinations have increased in order to assist in the differential diagnosis of different neurological manifestations of COVID‐19. The most common indications for neuroimaging appear to be associated with altered mental status, syncope/fall, and focal neurologic deficits. 12 On the other hand, to avoid the additional risk of exposing other patients or healthcare providers to the virus, not all the COVID‐19 patients with neurological symptoms have undergone neuroimaging studies. In particular, the American College of Radiology (ACR) and Centers for Disease Control and Prevention (CDC) agreed on postponing imaging studies that would not have an impact on physicians decision‐making. 13 Nonetheless, a range of interesting and helpful brain and spinal cord MRI and CT findings have been described across the globe, necessitating careful review of these data. In this systematic review, we aimed to provide an overview of the currently described neuroimaging features of CNS diseases that have been associated with COVID‐19. In addition, special emphasis was given to identify possible neuroimaging patterns that may raise the suspicion of COVID‐19‐associated neurological manifestations.
Methods
This systematic review adopted the Preferred Reporting Items for Systematic reviews and Meta‐Analyses guidelines (PRISMA) 14 and was compliant with the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) 15 recommendations. We searched the literature utilizing two different databases (PubMed and Scopus) and applied the following search strategy: “(“coronavirus” OR “SARS‐CoV” OR “COVID‐19” OR “SARS‐CoV‐2”) AND (“neurologic” OR “brain” OR “Central Nervous System” OR “Cerebral” OR “stroke” OR “encephalitis” OR “myelitis”) AND (“neuroimaging” OR “computed tomography” OR “CT” OR “magnetic resonance” OR “imaging”).” We screened the references of relevant studies and searched for preprints to ensure that we did not miss important published results by our database search. All different forms of published scientific articles and studies were abstracted. Last literature search was conducted by two independent authors (TL and CK) on August 7, 2020. All of the studies included in our review concern confirmed, and not probable or possible, COVID‐19 case definitions according to the WHO COVID‐19 case definitions. 16
Original contributions including case reports and case series, which presented CT and/or MRI findings of COVID‐19 patients with CNS involvement, were included. Review articles, letters to the editor, and other correspondence not containing original data were excluded from further analysis.
Data were extracted by three independent authors (TL, JM, and JCJ) with the use of standardized columns contained information about the authors, title, journal, date of publication, disease category, region, design (ie, case report of cohort study), number of COVID‐19 patients with documented CNS involvement, age, sex, neuroimaging modality, and described neuroimaging findings. Disease category was defined as follows: 1 = ischemic stroke, 2 = hemorrhagic stroke, 3 = cerebral microbleeds, 4 = cerebral and sinus thrombosis (CVST), 5 = Posterior Reversible Encephalopathy Syndrome (PRES), 6 = other cerebrovascular manifestations (eg. cervical artery dissection), 7 = meningo‐/encephalitis, 8 = demyelination/leukoencephalopathy, 9 = myelitis, and 10 = cranial nerve involvement. After reviewing all the included studies, three authors (TL, GT, and CK) reached consensus about the emerging common neuroimaging features in each disease category, which were frequently reported in COVID‐19 patients. Subsequently, two further columns were added to the standardized form, which included (1) information about the number of patients with reported individual findings and (2) the number of patients exhibiting the suggested typical neuroimaging feature. Descriptive statistical analysis was based on the patient numbers given in these two columns, as proportion of patients exhibiting the suggested typical neuroimaging feature was determined. Examples of neuroimaging findings of COVID‐19 patients are presented for most of the discussed neurological disease entities.
Results
The initial literature search resulted in a list of 497 total records. The removal of duplicates yielded 273 articles. After a manual screening of these articles based on their titles or abstracts, a total of 115 studies reporting neuroimaging findings on a total of 954 COVID‐19 patients met the inclusion criteria for this systematic review (Fig 1); 73 studies were single case reports, 22 were presented as case series, 19 were conducted as retrospective observational or cross‐sectional studies, and one article represents a prospective cohort study. Sample sizes ranged from 1 to 242 patients per study.
Fig 1.
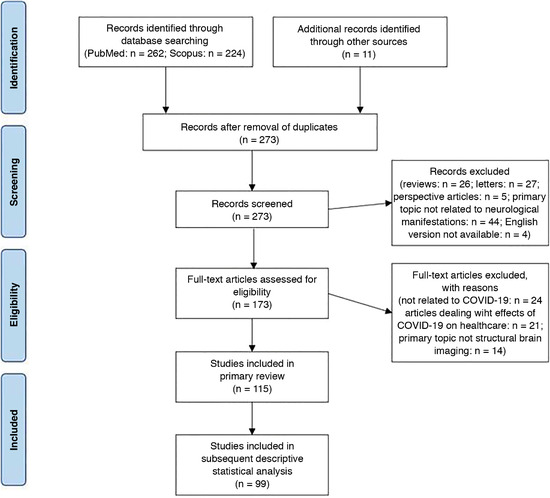
“Preferred Reporting Items for Systematic Reviews and Meta‐Analyses”—flow diagram of the study. n = number.
A total of 48 studies were conducted in North America (USA = 47; Canada = 1), two in South America (Brazil), eight studies in East Asia (China = 5, Taiwan = 1, Japan = 1, Singapore = 1), 13 in West Asia (Iran = 6, United Arab Emirates = 2, Turkey = 2, Qatar = 1, Kuwait = 1, Israel = 1), and 44 studies were conducted in Europe (France = 14, Italy = 11, Spain = 6, UK = 6, Germany = 3, Sweden = 2, Netherlands = 2, Switzerland = 1).
Out of the 115 original studies included in this review, 99 articles further met the criteria of reporting individual data. Thus, the data of 660 (69.2%) patients with individual information were considered for subsequent descriptive statistical analysis (Table 1).
Table 1.
Synopsis of Neuroimaging Features and Characteristics of Studies Reporting on COVID‐19 Patients with Associated CNS Manifestations
| Neurological Manifestation | References | Study Type | Origin | Patients | Typical Neuroimaging Features | Typical Neuroimaging Featurewith Possible Predominance in COVID‐19 Patients | Prevalence of Typical Feature |
|---|---|---|---|---|---|---|---|
| Ischemic stroke | 12,18,21‐24,30‐67 |
Total n = 45 Single case reports: n = 17 Case series: n = 9 Retrospective studies: n = 19 |
USA = 24 France = 9 China, Spain = 3 Italy = 2 Brazil, Iran, Sweden, Netherlands = 1 |
n = 483 Mean age, y; median (IQR): 64.5 (56‐72) Male sex = 64.4% (195/303) |
|
‐ LVO stroke, optionally with hemorrhagic transformation |
59.9% (238/397) |
| Hemorrhagic stroke | 12,18,21,22,26,48,56,57,59,65,70‐76 |
Total n = 18 Single case reports: n = 3 Case series: n = 6 Retrospective studies: n = 8 Prospective studies: n = 1 |
USA = 8 Italy, Spain = 2 Canada, France, Sweden, Iran, Germany, UK = 1 |
n = 135 Mean age, y; median (IQR): 60.8 (57‐66) Male sex = 67.0% (73/109) |
|
‐ Intraparenchymal ICH in non‐hypertension‐associated locations (cortical, cortical‐subcortical, lobar): |
69.2% (45/65) |
| Microbleeds | 56,60,64,65,79,81,82 |
Total n = 7 Single case reports: n = 1 Case series: n = 1 Retrospective studies: n = 4 Prospective studies: n = 1 |
France = 3 USA, Qatar, Switzerland, Sweden = 1 |
n = 135 Mean age, y; median (IQR): 62.5 (61‐68) Male sex = 80.5% (66/82) |
|
‐ Callosal and juxtacortical location |
58.7% (61/104) |
| Cerebral venous and sinus thrombosis (CVST) | 60,84‐87,89,90 |
Total n = 7 Single case reports: n = 5 Case series: n = 1 Retrospective studies: n = 1 |
USA, France = 2 Iran, UK, Italy = 1 |
n = 9 Mean age, y; Median (IQR): 44.3 (21‐65) Male sex = 55.6% (5/9) |
|
‐ Currently no indication of a special neuroimaging feature in patients with COVID‐19 ‐ However, CVST rarely with concurrent pulmonal thrombosis reported |
n/a |
| Posterior reversible encephalopathy syndrome (PRES) | 60,95.97,98 |
Total n = 4 Single case reports: n = 1 Case series: n = 2 Retrospective studies: n = 1 |
USA = 2 France, Italy = 1 |
n = 9 Mean age, y; Median (IQR): 62.5 (56‐69) Male sex = 44.4% (4/9) |
|
‐ Currently no indication of a special neuroimaging feature in patients with COVID‐19 | n/a |
| Meningo‐/encephalitis | 18,24,61,64,101‐106,108‐119,129 |
Total n = 21 Single case reports: n = 13 Case series: n = 3 Retrospective studies: n = 4 |
France, UK = 4 USA = 4 Japan = 2 China, Sweden, Germany, UAE, Italy, Turkey = 1 |
n = 65 Mean age, y; Median (IQR): 59 (39‐61) Male sex = 67.7% (21/31) |
|
‐ Features of hemorrhagic necrotizing encephalopathy (FLAIR hyperintensities in temporal lobe and/or thalamus with evidence of hemorrhage in SWI and post contrast ring enhancement) |
28.8% (15/52) |
| Demyelination/leukoencephalopathy | 24,56,60,65,80,113,118‐125 |
Total n = 14 Single case reports: n = 8 Case series: n = 1 Retrospective studies: n = 5 |
USA, France = 4 Italy = 2 Germany, Sweden, Turkey, Iran = 1 |
n = 78 Mean age, y; Median (IQR): 60.8 (57‐66) Male sex = 67.3% (70/104) |
|
‐ Currently no indication of a special neuroimaging feature in patients with COVID‐19 | n/a |
| Myelitis | 119,128‐131 |
Total n = 5 Single case reports: n = 5 |
USA, Germany, Spain, UK, UAE = 1 |
n = 5 Mean age, y; Median (IQR): 60 (32‐69) Male sex = 80.5% (66/82) |
|
‐ Currently no indication of a special neuroimaging feature in patients with COVID‐19 | n/a |
| Olfactory system involvement | 65,136,138 |
Total n = 3 Single case reports: n = 2 Retrospective studies: n = 1 |
Italy, Taiwan, Sweden = 1 |
n = 9 Mean age, y; Median (IQR): 23 (21‐25) Male sex = 50.0% (1/2) |
|
‐ Currently no indication of a special neuroimaging feature in patients with COVID‐19 ‐ However, due to the neurotropism with high prevalence of hyposmia (34‐68% of the patients), alterations of olfactory system should be indicative for SARS‐CoV‐2‐infection |
n/a |
n = number; y = year; n/a = not applicable; IQR = interquartile range; STRI = Short‐TI Inversion Recovery; DWI = diffusion weighted imaging; SWI = susceptibility‐weighted imaging; FLAIR = fluid‐attenuated inversion recovery; T2w = T2 weighted.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Cerebrovascular Diseases
Ischemic Stroke
In a large retrospective cohort study of a total of 3,218 COVID‐19 confirmed patients, acute ischemic lesions were the most common neuroimaging finding in the subgroup of 454 COVID‐19 patients with neurological symptoms (ie, stroke syndromes) who underwent neuroimaging. Acute stroke findings accounted for 92.5% of patients with positive neuroimaging studies and were present in .9‐1.1% of the total number of hospitalized patients infected with SARS‐CoV‐2. 17 , 18 In this context, Large Vessel Occlusion (LVO) was reported to be the most common presentation of acute ischemic stroke accounting for 21.6‐72.5% of the total acute ischemic strokes, with a 14.9% of the patients revealing multiple LVOs and only 7.5‐8.7% showing lacunar strokes. 19 , 20 , 21 , 22 , 23 , 24 , 25 Overall, the whole spectrum of known ischemic stroke etiologic subtypes according to TOAST criteria was reported in several studies, showing also increased incidence of hemorrhagic transformation 6 , 26 and worse outcomes with a mortality rate of up to 45%. 6 , 27 Most of the patients were suffering from cardiovascular risk factors and were older than 60 years old (mean age: 63.4 ± 13.1 years). However, LVO strokes were also reported in younger patients without any known cardiovascular risk factor. 19 , 20 , 28 Interestingly, infarction of the splenium of the corpus callosum, an oftentimes very rare condition, was observed occasionally in some COVID‐19 patients undergoing neuroimaging. 29 Noteworthy, a certain proportion of stroke cases were tested positive for antiphospholipid antibodies such as lupus anticoagulant (41.7%), anticardiolipin antibodies (20% IgM and 42.9% IgA), and anti‐β2‐glycoprotein I antibodies (10% IgM, 38.5% IgG, and 42.9% IgA). 6
An exemplary case with a typical neuroimaging pattern (LVO stroke with hemorrhagic transformation) for ischemic stroke in COVID‐19 patients is depicted in Figure 2.
Fig 2.
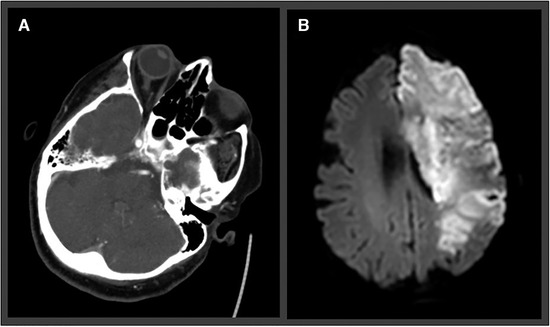
(A) CT angiography demonstrates left internal carotid artery occlusion (large vessel occlusion stroke) in a 79‐year‐old female patient. (B) Despite thrombolysis in cerebral infarction scale 2b recanalization, complete infarction of the left media and anterior cerebral artery was visible on follow‐up diffusion‐weighted imaging sequence.
Our systematic review revealed a total of 45 studies reporting imaging features of a total of 483 COVID‐19 patients suffering from acute ischemic stroke (AIS). 12 , 18 , 21 , 22 , 23 , 24 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 A total of 26 studies were presented as single case reports or as case series. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 The remaining 19 studies were conducted as retrospective studies reporting on neuroimaging findings of AIS patients. 12 , 18 , 21 , 22 , 23 , 24 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 Sample sizes ranged from 1 to 116 patients. The median of reported mean age was 64.5 years (interquartile range [IQR] = 56‐72 years; range = 43.5‐81 years). The sex of 303 patients was specified, 195 (64.4%) of them being males.
A total of 24 studies were conducted in the United States, one in Brazil, three in China, one in Iran, and 16 in Europe (France = 9, Spain = 3, Italy = 2, Sweden = 1, and Netherlands = 1).
From the total of these 45 reports, 38 studies reported individual patient data, with 397 (82.2%) of the 483 AIS patients being considered for additional descriptive statistical analysis. Of these, 238 (59.9%) revealed the typical finding of LVO stroke.
Hemorrhagic Stroke
A number of cases of intracerebral hemorrhage (ICH) were reported in patients with COVID‐19. It has been speculated that SARS‐CoV‐2 enters cells via the angiotensin‐converting‐enzyme (ACE)‐2 receptor and may cause dysregulation and fluctuations in cerebral blood pressure levels, resulting to an increased risk of hypertensive primary ICH. 68 It has been estimated that 17% of the total strokes in SARS‐CoV‐2‐infected patients are hemorrhagic. 25 However, many of the reported ICHs were located in a nontypical location for hypertensive hemorrhage such as lobar and/or cortical locations (loco atypico). Interestingly, in a British retrospective study of cases with COVID‐19 associated ICH, Benger et al report relatively young ages of affected patients (mean age = 52.2 years, range = 41‐64 years), with predominantly lobar location in the anterior circulation, even though most of the identified cases suffered from arterial hypertension. 69 According to Kvernland et al, 89.5% of the patients with hemorrhagic stroke were on anticoagulation. 70 In this context, an underlying COVID‐19‐induced endotheliopathy is discussed, complicating anticoagulation decision, as clinicians have to balance risk of thrombosis with risk of ICH.
One exemplary case with a large hypertensive ICH in a COVID‐19 patient is depicted in Figure 3.
Fig 3.
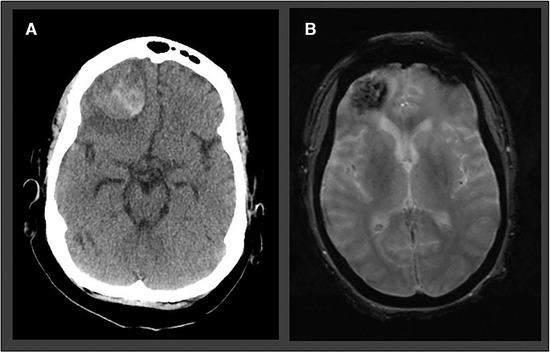
Atypical ICH in a 57‐year‐old woman with prolonged encephalopathy and hypoxic respiratory failure. Polymerase chain reaction for SARS‐CoV‐2 was positive in the nasopharyngeal swab. (A) Brain CT performed in the acute setting revealed a right frontal lobe hemorrhage with perifocal edema. (B) T2* image demonstrating the right frontal lobar intracerebral hemorrhage.
Our review documented a total of 18 studies reporting about imaging features of 135 COVID‐19 patients with ICH. 12 , 18 , 21 , 22 , 26 , 48 , 56 , 57 , 59 , 65 , 71 , 72 , 73 , 74 , 75 , 76 , 77 Eight studies were presented as single case reports or as case series. 48 , 59 , 71 , 72 , 73 , 74 , 75 , 77 Another eight studies were conducted as retrospective studies reporting on neuroimaging findings of ICH patients. 12 , 18 , 21 , 22 , 26 , 57 , 65 , 76 Sample sizes of these 18 studies ranged from 1 to 33 patients. Median of reported mean age was 60.8 years (IQR = 56.9‐66.2 years; range = 30‐74 years). The sex of 109 patients was specified, 73 (67.0%) of them being males.
Nine studies were conducted in North America (USA = 8; Canada = 1), one in Iran, and seven in Europe (Spain = 2, Italy = 2, France = 1, Germany = 1; Sweden = 1, UK = 1).
From the total of these 18 reports, 17 studies reported individual data of at least some of the included cases. Thus, data from 65 (48.1%) of the 135 ICH patients were considered for additional descriptive statistical analysis. Of these, 45 (69.2%) presented with the characteristic finding of an ICH in non‐hypertension‐associated locations (cortical, cortical‐subcortical, lobar).
Microbleeds
There is accumulating evidence that a high proportion of COVID‐19 patients may exhibit multiple cerebral microbleeds (CMB), mostly in atypical locations such as corpus callosum and juxtacortical white matter. 78 , 79 However, most of the reported cases were critically ill patients with Acute Respiratory Distress Syndrome and prolonged mechanical ventilation, raising concerns whether it could be a causal relationship of SARS‐CoV‐2 infection or incidental comorbidity of critical illness. In a large retrospective study, Radmanesh et al reported atypical CMB in 24% of COVID‐19 ICU patients. The authors also report in addition to CMB diffuse confluent T2 hyperintensities in the supratentorial white matter with mildly restricted diffusion, sparing characteristically the deep gray matter and the cortex and indicating a probable relation to delayed posthypoxic leukoencephalopathy. 80
Our systematic review revealed a total of seven studies reporting imaging features of a total of 135 COVID‐19 patients with evidence of CMB. 56 , 60 , 64 , 65 , 79 , 81 , 82 Only one study was presented as a single case report, and one further as case series (n = 9). 79 , 81 Most cases were reported in retrospective cross‐sectional studies (n = 4) 60 , 64 , 65 , 82 as well as in the only prospective cohort study.56 Sample sizes ranged from 1 to 39 patients. The median of reported mean ages was 62.5 years (range = 61‐67.7 years). Sex was specified in 82 patients, and 80.5% (66) of those were males.
One study was conducted in the United States, one in Qatar, and five studies were conducted in Europe (France = 3, Sweden = 1, and Switzerland = 1). All of these studies reported individual data of at least some of the included cases. Thus, data from 104 (77.0%) of the 135 CMB patients were considered for additional descriptive statistical analysis. Of these, 61 (58.7%) revealed the suggested typical finding of microbleed in callosal and/or juxtacortical location.
Cerebral Venous Sinus Thrombosis
CVST represents another occasionally observed cerebrovascular complication of SARS‐CoV‐2, constituting also a possible etiological association with the underlying coronavirus infection. Shahjouei et al reported in their multinational study a CVST prevalence of 4% among all cerebrovascular events. 25 The few case reports 83 , 84 , 85 , 86 and one case series 87 published to date in literature report CVST consistently in critically ill COVID‐19 patients. In most of these patients, CVST was accompanied with hemorrhagic venous infarction and the outcome in five (55.6%) of these nine cases was fatal. As in non‐COVID cases, sigmoid and transverse sinuses were predominantly affected, so that no unusual pattern of CVST has been observed. Notable, in some cases, concomitant cerebral arterial and venous thrombosis 88 or CVST with concurrent pulmonary arterial or venous thrombosis has been described. 84
An exemplary case of CVST in a COVID‐19 patient is depicted in Figure 4.
Fig 4.
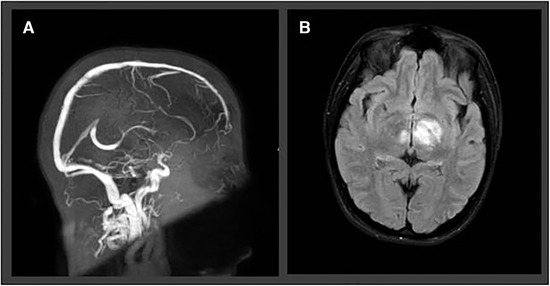
Two‐dimensional time‐of‐flight MR venography (A) with atypical internal cerebral vein thrombosis in a 30‐year‐old female SARS‐CoV‐2‐positive patient, presenting with severe headache, vomiting, and bilateral papilledema. Axial 2‐dimensional fluid‐attenuated inversion recovery revealed bilateral thalamic edema due to venous congestion in the same patient (B).
Our review revealed a total of seven studies reporting imaging features of a total of 9 COVID‐19 patients suffering from CVST. 60 , 84 , 85 , 86 , 87 , 89 , 90 Five studies were presented as single case reports, 83 , 84 , 85 , 86 , 88 one as case series (n = 3), 90 and one patient was the only CVST patient reported in a retrospective cross‐sectional study. 60 The mean age of these nine reported patients was 44.3 years (range = 21‐65 years). Five (55.6%) of the patients were male. Two studies were conducted in the United States, one in Iran, and five studies were conducted in Europe (France = 2, UK = 1, Italy = 1). All of these seven reports provided individual data, which were considered for further descriptive statistical analysis. However, as the involvement of deep and superficial veins and sinuses was equally described, no typical pattern of COVID‐associated CVST could be identified.
Posterior Reversible Encephalopathy Syndrome
PRES is a neurological entity that is characterized by vasogenic edema, most frequently in the parieto‐occipital regions, usually as a result of hypertensive emergencies or a direct effect of cytokines in the endothelium and subsequent disruption of it. 91 , 92 Interestingly, several COVID‐19 cases with PRES have been diagnosed and reported during the pandemic. In a case series of PRES in two COVID‐19 patients from Massachusetts, Kishfy et al describe a typical pattern of signal hyperintensities in fluid‐attenuated inversion recovery (FLAIR) images in occipital, posterior temporal lobes and the cerebellar hemispheres. 93 Diffusion‐weighted imaging abnormalities in occipital lobes and the splenium of corpus callosum indicative of the cytotoxic component of the edema were observed in one of the case reports. 94 Furthermore, there were three described cases of hemorrhagic transformation of the PRES‐associated lesions with petechial hemorrhages at the SWI images or CT imaging. 95 Notably, the first postmortem MRI study of COVID‐19 nonsurvivors supported the association of PRES‐like vasogenic lesions in at least four (21%) of the 19 patients of the study. 96 Interestingly, PRES in association with reversible cerebral vasoconstriction syndrome (RCVS) was reported in only one case. 97
Our systematic review revealed a total of four studies reporting about imaging features of a total of nine COVID‐19 patients with documented PRES. 60 , 95 , 97 , 98 Three studies were presented as case report/series (n = 1‐4) 95 , 97 , 98 and two further patients were identified in a retrospective cross‐sectional study. 60 The median of reported mean age of the patients was 62.5 years (range = 56‐69 years). At least four (44.4%) of the patients were male. Two studies were conducted in the United States, the others in France and Italy. Eight of these nine cases were eligible for further descriptive statistical analysis as they included patient‐level data. However, as PRES pattern did not differ from reported PRES pattern in non‐COVID patients, no typical feature of COVID‐associated PRES could be identified.
Other Cerebrovascular Manifestations
One case with bilateral and one with unilateral vertebral artery dissection were reported in two SARS‐Cov‐2‐positive female patients with a history of migraine. 99 , 100 The latter was also found to suffer from bilateral high frontal convexity subarachnoid hemorrhage (cSAH) on CT and evidence of RCVS in the anterior and middle cerebral circulation. Whether the endothelial dysfunction caused by the SARS‐CoV‐2 led to the vertebral artery dissection and subsequently to RCVS t caused the cSAH, or the underlying history of migraine was the crucial etiological factor with the coronavirus infection being incidental, remains speculative. Possibly, the cSAH could have caused secondary vasospasm. Two more cases of vertebral and internal carotid artery dissection have been reported from Hernandez‐Fernandez et al,21 both having evident intimal flaps in CT and MRI, with the latter causing secondary ischemic event after occlusion of the insular branch of the unilateral middle cerebral artery.
Inflammatory Processes
Meningo‐/Encephalitis
In reported COVID cases with additional encephalitis, the MRI abnormalities were considerably diverse and ranged from restricted diffusion and FLAIR hyperintensity in mesial temporal lobe and hippocampus in a patient presenting with seizures and neck stiffness 101 to mild encephalitis with a reversible splenial callosal lesion. 102 Splenium T2‐weighted signal changes and restricted diffusion were seen also in SARS‐CoV‐2‐infected children. 103 Many patients affected by COVID‐19 were reported with acute hemorrhagic necrotizing encephalopathy, a rare but typical CNS complication of viral respiratory infection. A case reported by Poyiadji et al demonstrated bilateral temporal lobe and thalamus FLAIR hyperintensities with evidence of hemorrhage in SWI images and postcontrast ring enhancement of the lesions, 104 whereas another patient was characterized mainly from progressive pons edema accompanied with bilateral T2 hyperintensities with intrinsic hemorrhage in the subcortical perirolandic spaces. This second patient had a history of aplastic anemia in her medical history. 105 Increased FLAIR signal intensity in subinsular regions, medial temporal lobes, hippocampi, cerebral peduncles, thalami, and brainstem; restriction of water diffusion in the same regions indicating cytotoxic edema; and faint contrast enhancement—seeing predominantly in the subinsular regions bilaterally—were also documented from other authors. SWI images showed evidence of petechial hemorrhages in thalami and external capsule. There was a substantial improvement of the abovementioned findings in the follow‐up MRI a week later. 106 Moreover, cortical involvement characterized by signal abnormalities in FLAIR images, cortical diffusion restriction, and sporadically leptomeningeal enhancement in postcontrast FLAIR could be a sign of infectious or autoimmune neurological manifestation of SARS‐CoV‐2 as reported by Kandemirli et al. 107 Notwithstanding, hypoxic injury, postictal phase, or even hypoglycemia should also be considered in the interpretation of these finding as the authors mentioned. An exemplary case of encephalitis in COVID‐19 patients is depicted in Figure 5.
Fig 5.
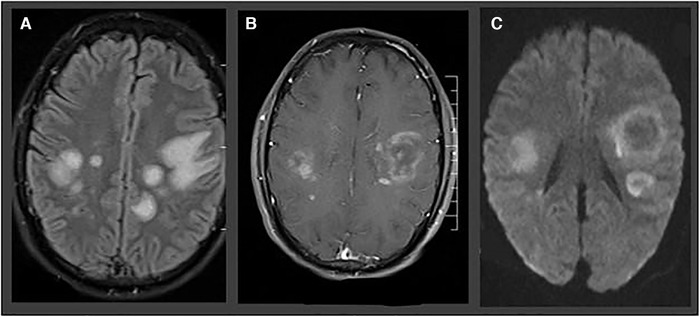
Acute necrotizing hemorrhagic encephalopathy in a 32‐year‐old man presenting with speech impairment, disorientation, and epileptic seizures. MRIs show predominantly subcortical fluid‐attenuated inversion recovery hyperintensities (A), with ring enhancement in T1‐weighted sequence (B) and ring‐shaped diffusion‐restriction (C).
Our review revealed a total of 21 studies reporting imaging features for a total of 65 COVID‐19 patients with evidence of meningo‐/encephalitis. 18 , 24 , 61 , 64 , 101 , 102 , 103 , 104 , 105 , 106 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 Thirteen studies were presented as single case report 101 , 102 , 104 , 105 , 106 , 108 , 109 , 110 , 111 , 112 , 113 , 114 and three as case series (n = 2‐4). 103 , 115 , 116 The other cases were identified in four retrospective cross‐sectional studies. 18 , 24 , 61 , 64 Sample sizes ranged from 1 to 22 patients. The median of reported mean age was 59 years (IQR = 39.5‐61 years; range = 12‐75 years). Sex was specified in 31 patients; 21 (67.7%) of the 31 patients were males.
Four studies were conducted in the United States, three in East Asia (Japan = 2; China = 1), two in West Asia (Turkey = 1; United Arab Emirates = 1), and 12 studies were conducted in Europe (France = 4, UK = 4, Italy = 1; Sweden = 1, Germany = 1). All of these 21 studies reported individual data of at least some of the included cases. Thus, data from 52 (80.0%) of the 65 patients were considered for additional descriptive statistical analysis. Of these, 15 (28.8%) revealed the suggested typical finding of acute hemorrhagic necrotizing encephalitis.
Demyelination/Leukoencephalopathy
The first reported demyelinating event in a 59‐year‐old COVID‐19 female patient supported by neuroimaging findings depicted periventricular confluent FLAIR hyperintensities adjacent to temporal, occipital, and frontal horns and across the spinal cord without the restriction of diffusion or contrast enhancement. 120 Following that, Radmanesh et al reported 10 cases of SARS‐CoV‐2‐positive and prolonged intubated patients that demonstrated diffuse leukoencephalopathy with symmetric and confluent T2 hyperintensities across the subcortical white matter, with equal or more limited restricted diffusion, sparing the deep gray matter structures as well as the juxtacortical white matter and the infratentorial regions in most cases. 80 These findings were interpreted by the authors as possible demyelination, probably in the context of delayed posthypoxic leukoencephalopathy. A more diverse imaging presentation of suspected demyelination in a patient tested positive to SARS‐CoV‐2 was described by Brun et al. 121 Asymmetrical periventricular and callosal white matter hyperintensities and bilateral Globus pallidum lesions with restricted diffusion sparing the infratentorial regions might be a typical manifestation of an acute disseminated encephalomyelitis (ADEM), whereas punctiform white matter lesions may be associated with a concurrent small‐vessel vasculitis as a combined secondary immune‐mediated phenomenon. Interestingly, contrast enhancement was observed only in a follow‐up MRI a few days later. Considering possible postinfectious ADEM, two more case reports were published, concerning two middle‐aged women critically and noncritically ill with asymmetrical FLAIR hyperintensities in subcortical white and gray matter structures, mildly restricted diffusion, and gadolinium enhancement. Both cases manifested neurological symptoms more than 10 days after the initiation of the SARS‐CoV‐2‐related symptoms, supporting the hypothesis of a postinfectious immune‐mediated pathogenesis. 122
An exemplary case of ADEM in a COVID‐19 patient is depicted in Figure 6.
Fig 6.
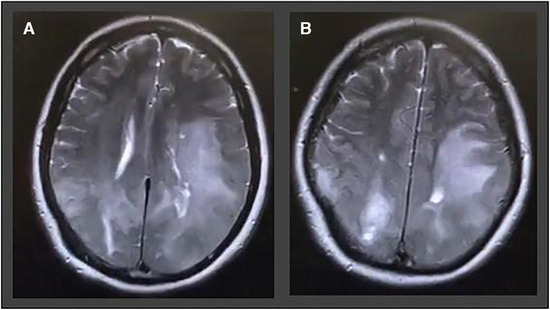
A 54‐year‐old man with no previous medical history admitted with headache, disorientation, nausea, vomiting, and blurred vision. T2‐weighted images show pronounced acute disseminated encephalomyelitis like leukoencephalopathy in the parietal and frontal lobes involving u‐fibers as well as cortical areas on both sides.
Our review revealed a total of 14 studies reporting imaging features of a total of 78 COVID‐19 patients analyzing the distribution of demyelination/leukencephalopathy. 24 , 56 , 60 , 65 , 80 , 113 , 118 , 120 , 121 , 122 , 123 , 124 , 125 , 126 Eight studies were presented as a single case report and one as case series (n = 11). 80 , 117 , 118 , 119 , 120 , 121 , 122 , 123 The other cases were identified in five retrospective cross‐sectional studies. 24 , 56 , 60 , 65 , 80 Sample sizes ranged from 1 to 17 patients. The median of reported mean age was 54 years (IQR = 52‐62.5 years; range = 21‐64 years). Sex was specified in 29 patients; a total of 16 (55.2%) out of the 29 patients were males.
Four studies were conducted in the United States, two in West Asia (Turkey = 1; Iran = 1), and eight studies were conducted in Europe (France = 4, Italy = 2; Sweden = 1, Germany = 1). All of these 14 studies reported detailed individual data of at least some of the included cases. However, as manifold patterns of demyelination and leukoencephalopathy were described, no typical pattern of COVID‐associated CVST could be identified.
Myelitis
Various patterns of acute or chronic myelitis have been causally linked to several viruses such as HSV‐1 or HSV‐2, West Nile Virus, Cytomegalovirus, Epstein‐Barr virus, Varicella zoster virus, etc. 127 The main pathophysiological pathway is usually immune‐ or autoimmune‐mediated processes triggered by the viral agent, with the neurotropic and/or cytolytic behavior of some pathogens, being far more infrequent. Overall, there were four published case reports of COVID‐19‐associated myelitis, with only 3 patients having received an MRI of the spinal cord. Early (after 2 days) 128 and late (after 7‐8 days) 129 development of neurological signs and symptoms after the first manifestation of the respiratory symptoms due to SARS‐CoV‐2 infection were described. Isolated or multifocal hyperintense lesions on STIR or T2‐weighted MRI images were detected in the cervical and thoracic cord. Some lesions were accompanied with tissue edema showing enlargement of spinal cord caliber. None of the patients demonstrated pathological gadolinium enhancement, whereas evidence of restricted diffusion on diffusion‐weighted imaging and apparent diffusion coefficient sequences supported the presence of cytotoxic edema.
Our review revealed a total of five single case reports studies reporting about imaging features of a total of five COVID‐19 patients with documented myelitis. 119 , 128 , 129 , 130 , 131 The median age of these patients was 60 years (range = 32‐69 years). At least two (40%) of the patients were male. The study group of these single case reports is from United States, United Arab Emirates, the United Kingdom, Spain, and Germany, respectively. Evaluating the pattern of these five patients, neuroimaging features did not differ from reported myelitis pattern in non‐COVID patients, thus, no typical feature of COVID‐associated myelitis could be identified.
Olfactory Pathway and Cranial Nerve Involvement
Anosmia/hyposmia is considered to be one of the principal symptoms of SARS‐CoV‐2 infection accounting for 33.9‐68.0% of the total cases. 132 The olfactory dysfunction in COVID‐19 patients can be quantified and objectively assessed. 133 Nasal epithelial injury is the leading proposed mechanism causing anosmia as manifestation. However, some MRI studies indicated bilateral olfactory bulb FLAIR signal hyperintensities and edema as well as signal abnormalities in cortical areas associated with the olfaction (eg, posterior gyrus rectus). 134 , 135 , 136 These findings underpin the initial hypothesis regarding the neurotropism of SARS‐CoV‐2, as CNS involvement could happen not only hematogenously in critically ill patients with viremia, but also via the nasal epithelium and the olfactory nerve. 137 In addition, olfactory bulb atrophy has been reported in COVID‐19 patients with persistent anosmia. 131
Our systematic review revealed a total of three studies reporting about imaging features of a total of nine COVID‐19 patients with alterations in the olfactory bulb and tracts. Two studies were presented as single case reports, and 7 patients were reported in a retrospective study. 65 , 136 , 138 The case reports were related to young patients (one 21‐year‐old male and one 25‐year‐old female).
Apart from the involvement of the olfactory nerve, some case reports have demonstrated alteration of the ophthalmokinetic nerves in the context of a presumptive Miller‐Fisher syndrome and facial nerve involvement. Contrast enhancement and T2 signal abnormalities were the hallmarks in the neuroimaging studies in such cases. 4 , 139
Our review revealed a total of seven studies reporting about imaging features of a total of 26 COVID‐19 patients with neuroimaging alterations of other cranial nerves than the olfactory bulb and tracts. 65 , 139 , 140 , 141 , 142 , 143 , 144 Six studies were presented as single case reports, 137 , 138 , 139 , 140 , 141 , 142 whereas 20 cases were identified in one retrospective cohort study from Sweden. 65 The case report studies were conducted in the United States (n = 4) as well as in Brazil and in Singapore (n = 1, respectively). The median age of the six patients presented in the case reports was 34 years (range = 21‐69), all (100%) of them being males. Mainly ophthalmokinetic nerves like trochlear and abducens were involved in these cases revealing to corresponding nerve palsies. Rarely, facial nerve palsy has been reported in two COVID‐19 patients, one in association with Miller‐Fisher‐Syndrome.
Discussion
Since the beginning of the COVID‐19 pandemic, various clinical neurological manifestations with manifold neuroimaging features have been described in patients with SARS‐Cov‐2 infection. In our systematic review, we summarized for a total of nine disease entities with documented neuroimaging characteristics of the pandemic. Additionally, we sought to analyze possible typical neuroimaging patterns that could indicate a COVID‐19‐associated manifestation. In four of the nine disease entities, we could identify the following typical neuroimaging features: (a) pattern of LVO infarction, frequently with hemorrhagic transformation in ischemic stroke; (b) ICH in non‐hypertension‐associated locations (lobar and/or cortical) in hemorrhagic stroke; (c) callosal and juxtacortical location in patients with CMB; and (d) hemorrhagic necrotizing encephalopathy in patients with meningo‐/encephalitis.
Although nonspecific symptoms of encephalopathy such as dizziness, headache, and confusion are common in COVID‐19 patients, cerebral neuroimaging evaluation is performed in less than 15% of the patients. 18 This raises a probable selection and reporting bias of underreporting cases with presumable neuroimaging findings but mild clinical symptoms, where a brain MRI or CT was considered unnecessary. Furthermore, as some of the aforementioned neuroimaging findings (eg, PRES‐like lesions, microhemorrhages, and ischemic lesions) are also found in many other constellations such as sepsis‐associated encephalopathy, carefully planned and more systematic studies are needed to clarify if observed imaging patterns are attributed to direct COVID‐19 pathophysiology. 166
The vast majority of the reported neuroimaging findings in the current COVID‐19 literature represent cases of cerebrovascular diseases because they are by far the leading cause of disability‐adjusted life years among all neurological disorders 145 and the initial work‐up of these entities always requires neuroimaging studies in the emergency setting. Noteworthy, due to this considerable incidence of SARS‐CoV‐2 infection between stroke patients, it has been proposed to incorporate chest CT into the emergency imaging protocol in order to reduce the probability of exposing all medical profesionals. 167 The most common indications for neuroimaging appear to be associated with altered mental status and focal neurologic deficits, and this may introduce bias as the latter is often associated with stroke. An association between COVID‐19 and several cerebrovascular events as well as other vascular complications has been repeatedly reported already from the beginning of the pandemic. The prevalence of reported ischemic and hemorrhagic stroke in COVID‐19 patients ranges between .2% and 2.7%. 6 , 10 , 21 Several studies reported a higher mortality despite the lower baseline modified Rankin scale score. 6 , 26 , 170 Underlying hypercoagulable condition, 146 blood pressure dysregulation, hypoxia, viral neurotropism, endothelial dysfunction, 147 presence of antiphospholipid antibodies, 148 virus‐associated myocardial injury, 149 immobilization, triggering of atrial fibrillation, and rupture of an unstable carotid plaque through infectious processes 150 are some of the proposed pathophysiological aspects increasing the risk of thromboembolic complications 151 and being indicated to play an important role in cerebrovascular complications during the pandemic. A plausible mechanism of blood clot formation including oxidative stress triggered by the viral‐induced endothelial damage, formation of antiphospholipid antibody complexes (mainly with beta2‐Glycoprotein), and subsequent platelet adhesion is proposed by Janardhan et al. 168 A high proportion of ischemic stroke cases is estimated to be caused by LVO. The prevalence of LVO in a noninfected population compromises only 24‐39% of acute ischemic stroke. 152 , 153 In our subsequent descriptive analysis, we could identify this pattern in 60.0% of the reported COVID‐19 patients. Even if we take into account the possible bias of underreporting of minor stroke cases, SARS‐CoV‐2 might be a risk factor for LVO stroke. It is worth noting that the prevalence of lacunar strokes in our review (8%) is consistent with the findings of Shahjouei et al multinational study (7.5%). 25
Regarding hemorrhagic stroke in COVID‐19 patients, the atypical lobar location of hypertension‐related hemorrhages should be underscored. A plausible explanation, as indicated by Kvernland et al, might be the empiric therapeutic anticoagulation of most patients in the context of the hypercoagulable state caused by the COVID‐19. Up to 89.5% of the patients positive to SARS‐CoV‐2 with ICH were under empiric anticoagulation. 70 As frequently reported in the literature, anticoagulant‐related ICH occurs predominantly in a lobar and/or cortico‐subcortical location. 154 , 155 In line with it, a greater prevalence of hemorrhagic transformation of ischemic strokes was reported in SARS‐CoV‐2‐positive patients. 6 , 26 Considering the delay in the diagnosis 156 , 157 by isolation measures and the fact that a differential diagnosis based only on a CT is not always possible, some hemorrhagic transformations might have been misclassified as ICH.
Because of the relatively low total number of SARS‐CoV‐2‐associated CVST cases in current literature reported so far, no safe conclusions can be withdrawn. We could not identify any typical neuroimaging patterns in CVST cases. Similarly, PRES imaging characteristics in COVID‐19 patients seem to not differ substantially from non‐COVID‐19 individuals. However, further investigation via larger observational studies is needed for these two neurological manifestations.
Apart from cerebrovascular events, there were many cases of inflammatory phenomena that became evident during the pandemic. A significant number of meningitis/encephalitis cases implied a possible neurotropism 158 of the virus or its ability to trigger secondary immune‐mediated responses. However, the so far performed neuropathological and neurochemical studies have produced inconsistent results and the manifold alternative clinical explanations suggest that direct brain invasion of the virus is a possible but rare cause for the aforementioned pathology. 169 It is worth noting that many of the cases (in our limited analysis 28.8% of these patients) represented the rare neuroimaging feature of acute hemorrhagic necrotizing encephalitis. Interestingly, this finding has been previously reported as secondary to respiratory virus infections, mainly influenza virus and HHV‐6. However, the exact etiology and pathogenesis of this disorder is purely defined, with some evidence indicating cytokine storm conditions, such as severe SAR‐CoV‐2 infection,159 as a possible key factor. 160
Animal, as well as human neuropathological, studies suggest a possible causality of SARS‐CoV‐2 and other coronaviruses and demyelinating events of the CNS. 161 , 162 , 163 , 164 On the other hand, hypoxic ischemic leukoencephalopathy in critically ill patients could also be a leading cause of demyelination in the subcortical white matter. 165 Overall, we could not identify any typical neuroimaging pattern regarding the demyelinating events and the myelitis cases in COVID‐19 patients.
Olfactory nerve and olfactory pathway involvement have been assumed from the very early days of the pandemic, indicating a nonhematogenous virus entry in the human body. Indeed, some patients appear to have relevant MRI findings with, however, to date unknown clinical significance. Furthermore, other cranial nerves have been sporadically affected in the course of SARS‐CoV‐2 infection, demonstrating a probable predominant involvement of ophthalmokinetic nerves. However, these neuroimaging features appear seldomly, so that such a finding might represent itself a typical feature of SARS‐CoV‐2 infection.
In conclusion, having summarized the so far documented neuroimaging characteristics of the pandemic in our narrative review, we identified four typical neuroimaging patterns that might indicate a possible COVID‐19 associated manifestation: (a) pattern of LVO infarction, frequently with hemorrhagic transformation in ischemic stroke; (b) ICH in non‐hypertension‐associated locations (lobar and/or cortical) in hemorrhagic stroke; (c) callosal and juxtacortical location in patients with CMB; and (d) hemorrhagic necrotizing encephalopathy in patients with meningo‐/encephalitis. Familiarity with these imaging patterns appears reasonable because manifold CNS involvement is increasingly being reported in COVID‐19 patients. However, because many of these patterns are also seen in many critical ill patients with alternative explanations such as multiple organ dysfunction syndrome and sepsis, further studies are needed to elucidate if these neuroimaging patterns are directly associated with COVID‐19 or are confounding results in the context of critical illness. The classification according to imaging findings and not according to etiopathogenetic criteria, together with the author‐based consensus regarding the identification of typical neuroimaging patterns, represents a further limitation of our study. In view of the abundance of case reports and the lack of prospective etiopathogenetically oriented studies, this approach seems appropriate at the current time point of the pandemic. As evaluation at this early stage is likely to be influenced by different types of bias, further research on this topic is required to identify neuroimaging findings that are either sensitive or specific of COVID‐19 CNS manifestations.
Acknowledgements and Disclosure: We would like to thank Professor Ralf Gold (Dean of the Medical Faculty of the Ruhr University of Bochum, and Director of the University Department of Neurology, St. Josef‐Hospital Bochum) for his support in the implementation and completion of this review. All authors declare no conflicts‐of‐interest relevant to this work. The study was not industry sponsored and did not receive any funding.
Open access funding enabled and organized by Projekt DEAL.
References
- 1. Renu K, Prasanna PL, Valsala Gopalakrishnan A. Coronaviruses pathogenesis, comorbidities and multi‐organ damage ‐ a review. Life Sci 2020;255:117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med 2020;26:1017‐32. [DOI] [PubMed] [Google Scholar]
- 4. Tsivgoulis G, Palaiodimou L, Katsanos AH, et al. Neurological manifestations and implications of COVID‐19 pandemic. Ther Adv Neurol Disord 2020;13:1756286420932036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan YK, Goh C, Leow AST, et al. COVID‐19 and ischemic stroke: a systematic review and meta‐summary of the literature. J Thromb Thrombolysis 2020;50:587‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS‐CoV‐2: understanding the neurological manifestations in COVID‐19 patients. Neurol Sci. 2020,. 28:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Getts DR, Chastain EM, Terry RL, et al. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev 2013;255:197‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou C, Wu L, Ni F, et al. Critical illness polyneuropathy and myopathy: a systematic review. Neural Regen Res 2014;9:101‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol 2020;19:767‐83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ng Kee Kwong KC, Mehta PR, Shukla G, et al. COVID‐19, SARS and MERS: a neurological perspective. J Clin Neurosci 2020;77:13‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radmanesh A, Raz E, Zan E, et al. Brain imaging use and findings in COVID‐19: a single academic center experience in the epicenter of disease in the United States. AJNR Am J Neuroradiol 2020;41:1179‐83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalili N, Haseli S, Bahrami‐Motlagh H, et al. Neurologic Involvement in COVID‐19: radiologists' perspective. Acad Radiol 2020;27:1051‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008‐12 [DOI] [PubMed] [Google Scholar]
- 16. WHO . Coronavirus disease 2019 (COVID‐19): situation report, 61. Geneva, Switzerland: World Health Organization, 2020. [Google Scholar]
- 17. Tsivgoulis G, Katsanos AH, Ornello R, et al. Ischemic stroke epidemiology during the COVID‐19 pandemic: navigating uncharted waters with changing tides. Stroke 2020;51:1924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jain R, Young M, Dogra S, et al. COVID‐19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci 2020;414:116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID‐19. J Neurol Neurosurg Psychiatry 2020;91:889‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oxley TJ, Mocco J, Majidi S, et al. Large‐vessel stroke as a presenting feature of COVID‐19 in the young. N Engl J Med 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hernandez‐Fernandez F, Valencia HS, Barbella‐Aponte RA, et al. Cerebrovascular disease in patients with COVID‐19: neuroimaging, histological and clinical description. Brain 2020;143:3089‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rothstein A, Oldridge O, Schwennesen H, et al. Acute cerebrovascular events in hospitalized COVID‐19 patients. Stroke 2020;51:e219‐e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kihira S, Schefflein J, Mahmoudi K, et al. Association of Coronavirus disease (COVID‐19) with large vessel occlusion strokes: a case‐control study. AJR Am J Roentgenol 2020;29:1‐6. [DOI] [PubMed] [Google Scholar]
- 24. Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in COVID‐19 patients: a retrospective multicenter study. Neurology 2020;95:e1868‐e82. [DOI] [PubMed] [Google Scholar]
- 25. Shahjouei S, Naderi S, Li J, et al. Risk of stroke in hospitalized SARS‐CoV‐2 infected patients: a multinational study. EBioMedicine 2020;59:102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dogra S, Jain R, Cao M, et al. Hemorrhagic stroke and anticoagulation in COVID‐19. J Stroke Cerebrovasc Dis 2020;29:104984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Sun W, Wang Y, et al. Clinical course and mortality of stroke patients with coronavirus disease 2019 in Wuhan, China. Stroke 2020;51:2674‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al Saiegh F, Ghosh R, Leibold A, et al. Status of SARS‐CoV‐2 in cerebrospinal fluid of patients with COVID‐19 and stroke. J Neurol Neurosurg Psychiatry 2020;91:846‐8. [DOI] [PubMed] [Google Scholar]
- 29. Sparr SA, Bieri PL. Infarction of the splenium of the corpus callosum in the age of COVID‐19: a snapshot in time. Stroke 2020;51:e223‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saggese CE, Del Bianco C, Di Ruzza MR, et al. COVID‐19 and stroke: casual or causal role? Cerebrovasc Dis 2020;49:341‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kariyanna PT, Chandrakumar HP, Jayarangaiah A, et al. Apical Takotsubo cardiomyopathy in a COVID‐19 patient presenting with stroke: a case report and pathophysiologic insights. Am J Med Case Rep 2020;8:350‐7.32704530 [Google Scholar]
- 32. Moshayedi P, Ryan TE, Mejia LLP, et al. Triage of acute ischemic stroke in confirmed COVID‐19: large vessel occlusion associated with Coronavirus infection. Front Neurol 2020;11:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou B, She J, Wang Y, et al. A case of coronavirus disease 2019 with concomitant acute cerebral infarction and deep vein thrombosis. Front Neurol 2020;11:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruggemann R, Gietema H, Jallah B, et al. Arterial and venous thromboembolic disease in a patient with COVID‐19: a case report. Thromb Res 2020;191:153‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viguier A, Delamarre L, Duplantier J, et al. Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID‐19 infection. J Neuroradiol 2020;47:393‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salahuddin H, Castonguay AC, Zaidi SF, et al. Interventional stroke care in the era of COVID‐19. Front Neurol 2020;11:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valderrama EV, Humbert K, Lord A, et al. Severe acute respiratory syndrome Coronavirus 2 infection and ischemic stroke. Stroke 2020;51:e124‐e7. [DOI] [PubMed] [Google Scholar]
- 38. Goldberg MF, Goldberg MF, Cerejo R, et al. Cerebrovascular disease in COVID‐19. AJNR Am J Neuroradiol 2020;41:1170‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azouz E, Yang S, Monnier‐Cholley L, et al. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID‐19. Intensive Care Med 2020;46:1464‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gill I, Chan S, Fitzpatrick D. COVID‐19‐associated pulmonary and cerebral thromboembolic disease. Radiol Case Rep 2020;15:1242‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rudilosso S, Esteller D, Urra X, et al. Thalamic perforating artery stroke on computed tomography perfusion in a patient with coronavirus disease 2019. J Stroke Cerebrovasc Dis 2020;29:104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soldatelli MD, Amaral LFD, Veiga VC, et al. Neurovascular and perfusion imaging findings in coronavirus disease 2019: case report and literature review. Neuroradiol J 2020;33:368‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hanafi R, Roger PA, Perin B, et al. COVID‐19 Neurologic complication with CNS vasculitis‐like pattern. AJNR Am J Neuroradiol 2020;41:1384‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischer D, Threlkeld ZD, Bodien YG, et al. Intact brain network function in an unresponsive patient with COVID‐19. Ann Neurol 2020;88:851‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rasmussen C, Niculescu I, Patel S, et al. COVID‐19 and involvement of the corpus callosum: potential effect of the cytokine storm? AJNR Am J Neuroradiol 2020;41:1625‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gunasekaran K, Amoah K, Rajasurya V, et al. Stroke in a young COVID‐19 patient. QJM 2020;113:573‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Avula A, Nalleballe K, Narula N, et al. COVID‐19 presenting as stroke. Brain Behav Immun 2020;87:115‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morassi M, Bagatto D, Cobelli M, et al. Stroke in patients with SARS‐CoV‐2 infection: case series. J Neurol 2020;267:2185‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang A, Mandigo GK, Yim PD, et al. Stroke and mechanical thrombectomy in patients with COVID‐19: technical observations and patient characteristics. J Neurointerv Surg 2020;12:648‐53. [DOI] [PubMed] [Google Scholar]
- 50. Lapergue B, Lyoubi A, Meseguer E, et al. Large vessel stroke in six patients following SARS‐CoV‐2 infection: a retrospective case study series of acute thrombotic complications on stable underlying atherosclerotic disease. Eur J Neurol 2020. 10.1111/ene.14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Escalard S, Maier B, Redjem H, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID‐19: experience from Paris. Stroke 2020;51: 2540‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Diaz‐Segarra N, Edmond A, Kunac A, et al. COVID‐19 ischemic strokes as an emerging rehabilitation population: a case series. Am J Phys Med Rehabil 2020;99:876‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. O'Shea A, Parakh A, Hedgire S, et al. Multisystem assessment of the imaging manifestations of coagulopathy in hospitalized patients with COVID‐19. AJR Am J Roentgenol. 2020. 10.2214/AJR.20.24132. [DOI] [PubMed] [Google Scholar]
- 54. Ashrafi F, Zali A, Ommi D, et al. COVID‐19‐related strokes in adults below 55 years of age: a case series. Neurol Sci 2020;41:1985‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mohamud AY, Griffith B, Rehman M, et al. Intraluminal carotid artery thrombus in COVID‐19: another danger of cytokine storm? AJNR Am J Neuroradiol 2020;41:1677‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Helms J, Kremer S, Merdji H, et al. Delirium and encephalopathy in severe COVID‐19: a cohort analysis of ICU patients. Crit Care 2020;24:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Katz JM, Libman RB, Wang JJ, et al. Cerebrovascular complications of COVID‐19. Stroke 2020;51:e227‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Conklin J, Frosch MP, Mukerji S, et al. Cerebral microvascular injury in severe COVID‐19. medRxiv 2020. 10.1101/2020.07.21.20159376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pons‐Escoda A, Naval‐Baudin P, Majos C, et al. Neurologic involvement in COVID‐19: cause or coincidence? A neuroimaging perspective. AJNR Am J Neuroradiol 2020;41:1365‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chougar L, Shor N, Weiss N, et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS‐CoV‐2 infection and neurological manifestations. Radiology 2020. 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scullen T, Keen J, Mathkour M, et al. Coronavirus 2019 (COVID‐19)‐associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg 2020;141:e437‐46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xiong W, Mu J, Guo J, et al. New onset neurologic events in people with COVID‐19 infection in three regions in China. Neurology 2020;95:e1479‐e87. [DOI] [PubMed] [Google Scholar]
- 63. Yaghi S, Ishida K, Torres J, et al. SARS‐CoV‐2 and stroke in a New York healthcare system. Stroke 2020;51:2002‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kremer S, Lersy F, de Seze J, et al. Brain MRI findings in severe COVID‐19: a retrospective observational study. Radiology 2020;297:E242‐E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Klironomos S, Tzortzakakis A, Kits A, et al. Nervous system involvement in COVID‐19: results from a retrospective consecutive neuroimaging cohort. Radiology 2020;297:E324‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liang JW, Reynolds AS, Reilly K, et al. COVID‐19 and Decompressive hemicraniectomy for acute ischemic stroke. Stroke 2020;51:e215‐e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhai P, Ding Y, Li Y. The impact of COVID‐19 on ischemic stroke. Diagn Pathol 2020;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang S, Wang J, Liu F, et al. COVID‐19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res 2020;43:824‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Benger M, Williams O, Siddiqui J, et al. Intracerebral haemorrhage and COVID‐19: clinical characteristics from a case series. Brain Behav Immun 2020;88:940‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kvernland A, Kumar A, Yaghi S, et al. Anticoagulation use and hemorrhagic stroke in SARS‐CoV‐2 patients treated at a New York healthcare System. Neurocrit Care 2020. 10.1007/s12028-020-01077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Doo FX, Kassim G, Lefton DR, et al. Rare presentations of COVID‐19: PRES‐like leukoencephalopathy and carotid thrombosis. Clin Imaging 2020;69:94‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vu D, Ruggiero M, Choi WS, et al. Three unsuspected CT diagnoses of COVID‐19. Emerg Radiol 2020;27:229‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Haddadi K, Ghasemian R, Shafizad M. Basal ganglia involvement and altered mental status: a unique neurological manifestation of Coronavirus disease 2019. Cureus 2020;12:e7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nicholson P, Alshafai L, Krings T. Neuroimaging findings in patients with COVID‐19. AJNR Am J Neuroradiol 2020;41:1380‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Argiro R, Cirelli C, Sgreccia A, et al. Cerebral hemorrhage related to vein thrombosis in COVID‐19 patients in different Italian hospitals: view point for clinical and imaging implications. J Neurol Sci 2020;416:117023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Altschul DJ, Unda SR, de La Garza Ramos R, et al. Hemorrhagic presentations of COVID‐19: risk factors for mortality. Clin Neurol Neurosurg 2020;198:106112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muhammad S, Petridis A, Cornelius JF, et al. Letter to editor: severe brain haemorrhage and concomitant COVID‐19 Infection: a neurovascular complication of COVID‐19. Brain Behav Immun 2020;87:150‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. De Stefano P, Nencha U, De Stefano L, et al. Focal EEG changes indicating critical illness associated cerebral microbleeds in a COVID‐19 patient. Clin Neurophysiol Pract 2020;5:125‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fitsiori A, Pugin D, Thieffry C, et al. COVID‐19 is associated with an unusual pattern of brain microbleeds in critically ill patients. J Neuroimaging 2020;30:593‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Radmanesh A, Derman A, Lui YW, et al. COVID‐19 ‐associated diffuse leukoencephalopathy and microhemorrhages. Radiology 2020;297:E223‐E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Vattoth S, Abdelhady M, Alsoub H, et al. Critical illness‐associated cerebral microbleeds in COVID‐19. Neuroradiol J 2020;33:374‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Agarwal S, Jain R, Dogra S, et al. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID‐19. Stroke 2020;51:2649‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chougar L, Mathon B, Weiss N, et al. Atypical deep cerebral vein thrombosis with hemorrhagic venous infarction in a patient positive for COVID‐19. AJNR Am J Neuroradiol 2020;41:1377‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Garaci F, Di Giuliano F, Picchi E, et al. Venous cerebral thrombosis in COVID‐19 patient. J Neurol Sci 2020;414:116871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hughes C, Nichols T, Pike M, et al. Cerebral venous sinus thrombosis as a presentation of COVID‐19. Eur J Case Rep Intern Med 2020;7:001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hemasian H, Ansari B First case of COVID‐19 presented with cerebral venous thrombosis: a rare and dreaded case. Revue Neurol 2020;176:521‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Klein DE, Libman R, Kirsch C, et al. Cerebral venous thrombosis: a typical presentation of COVID‐19 in the young. J Stroke Cerebrovasc Dis 2020;29:104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Malentacchi M, Gned D, Angelino V, et al. Concomitant brain arterial and venous thrombosis in a COVID‐19 patient. Eur J Neurol 2020. 10.1111/ene.14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Roy‐Gash F, M DEM, Devys JM, et al. COVID‐19‐associated acute cerebral venous thrombosis: clinical, CT, MRI and EEG features. Crit Care 2020;24:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cavalcanti DD, Raz E, Shapiro M, et al. Cerebral venous thrombosis associated with COVID‐19. AJNR Am J Neuroradiol 2020;41:1370‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol 2017;264:1608‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914‐25. [DOI] [PubMed] [Google Scholar]
- 93. Kishfy L, Casasola M, Banankhah P, et al. Posterior reversible encephalopathy syndrome (PRES) as a neurological association in severe COVID‐19. J Neurol Sci 2020;414:116943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kaya Y, Kara S, Akinci C, et al. Transient cortical blindness in COVID‐19 pneumonia; a PRES‐like syndrome: case report. J Neurol Sci 2020;413:116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Franceschi AM, Ahmed O, Giliberto L, et al. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID‐19 infection. AJNR Am J Neuroradiol 2020;41:1173‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID‐19 non‐survivors. Neurology 2020;95:e2016‐e27. [DOI] [PubMed] [Google Scholar]
- 97. Princiotta Cariddi L, Tabaee Damavandi P, Carimati F, et al. Reversible encephalopathy syndrome (PRES) in a COVID‐19 patient. J Neurol 2020;267:3157‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Parauda SC, Gao V, Gewirtz AN, et al. Posterior reversible encephalopathy syndrome in patients with COVID‐19. J Neurol Sci 2020;416:117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dakay K, Kaur G, Gulko E, et al. Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID‐19 infection. J Stroke Cerebrovasc Dis 2020;29:105011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Patel P, Khandelwal P, Gupta G, et al. COVID‐19 and cervical artery dissection‐ A causative association? J Stroke Cerebrovasc Dis 2020;29:105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis 2020;94:55‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hayashi M, Sahashi Y, Baba Y, et al. COVID‐19‐associated mild encephalitis/encephalopathy with a reversible splenial lesion. J Neurol Sci 2020;415:116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Abdel‐Mannan O, Eyre M, Lobel U, et al. Neurologic and radiographic findings associated with COVID‐19 infection in children. JAMA Neurol 2020;77:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Poyiadji N, Shahin G, Noujaim D, et al. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology 2020;296:E119‐E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dixon L, Varley J, Gontsarova A, et al. COVID‐19‐related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm 2020;7:e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Virhammar J, Kumlien E, Fallmar D, et al. Acute necrotizing encephalopathy with SARS‐CoV‐2 RNA confirmed in cerebrospinal fluid. Neurology 2020;95:445‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI findings in patients in the intensive care unit with COVID‐19 infection. Radiology 2020;297:E232‐E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pilotto A, Odolini S, Masciocchi S, et al. Steroid‐responsive encephalitis in Coronavirus disease 2019. Ann Neurol 2020;88:423‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Al‐Olama M, Rashid A, Garozzo D. COVID‐19‐associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir 2020;162:1495‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Afshar H, Yassin Z, Kalantari S, et al. Evolution and resolution of brain involvement associated with SARS‐ CoV2 infection: a close clinical‐paraclinical follow up study of a case. Mult Scler Relat Disord 2020;43:102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Efe IE, Aydin OU, Alabulut A, et al. COVID‐19‐associated encephalitis mimicking glial tumor. World Neurosurg 2020;140:46‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Collange O, Tacquard C, Delabranche X, et al. Coronavirus disease 2019: associated multiple organ damage. Open Forum Infect Dis 2020;7:ofaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Westhoff TH, Seibert FS, Bauer F, et al. Allograft infiltration and meningoencephalitis by SARS‐CoV‐2 in a pancreas‐kidney transplant recipient. Am J Transplant 2020. 10.1111/ajt.16223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Grimaldi S, Lagarde S, Harle JR, et al. Autoimmune encephalitis concomitant with SARS‐CoV‐2 infection: insight from (18)F‐FDG PET imaging and neuronal autoantibodies. J Nucl Med 2020. 10.2967/jnumed.120.249292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hosseini AA, Shetty AK, Sprigg N, et al. Delirium as a presenting feature in COVID‐19: neuroinvasive infection or autoimmune encephalopathy? Brain Behav Immun 2020;88:68‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Benameur K, Agarwal A, Auld SC, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and Coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis 2020;26:2016‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID‐19. Brain Behav Immun 2020;88:945‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med 2020;382:2268‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wong PF, Craik S, Newman P, et al. Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID‐19 infection. Clin Med 2020;20:293‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zanin L, Saraceno G, Panciani PP, et al. SARS‐CoV‐2 can induce brain and spine demyelinating lesions. Acta Neurochir 2020;162:1491‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Brun G, Hak JF, Coze S, et al. COVID‐19‐white matter and globus pallidum lesions: demyelination or small‐vessel vasculitis? Neurol Neuroimmunol Neuroinflamm 2020;7:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Parsons T, Banks S, Bae C, et al. COVID‐19‐associated acute disseminated encephalomyelitis (ADEM). J Neurol 2020;267:2799‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Demirci Otluoglu G, Yener U, Demir MK, et al. Encephalomyelitis associated with COVID‐19 infection: case report. Br J Neurosurg 2020. 10.1080/02688697.2020.1787342 [DOI] [PubMed] [Google Scholar]
- 124. Radmanesh A, Derman A, Ishida K. COVID‐19‐associated delayed posthypoxic necrotizing leukoencephalopathy. J Neurol Sci 2020;415:116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Novi G, Rossi T, Pedemonte E, et al. Acute disseminated encephalomyelitis after SARS‐CoV‐2 infection. Neurol Neuroimmunol Neuroinflamm 2020;7:e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zoghi A, Ramezani M, Roozbeh M, et al. A case of possible atypical demyelinating event of the central nervous system following COVID‐19. Mult Scler Relat Disord 2020;44:102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Asundi A, Cervantes‐Arslanian AM, Lin NH, et al. Infectious myelitis. Semin Neurol 2019;39:472‐81. [DOI] [PubMed] [Google Scholar]
- 128. AlKetbi R, AlNuaimi D, AlMulla M, et al. Acute myelitis as a neurological complication of COVID‐19: a case report and MRI findings. Radiol Case Rep 2020;15:1591‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Munz M, Wessendorf S, Koretsis G, et al. Acute transverse myelitis after COVID‐19 pneumonia. J Neurol 2020;267:2196‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sotoca J, Rodriguez‐Alvarez Y COVID‐19‐associated acute necrotizing myelitis. Neurol Neuroimmunol Neuroinflamm 2020;7:e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Valiuddin H, Skwirsk B, Paz‐Arabo P. Acute transverse myelitis associated with SARS‐CoV‐2: a case‐report. Brain Behav Immun Health 2020;5:100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Meng X, Deng Y, Dai Z, et al. COVID‐19 and anosmia: a review based on up‐to‐date knowledge. Am J Otolaryngol 2020;41:102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tsivgoulis G, Fragkou PC, Delides A, et al. Quantitative evaluation of olfactory dysfunction in hospitalized patients with Coronavirus [2] (COVID‐19). J Neurol 2020;267:2193‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulbs edema during COVID‐19‐related anosmia. Neurology 2020;95:224‐5. [DOI] [PubMed] [Google Scholar]
- 135. Galougahi MK, Ghorbani J, Bakhshayeshkaram M, et al. Olfactory bulb magnetic resonance imaging in SARS‐CoV‐2‐induced anosmia: the first report. Acad Radiol 2020;27:892‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with Coronavirus disease 2019 (COVID‐19) and anosmia. JAMA Neurol 2020;77:1028‐9. [DOI] [PubMed] [Google Scholar]
- 137. Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host‐virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11:995‐8. [DOI] [PubMed] [Google Scholar]
- 138. Li CW, Syue LS, Tsai YS, et al. Anosmia and olfactory tract neuropathy in a case of COVID‐19. J Microbiol Immunol Infect. 10.1016/j.jmii.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Goh Y, Beh DLL, Makmur A, et al. Pearls and oysters: facial nerve palsy as a neurological manifestation of COVID‐19 infection. Neurology 2020;95:364‐7. [DOI] [PubMed] [Google Scholar]
- 140. Lantos JE, Strauss SB, Lin E. COVID‐19‐associated Miller Fisher syndrome: MRI findings. AJNR Am J Neuroradiol 2020;41:1184‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Dinkin M, Gao V, Kahan J, et al. COVID‐19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology 2020;95:221‐3. [DOI] [PubMed] [Google Scholar]
- 142. Hutchins KL, Jansen JH, Comer AD, et al. COVID‐19‐associated bifacial weakness with paresthesia subtype of Guillain‐Barre syndrome. AJNR Am J Neuroradiol 2020;41:1707‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Falcone MM, Rong AJ, Salazar H, et al. Acute abducens nerve palsy in a patient with the novel coronavirus disease (COVID‐19). J AAPOS 2020. 10.1016/j.jaapos.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Oliveira RMC, Santos DH, Olivetti BC, et al. Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID‐19 infection. Arq Neuropsiquiatr 2020;78:385‐6. [DOI] [PubMed] [Google Scholar]
- 145. GBD 2015 Neurological Disorders Collaborator Group . Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017;11:877‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with COVID‐19. J Thromb Haemost 2020;18:1324‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395:1417‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID‐19. N Engl J Med 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID‐19: evidence from front‐line clinical observation in Wuhan, China. Int J Cardiol 2020;311:116‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Emsley HC, Hopkins SJ Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol 2008;7:341‐53. [DOI] [PubMed] [Google Scholar]
- 151. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Dozois A, Hampton L, Kingston CW, et al. PLUMBER Study (Prevalence of Large Vessel Occlusion Strokes in Mecklenburg County Emergency Response). Stroke 2017;48:3397‐9. [DOI] [PubMed] [Google Scholar]
- 153. Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large‐vessel occlusions contribute disproportionately to stroke‐related dependence and death: a Review. Front Neurol 2017;8:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lioutas VA, Goyal N, Katsanos AH, et al. Clinical outcomes and neuroimaging profiles in nondisabled patients with anticoagulant‐related intracerebral hemorrhage. Stroke 2018;49:2309‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tsivgoulis G, Wilson D, Katsanos AH, et al. Neuroimaging and clinical outcomes of oral anticoagulant‐associated intracerebral hemorrhage. Ann Neurol 2018;84:694‐704. [DOI] [PubMed] [Google Scholar]
- 156. Teo KC, Leung WCY, Wong YK, et al. Delays in stroke onset to hospital arrival time during COVID‐19. Stroke 2020;51:2228‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nguyen‐Huynh MN, Tang XN, Vinson DR, et al. Acute stroke presentation, care, and outcomes in community hospitals in northern California during the COVID‐19 pandemic. Stroke 2020;51:2918‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhou Z, Kang H, Li S, et al. Understanding the neurotropic characteristics of SARS‐CoV‐2: from neurological manifestations of COVID‐19 to potential neurotropic mechanisms. J Neurol 2020;267:2179‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mangalmurti N, Hunter CA. Cytokine storms: understanding COVID‐19. Immunity 2020;53:19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wu X, Wu W, Pan W, et al. Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm 2015;2015:792578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Savarin C, Bergmann CC Viral‐induced suppression of self‐reactive T cells: lessons from neurotropic coronavirus‐induced demyelination. J Neuroimmunol 2017;308:12‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wu GF, Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune‐mediated demyelination following murine infection with a neurotropic coronavirus. J Virol 1999;73:8771‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Reichard RR, Kashani KB, Boire NA, et al. Neuropathology of COVID‐19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)‐like pathology. Acta Neuropathol 2020;140:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Murray RS, Cai GY, Hoel K, et al. Coronavirus infects and causes demyelination in primate central nervous system. Virology 1992;188:274‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ginsberg MD, Hedley‐Whyte ET, Richardson EP Jr. Hypoxic‐ischemic leukoencephalopathy in man. Arch Neurol 1976;33:5‐14. [DOI] [PubMed] [Google Scholar]
- 166. Stubbs DJ, Yamamoto AK, Menon DK. Imaging in sepsis‐associated encephalopathy ‐ insights and opportunities. Nat Rev Neurol 2013;9:551‐61 [DOI] [PubMed] [Google Scholar]
- 167. Qureshi AI, French BR, Siddiq F, et al. COVID‐19 screening with chest CT in acute stroke imaging: a clinical decision model. J Neuroimaging 2020;30:617‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Janardhan V, Janardhan V, Kalousek V. COVID‐19 as a blood clotting disorder masquerading as a respiratory illness: a cerebrovascular perspective and therapeutic implications for stroke thrombectomy. J Neuroimaging 2020;30:555‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Iadecola C, Anrather J, Kamel H. Effects of COVID‐19 on the nervous S‐system. Cell 2020;183:16‐27.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Naval‐Baudin P, Rodriguez Caamaño I, Rubio‐Maicas C et al. COVID‐19 and ischemic stroke: clinical and neuroimaging findings. J Neuroimaging 2020. 10.1111/jon.12790. [DOI] [PubMed] [Google Scholar]


