Abstract
The arrival of novel health crisis by a novel member of coronavirus group named as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) by World Health Organization took the whole world in global emergency by affecting 206 countries. The virus infects 206 countries with 86,839,226 confirmed cases, 61,565,949 recoveries, and 1,876,243 deaths as on January 6, 2021. Evidence pointed out the fact that virus might first originated in bats in China and it took only 2 months to spread over almost every country of the world. SARS‐CoV‐2 belongs to beta coronavirus and is enveloped, positive sense, and single‐stranded RNA virus. The treatment would be difficult as SARS‐CoV‐2 is an RNA virus and thus the mutation rate is higher in comparison with the DNA viruses. The virus infection also leads to generation of effective protective immune response of tumor necrosis factor, interleukin (IL)‐1β, IL‐6, IL‐8, granulocyte colony‐stimulating factor, granulocyte‐macrophage colony‐stimulating factor, and so on that may help in virus elimination. The speed of the global spread of the current pandemic is of major concern and it has created a significant threat to economic and human health across the world. In India, the infection spreads with an infection and fatality rates of the disease are 1.7% and 2.8%, respectively. By this review, we want to emphasize the actual situation and major factors associated with COVID‐19 pandemic, its significance, destructions, important findings, treatments, and preventive measures taken by all nations to provide better cure without having much loss.
Keywords: coronavirus, COVID‐19, India, pandemic, SARS‐CoV‐2
Graphical Abstract
The novel coronavirus has established its infection worldwide and has created a pandemic. In India, the condition is more dangerous due to its high population. After employing much novel research strategies toward its diagnosis and treatment, the disease comes out with several questions like: When will the adequate vaccine being commercialized for public benefit? Why in some patients the disease is asymptomatic? Why there is reoccurrence of infection? and The health condition after its treatment?
Abbreviation
- Ang
Angiotensin
- ACE2
angiotensin‐converting enzyme 2
- COVID‐19
coronavirus disease 2019
- CoV
coronavirus
- CDC
Centers for Disease Control and Prevention 2019
- CPAP
continuous positive airway pressure
- DPP4
dipeptidyl peptidase‐4
- ECs
epithelial cell
- ExoN
exoribonuclease
- EUA
emergency use authorization
- ICMR
Indian Council of Medical Research
- MERS
Middle East Respiratory Disorder
- mAbs
monoclonal antibodies
- Neo CoV
Neoromicia capensis Bat Coronavirus
- nsps
nonstructural proteins
- RdRp
RNA‐dependent RNA polymerase protein
- RTC
replication transcription complex
- RBD
receptor‐binding domain
- S protein
spikes glycoprotein
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
1. Introduction
Progressions of devastated pneumonia like cases were accounted in China in December 2019 that made the scourge circumstance in the nation. In a brief period, this epidemic changed into a perilous pandemic and got spread to different nations [1]. In January 2020, World Health Organization (WHO) explained the circumstance and declared this pandemic as an international health emergency. WHO reported that this pandemic is because of novel coronavirus (2019‐CoV) that causes disease known as COVID‐19 [2]. International Committee on Taxonomy of Viruses named 2019‐nCoV as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) [3]. Coronaviruses (CoVs) are critical pathogen that can depend on human and vertebrate for infection. They can invade into respiratory, gastrointestinal, hepatic, and focal sensory system of human, bat, mouse, and many other wild creatures [4, 5]. This COVID‐19 flare‐up in the whole world and breaks all the parameters and measures that had happened because of SARS in 2002/2003 and the Middle East respiratory disorder (MERS) in 2012, the two pandemics that have occurred due to SARS group virus [6]. SARS and MERS are two other CoVs that also caused nosocomial infections and were the killer of over thousand people [7]. MERS is the extended form of beta CoV and more like African Neoromicia capensis bat CoV (Neo CoV) [8, 9]. Although the infection patterns of these three viruses are almost similar but they evolved from different ancestries. SARS infected the population of more than 20 countries in North America, South America, Europe, and Asia before its global outbreak was controlled, whereas MERS was mostly prominent in most of the region of Saudi Arabia [10]. Bat and human CoVs have almost 79.5% genome similarity and therefore the disease could be predicted to be transmitted from bats to humans. There are several similarities among these infections, but they also differ in many respects. SARS, also known as Acute Respiratory Distress Syndrome (ARDS), is a zoonotic disease that was thought to be caused by novel CoV and transmitted from bats via civet cats [11]. MERS is also a zoonotic virus that originated from bats via dromedary camel. All infections show common symptoms like fever and cough, which frequently lead to lower respiratory tract disease with poor clinical outcomes associated with older age and underlying severe health conditions. This can emphasize from the fact that SARS virus can be aerosolized and can sustain into airborne transmission, but MERS viruses had not reported any human‐to‐human transmission. The major transmission originator for MERS viruses in human are camel. A list of contrasting features between these three epidemics is listed in Table 1 [12].
TABLE 1.
Comparative and contrasting features of SARS‐CoV, MERS, and SARS‐CoV‐2
| SARS | MERS | SARS‐CoV‐2 (January 6, 2021) | |
|---|---|---|---|
| Countries | 29 | 23 | 206 |
| Reported cases | 8,098 | 935 | 86,839,226 |
| Deaths | 774 | 371 | 1,876,243 |
| Mean incubation period (days) | 4.7 | 5.8 | 5.1 |
| Origin country | China | Saudi Arabia | China |
| Origin source | Bats via Civet cats | Bats via Camels | Bats, Pangolins |
| Fatality rate | 10% | 37% | 4.39% |
Eventually, this pandemic grasped everyone's attention due to tremendous increase in the number of incidences and death cases in China. The infrequent arrival and epidemics prompt us that CoVs are an unembellished universal threat to health. It is extremely possible that novel CoV emergence is unescapable in the future due to changes in the genome, climate and ecology, and the increased interactions of human with animals [13]. Therefore, there is an imperative prerequisite to developed advanced and effective therapies and vaccines against CoVs.
Highlights
The novel pandemic is due to novel coronavirus (2019‐CoV) that causes disease known as COVID‐19.
India, being the second most populated country after China, is massively hit by novel coronavirus in many ways.
The virus infects 206 countries with 86,839,226 confirmed cases, 61,565,949 recoveries, and 1,876,243 deaths as on January 6, 2021.
Six Indian organizations (Zydus Cadila, Serum Institute, Biological E, Bharat Biotech, Indian Immunologicals, and Mynvax) are preparing immunization for COVID‐19.
2. Establishment and Story Behind the Epidemic of the Disease
The first case of the disease was found in Wuhan, the capital city of China (Hubei Province). Like two earlier viral outbreaks SARS and MERS, SARS‐CoV‐2 origin was also found to be from bat. But how it transmitted from bat to human is still not clear. Chinese clinics began witnessing cases with extreme pneumonia of obscure reason. A significant number of the underlying cases had a typical relation or history of traveling to the Huanan market that also supplied live animals [14, 15]. The reconnaissance framework that was also started for SARS outbreak was initiated and respiratory samples were tested. On last day of 2019, China told the outbreak to the WHO and on first January the Huanan market was shut [16, 17]. After some time, human‐to‐human transmission of this disease started to occur as patients who did not have any contact with the live animals were reported. The spread increased after massive traveling of Chinese during Chinese New Year, which powered the epidemic and changed it into a global pandemic after cases reported in Thailand, South Korea, and Japan from travelers that returned from Wuhan. Soon, the cases were reported in the patients that did not have any travel history with China, which suggested local human‐to‐human transmission is also possible. Notably, airport authorities also started screening of travelers to identify possible patients. However, the further increase in reported cases and even after clearing the screening barriers at airports suggested that local asymptomatic people could possibly transmit the disease. Till January 6, 2021, 86,839,226 confirmed cases, 61,565,949 recoveries, and 1,876,243 deaths were reported in around 206 countries areas and territories [18]. India being the second most populated country after China faces a great threat due to novel CoV [19].
3. SARS‐CoV‐2 Epidemic: The Indian Horizon
India faces a great threat due to novel CoV. The first case of COVID‐19 in India was reported on January 30, 2020 and since then the number of incidences has been increasing. The case fatality rate of the disease was noted as 1.7% in the month of March 2020, which increased to 2.8% by June 2020. Although the fatality rate is low in comparison with worldwide infection rate, which is 6.13%, the severity is much higher in the nation due to the large population [20]. In India, the total number of reported cases are 10,375,478 of which 9,997,272 have been recovered and 150,151 have died till January 6, 2021 (Fig. 1) [21]. The country had its first introduction with disease in the state of Kerala where three individuals who had traveled to Wuhan, China confirmed the infection [22]. Infection escalated in the month of March when cases were reported from individuals who traveled from severely infected countries and the month also witnessed its first death from COVID‐19 who had a recent travel history to Saudi Arabia. A case of super spreading was also noticed in March where a person who had traveled to Italy and Germany attended a festival in India and caused a spread to 19 other people. After the incidence, 20 villages of state were quarantined to contain the spread [23, 24]. The outbreak was then pronounced as an epidemic in almost every state and union territory with most of the cases reported in Maharashtra (1,950,171), Karnataka (923,353), Andhra Pradesh (883,587), Tamil Nadu (822,370), Kerala (784,488), and Delhi (627,698). The situation worsened to its peak and caused the implementation of Epidemic Diseases Act 1897 in the whole nation. All tourist visas and import–export system was suspended. The nationwide lockdown was implemented from end March to end May and this lockdown has helped in flattening the peak of incidences, which was otherwise predicted to be very high and uncontrollable [25]. The lockdown resulted in slowing the doubling rate to 6 days, which was 3 days prior to lockdown. Most of the infection cases are self‐limited as the disease itself has its distinguished symptoms and diagnosis is require only in severe cases. In India, the first case was introduced in late January and since then preventive measures has been started to cure local population. Preventive measure includes screening of the immigrants at the airport as this disease started by travelers between China and other countries [26]. Any suspected person was sent to the isolation ward of the hospital or said to be home quarantined for a period of at least 15 days. But the infection rate of the virus is very high to control when there are no symptoms in the starting days. Some immigrants behaved as super spreaders and irresponsible citizens by not following the quarantine period when they were told to do so. Super spreaders can be defined as people who transferred the infection to at least 8 people. Shut down of all public as well as government sectors and lockdown of a month were important steps for curative procedure of local population. Several possible steps have been taken by the government to support their residents of all over country from supplying food to providing their essential needs. These steps were needed as India is a developing country and two‐third of the population belongs to below the poverty line [27].
FIG 1.
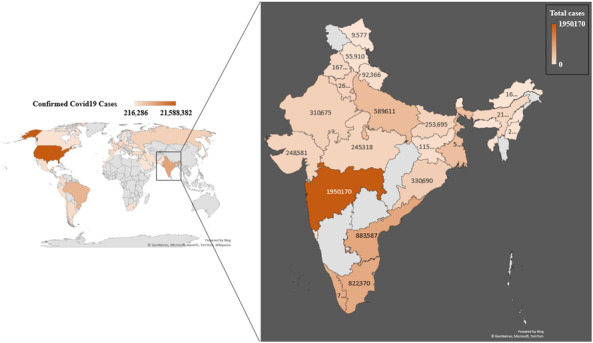
Total number of incidence cases: countries, areas, and territories with total number of incidences till January 6, 2021.
Further, the entire nation was divided into green zone, red zone, and orange zone. The red zone comprised of districts with high doubling rate and high number of cases and this is followed by orange zone with lesser number of cases and green zone with very few or no new reported incidences. The red zone states were Mumbai, Delhi, Uttar Pradesh, Chennai, Pune, and Kolkata [28]. The steps were taken by the recommendation of Michael Ryan, the executive director of WHO, who noticed the cruelty of pandemic in India and emphasized that India comprises a marvelous capacity of carrying the disease and the situation in the country could be more devastating as compared with any other country [22]. Apart from these necessary steps, information technology was also used to contain the spread of the virus. This was done by Aarogya setu App that helps a person in finding hotspot areas and prevents them from encountering the infected person [29].
National Institute of Virology, India (NIV) along with other 65 laboratories started testing system for COVID‐19, and in March, the institute isolated a strain or novel CoV after which India comes to fifth position after China, Japan, Thailand, and the United States in having a pure virus sample. NIV also generated a rapid testing diagnostic kit based on enzyme‐linked immunosorbent assay principle [30]. Initially, the testing was done to only those suspects who have travel history to countries with high COVID risk or those who have come in contact with the suspected person. After the country saw a sharp increase in incidences, the testing strategy was revised with testing people having pneumonia and people showing symptoms for more than a week and belonged to hotspot area [31]. After encountering a huge increase in cases, the testing facility was also provided to National Accreditation Board for Testing and Calibration Laboratories‐accredited private laboratories. Apart from rapid research strategies in the direction of vaccine development and drug designing, plasma therapy was also used to treat patients. Initially, the experiment gave satisfactory results when a patient suffering from the disease and on ventilator support had recovered after giving plasma therapy. But after getting no robust evidence for the use of the therapy on routine basis and having life‐threatening allergies and lung injuries, Indian Council of Medical Research (ICMR) decided to discontinue the therapy [32].
India became a known figure worldwide due to various reasons one of which is export of hydroxychloroquine and paracetamol to the United States and other countries including Brazil, Spain, France, UK, Germany, Australia, and the SAARC countries. Because of more effective curative procedures and timely control, more patients have recovered in India. As on January 6, 2021, a total of 9,997,272 COVID‐19 patients had recovered. This is 96.35% of the total cases reported in India [33, 34].
4. Impact of the COVID‐19 Situation and Factors Affecting its Eradication in India
The current pandemic situation is creating a deep negative impact on the local population of India. Being a developing country and the second most populated country in the world, India is facing sever impacts of this pandemic. Although the government and the healthcare industry of the country are working at their best, but the situation demands more [35, 36, 37]. The pandemic severely affected the economy, commercial establishments, education, entertainment, sports, and transport industries. Moreover, the situation would be remembered as a bitter story by the labor class workers as they were homeless in initial time. After so much efforts, there are still some factors that hinder the controlling and proper eradication of the infection. The unnecessary gathering, panic buying of goods, suspected people escaping from hospitals, misinformation regarding the disease, unapproved treatments, local treatments, and discrimination are the most drastic factors that hinder the process of containing/controlling the infection [38, 39]. In this context, it becomes necessary that people should organize themselves with proper cure and know the fact behind the information. In addition to the above‐mentioned factors, the situation in India can be more severe due to several other comorbid conditions like tuberculosis and diabetes. Therefore, to treat the combined disease becomes the major issue in the treatment [40].
5. CoV Structure and its Genomic Integrity
Coronaviruses are named due to their crown like spikes that covers the outermost surface of the virion particle. These viruses are one of the largest virus groups of order Nidovirales. Nidovirales consists of Coronaviridae, Arteriviridae, Mesoniviridae, and Roniviridae families. Coronaviridae family is further subdivided into Coronavirinae and Toronavirinae. Coronavirinae further comprises four genera, that is, alpha, beta, gamma, and delta [41]. SARS‐CoV‐2, like other CoVs including SARS CoV and MERS CoV, is a part of beta CoV cluster. Moreover, novel CoV is closely related to two relative bat CoVs that were collected from eastern China in 2018 and known as bat‐SL‐CoVZC45 and bat‐SL‐CoVZXC21 with almost 88% similarity. The genome sequence similarity thus more distant from the SARS CoV (79%) and MERS CoV (50%). Moreover, one of the recent reports suggest pangolins as the possible virus reservoir. Pangolin‐CoV is 91.02% and 90.55% identical to SARS‐CoV‐2 and one bat CoV RaTG13, respectively, and the S1 protein of CoV is very much identical to the virus samples obtained from dead pangolin lung samples [42]. The genome is single‐stranded RNA of positive sense (+ssRNA) of approximately 30 kb size [43]. Along with this genome, the virus also contains 5′ capping and 3′ poly A tail sequence. There are mainly two types of proteins that are characterized as structural and nonstructural proteins. Structural proteins include spikes, nucleocapsids, matrix, and envelope proteins, whereas nonstructural proteins (nsps) comprise RNA‐dependent RNA polymerase protein (RdRp) that is coded by nsp12 gene. RdRp is an essential enzyme for running life cycle of various RNA viruses and continuously being a target to eradicate RNA viruses. Previous literatures reported that active site of RdRp is a conserved sequence containing two essential aspartate residues. These nsps along with other proteins form replication transcription complex (RTC), that is, packed into double membrane vesicles. RTC utilizes discontinuous transcription to synthesize a set of subgenomic RNA that were then used for production of subgenomic mRNAs that possess common 5′ and 3′ leader and terminal sequences, respectively. The overall genome of CoV contains 6 ORFs. The first ORF covers almost two‐third of the whole genome length that encodes 16 nsps. Ribosomal frameshifting between ORF1a and ORF1b leads to the production of polyproteins 1a and polyprotein 1b, respectively. These polypeptides are processed by viral chymotrypsin, papain, and main proteases to design 16 nsps. The 3′ terminal of the genome encodes structural proteins as mentioned above [44].
The genomic sequence alignment studies had showed 58% and 43% of sequence identity in nonstructural and structural proteins, respectively, among different CoVs species. This result suggests that nonstructural part of the virus is more conserved than structural part, which shows more diverse characteristic. Usually, genome size of RNA viruses is smaller than DNA viruses (less than 10 kb) but this is not the case with CoVs that have a genome of 30 kb. The large genomic size of CoVs that enhance their capability of mutation, which are already higher in case of RNA viruses, which is a major concern in developing therapeutic against these viruses [45, 46, 47].
6. Pathogenesis and Pathophysiology
Coronaviruses cause severe respiratory disease by primarily affecting lower respiratory tract. Although the symptoms are milder like cough, sneezing, coughing, sore throat, and so on, the symptoms worsen in immunecompromised conditions where patient could get multiple organ failure that ultimately leads to death. Cases with COVID‐19 also have higher morbidity and mortality rate in compromised state of the patients that may have developed due to diabetes, cancer, arthritis, and pulmonary diseases like tuberculosis, cardiac disease, kidney disease, hypertension, and so on. Various previous literatures had confirmed that this population is the most vulnerable one [48]. People of all ages are susceptible to infection as it spreads through coughing, sneezing, or touching any object that had a previous exposure to the virus, but the situation is more critical for elder patients due to weak immune capacity to deal with the pathogen. Symptomatic patient has higher viral load in the nasal cavity in comparison with the throat, which can predict that sneezing has the highest probability of infection than coughing. Few reports of China had also confirmed that asymptomatic people even after recovery can transmit the disease to a healthy person therefore patient should be quarantined for a definite period of 12–15 days after recovery.
Clinical features include higher leukocyte count, abnormal functioning of respiratory organs, a tremendous increase in proinflammatory cytokines interleukin (IL)2, IL7, granulocyte colony‐stimulating factor (G‐CSF), inducible protein (IP)10, macrophage chemotactic protein (MCP)1, macrophage inflammatory protein (MIP)1A, and tumor necrosis factor (TNF)α, whereas some of the severe cases also presented with higher level of cytokines and reactive chemokines like IL1‐β, IL1RA, IL7, IL8, IL9, IL6, IL10, basic FGF2, G‐CSF, granulocyte‐macrophage colony‐stimulating factor, interferon‐γ, IP10, MCP1, MIP1α, MIP1β, platelet‐derived growth factor B, TNFα, and vascular endothelial growth factor A. In case of immunocompromised state, the host is unable to secrete any protective response in form of cytokines and lymphocytes thus virus replicates and forms its progeny virion family to hijack host machinery (Fig. 2) [49]. The most common symptoms were fever (50%) and cough (38%). COVID‐19 patient also contains lower circulation of B cells and T cells and relatively high amount of circulating neutrophils. Complications witnessed include acute lung injury, ARDS, shock and acute kidney injury, or cardiac arrest conditions. Recovery may start in the second or third week of the treatment [50].
FIG 2.
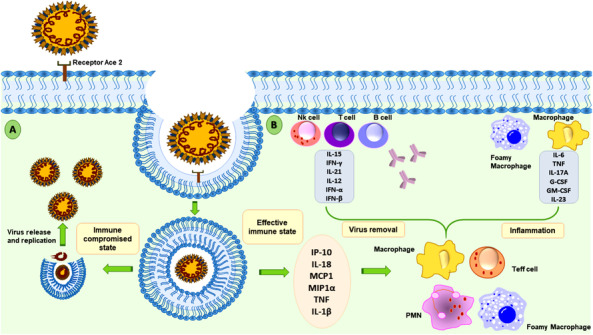
Protective effect of host immune system in novel coronavirus infection: interaction between virus particle and host cell occurs at the receptor Ace2 after a normal person meets the infected person. After recognition of the spike protein, virus membrane is fused with host cellular membrane, which then enclose virus in a double membrane vesicle. After this stage, the virus either bursts the vesicle and releases its progeny in case of immunocompromised person (A) or it can activate a protective immune response of cytokines and lymphocytes in normal person, which causes inflammation or favors virus removal (B).
Like different CoVs, SARS‐CoV‐2 particles are circular and have proteins called spike glycoprotein (S protein) projecting from their surface. These spike hook onto human cells and at that point, experience structural change that permits the viral layer to combine with the human cell surface. The viral genomic material would then be able to enter the host cell and start duplication for hijacking host machinery to replicate itself to deliver more infections. Angiotensin‐converting enzyme 2 (ACE2) and dipeptidyl peptidase‐4 (DPP4) are already studied host receptors for the entry of SARS and MERS CoV [51]. Previous literatures reported that like the infection that caused the 2002 SARS episode, SARS‐CoV‐2 spikes bind to receptors on the human cell surface called ACE2. The normal function of ACE2 in humans is maturation of angiotensin (Ang) that participates in vasoconstriction and regulates blood pressure [52]. ACE2 is a type I membrane protein expressed in type II alveolar cells in the lungs, heart, kidneys, and intestine whose lower expression can lead to cardiovascular disease. In case of SARS CoV infection, the Spike glycoprotein (S protein) of virion carries membrane fusion and virus integration into the host cell. The initiation of viral infection occurs by cleavage of trimeric S protein into its subunits S1 and S2, where S1 participate in post fusion events and S2 substantially undergoes structural rearrangement and leads to membrane fusion events. S1 contains the receptor‐binding domain (RBD), which directly binds to the peptidase domain of ACE2. When S1 integrates with host receptors, S2 also undergoes multiple cleavages by host proteases to carry out a process that is crucial for viral infection. The integration of S1 with host receptors, the RBD of S1 undergoes hinge‐like conformational change that exposes or hides molecular and structural determinant of receptor binding. Because of its essential nature to expose or hide the receptor determinants, receptor sites might be a significant therapeutic target or vaccine development [53]. Another responsible protein is Valocin‐containing protein that also plays an important role during infection, which was proved by mutagenesis. The event of postinfection replication, transcription, and translation of viral genome requires multisubunit complexes. For example, nsp14 is a complex that acts as an exoribonuclease (ExoN) with proofreading ability. Its C‐terminal is involved in viral mRNA capping by N7 methyl transferase (N7MTase) activity, whereas its N terminal is indulged in proofreading activity by ExoN and prevents it from lethal mutagenesis. This ExoN activity is also involved in boosting the RdRp activity, which is encoded by nsp12. Further evaluation of nsp14 by crystal structure determination found its strong interaction with nsp10, which strongly enhances the ExoN activity [54].
When the viral particles enter in human body, it interacts with ACE2 receptor and discharges its RNA particle in the epithelial cell (ECs) where replication of viral proteins occurs, which is followed by the spread of infection to the neighboring cells such as alveolar area of lung. Due to this infection, the gaseous exchange driven by alveoli is severely affected because of increased permeability and leakage. This condition causes pulmonary edema, activation of disseminated intravascular coagulation, pulmonary ischemia, hypoxic respiratory failure, and progressive lung damage. The viral particles can also travel to different parts of the body including the brain, gastrointestinal tract, heart, kidney, and liver that may lead to cerebral hemorrhage, neural disorder, ischemic stroke, coma, paralysis, and eventually death [55]. The viral infection also causes immune system activation and release of cytokines known as cytokine storm, which includes MCP‐1, IL‐8, and reduced expression of E‐cadherins on ECs that promotes leakage and vascular permeability. These conditions lead to hypotension and pulmonary dysfunction in ARDS. Most of the COVID‐19 patients are dying due to ARDS. The direct contact to ECs, the binding with ACE2 receptor and its activation, activation of Kallikrein bradykinin pathway, activated neutrophils that produce ROS, activation of immune cells, and release of cytokine IL‐1B and TNF that degrades glycocalyx are the major reasons behind increased vascular permeability and leakage in case of SARS‐CoV‐2 infection [56].
7. The Need of Oxygen Balance
The currently reported cases of fatality rate (number of deaths in disease positive patients divided by total number of disease positive patients) of each country is although low but it majorly depends upon the number of tests applied in that area. For example, in the greatest number of test applied countries like South Korea, the fatality rate is low due to large denominator that also includes mild infection (can be curated). The oxygen requirement occurs when lungs become infrequent in doing their putative work, that is, inhale oxygen and exhale carbon dioxide, and this situation results in pneumonia like condition [57]. Oxygen requirement in case of COVID‐19 is due to its infection in lungs that on severe conditions become necessary for one's survival. Oxygen requirement can be fulfilled in three stages: continuous positive airway pressure (CPAP), Non Invasive Ventilation (NIV), and extracorporeal membrane oxygenation (ECMO). The CPAP is the initial, noninvasive, and mild way requirement of oxygen. Patients of young age or patient without having any comorbidity could recover by applying CPAP and NIV. If the condition continued to deteriorate even after applying CPAP and NIV, mechanical ventilation by applying invasive ventilation and ECMO would be a savior. Fulfillment of oxygen requirement would be permissive in developed country but in developing countries like India, the situation would be difficult or worse if not cured at the right time [58].
8. Diagnosis and Ongoing Possible Preventive Treatments
Despite the specified symptoms of the disease to clarify the presence of actual situation, Centers for Disease Control and Prevention 2019 (CDC) and many Chinese and German diagnostic companies had developed a new laboratory test kit for use in testing patient specimens for SARS‐CoV‐2 [59]. All diagnostic kits are based on the principle of PCR. The kit basically comprises oligonucleotides that can identify and bind to viral genes and amplify it and the amplification level is the measure of infection. The most utilized test kit was named as the “Centers for Disease Control and Prevention (CDC) 2019‐Novel Coronavirus (2019‐nCoV) Real‐Time Reverse Transcriptase (RT)‐PCR Diagnostic Panel.” It is proposed for practice with the Applied Biosystems 7500 Fast DX Real‐Time PCR Instrument with SDS 1.4 software [60]. Various pharma companies are now participating in the development of efficient diagnostic test by considering the above‐mentioned genes (Table 2).
TABLE 2.
Diagnostic methods employed for detection of COVID‐19
| S. No. | Test | Time duration | Mechanism | Manufacturer/represented |
|---|---|---|---|---|
| 1. | NAAT | 3–4 H | Viral RNA | CDC |
| 2. | Abbott Rapid Test | 5 Min | RdRp | Abbott |
| 3. | RT‐PCR | 2–5 H | RdRp &E | [72] |
| 4. | SHERLOCK | 3–4 H | CAS 3 | Sherlock Biosciences |
| 5. | DETECTR | – | E&N | Mammoth Biosciences |
| 6. | Amplicon‐based metagenomic sequencing | NGS | [73] | |
| 7. | Serological test | 10 Min | IgG/IgM | CDC |
Researchers have applied many preventive strategies to establish a cure of this disease. Curative strategies include inhibition of cell entry and fusion. As virus enters the host cell by using its S protein and binds ACE2 receptor present on the host cell, this interaction is a potential target for developing therapeutic against SARS COV‐2. ACE2 is not the only critical parameter of viral entry; a host cellular serine protease known as TMPRSS2 was also supposed to be involved in interaction. Additionally, several other approaches like inhibition of viral replication by targeting RdRp enzyme, suppressing nonessential inflammatory and anti‐inflammatory response, and viral entry into the endosome by targeting AP‐2‐associated protein kinase 1 that regulates clathrin‐dependent endocytosis have been targeted for developing therapeutic target [32]. Most popularly, chloroquine was supposed to be the effective drug for this viral infection, but the molecular mechanism behind its effectiveness is still unclear. But it was proposed that rather defining a clear treatment from virus infection, chloroquine phosphate is probably effective to control pneumonia. Hydroxychloroquine was supposed to be more effective in comparison with chloroquine as it is reported that it could impair the endosome‐mediated viral entry in the late stages of viral replication. However, in June 2020, United States Food and Drug Administration (US FDA) cancelled the emergency use authorization (EUA) for the use of hydroxychloroquine to treat COVID‐19. US FDA decided and found that hydroxychloroquine is not likely to be effective for patients of COVID‐19 and the side effects of this medicine is a topic of concern. Moreover, remdesivir, lopinavir, ritonavir, and so on are some other drugs used in the treatment from COVID‐19 (Table 3) [61].
TABLE 3.
Antiviral drugs used or in trial to treat COVID‐19
| S. No. | Drug/vaccine | Mechanism of action | Manufacturer/presented |
|---|---|---|---|
| 1. | Remdesivir | Nucleotide prodrug | Gilead Sciences |
| 2. | Lopinavir | HIV‐1 protease inhibitor | AbbVie |
| 3. | Ritonavir | HIV protease inhibitors | Ascletis Pharma |
| 4. | Favipiravir | Broad‐spectrum antiviral drug developed to selectively and potently inhibit the RNA‐dependent RNA polymerase (RdRp) of RNA viruses. | Fujifilm Holdings and Zhejiang Hisun Pharmaceutical |
| 5. | Fingolimod | A sphingosine‐1‐phosphate receptor modulator, which sequesters lymphocytes in lymph nodes, preventing them from contributing to an autoimmune reaction. | Gilenya |
| 6. | Chloroquine phosphate | A quinoline compound with antimalarial and anti‐inflammatory properties | Bayer and numerous Chinese manufacturers |
| 7. | Hydroxychloroquine sulfate | Quinoline compound with antimalarial and anti‐inflammatory properties works like chloroquine but more effectively. | Bayer and numerous Chinese manufacturers |
| 8. | Hydroxychloroquine and azithromycin | Azithromycin is a bacteriostatic drug that acts by inhibiting protein synthesis. It binds reversibly to 50S ribosomal subunit and interfere with transpeptidation. | CDC, Trial |
| 9. | Equivir | Capable to bind angiotensin‐converting enzyme 2 (ACE2) and block SARS‐CoV‐2 entry into cells. | Impact BioMedical |
Apart from these medical therapies, there are some natural remedies that may be used to cure the disease caused by Cynara scolymus, Allium sativam, Embelia ribes, Nigella sativum, Clitoria ternatea, and so on. Although there are only a handful of researches present on these remedies, but it was already reported that these all boost the immune system for sure [62].
Passive antibody therapy is another putative cure to stop COVID‐19 condition. Passive immunization of antibody distinguishes the epitopic sections in the virus particle and reduces the virus replication and disease severity. Passive immunotherapy can be done by taking infected patient blood (plasma) or it can be synthesized in the laboratory. In view of the current evidences and related knowledge in treating other viral infections, for example, influenza, SARS, MERS, and Ebola, the early organization of healing plasma or hyperinsusceptible immunoglobulin from patients that contain critical antibody titers can almost certainly diminish the viral burden and disease mortality. However, the key difficulties, for example, accessibility of adequate benefactors, clinical condition, viral energy, and host intersection of SARS‐CoV‐2, should be clarified before thinking about healing plasma as an option for therapeutics [63].
Monoclonal antibodies (mAbs) are another promising target from decades to treat infectious diseases, and in case of COVID‐19 also, these are of great use and importance. The SARS‐CoV‐2 Spike (S) glycoprotein helps in the viral entry into the host cells and therefore it is the main target for the production of neutralizing mAbs. A report described the binding of mAbs S303, S304, S309, and S315 with SARS‐CoV‐2 and SARS‐CoV RBDs with nano‐ to subpicomolar affinity. A monoclonal antibody named S309 potently neutralizes pseudo‐SARS‐CoV, SARS‐CoV‐2, and active SARS‐CoV‐2 by engaging with the RBD of S glycoprotein. S309 can promote effector machineries and presented increased neutralization in amalgamation with weakly neutralizing mAbs, which may alleviate the risk of viral escape [64].
As in January 2021, European Union have authorized tozinameran, produced by Pfizer‐BioNTech vaccine, for EUA. UAE grant BBIBP‐CorV, which is manufactured by Sinopharm. There are total 321 vaccine candidate till date and the vaccine that are in use or in Phase II and Phase III trial are listed in Table 4 [65].
TABLE 4.
Antiviral vaccines used or in trial to treat COVID‐19
| 1. | Native S trimer antigen | Diagnosis of antigen by detecting neutralizing antibodies in fully recovered COVID‐19 patients | Clover Biopharmaceuticals |
| 2. | DNA plasmid vaccine | Express spike protein and activate T cell against COVID‐19 | Inovio Pharmaceuticals |
| 3. | In silico‐based mRNA vaccine | Advantageous over DNA vaccines | Moderna Inc. & NIAID |
| mRNA‐1273 | Novel lipid nanoparticle (LNP)‐encapsulated mRNA vaccine against the COVID‐19 encoding for a prefusion stabilized form of the Spike (S) protein | Moderna (EUA in Israel) | |
| 4. | EIDD‐2801 | It introduces genetic mutation in virus RNA that produces mutated virus progeny and reduces infection | Published in Science Translational Medicine |
| 5. | rSARSCoV‐E | Inactivated virus | CNB‐CSIC; University of Iowa |
| 6. | SARS VLPs S protein and influenza M1 protein | Virus‐like particle | Novavax |
| 7. | SARS recombinant spike protein plus delta inulin | Protein subunit | Vaxine Pty Ltd, Australia |
| 8. | ISCV | Inactivated virus | Sinovac Biotech (/Beijing Kexing Bioproduct), Chinese Centre for Disease Control and Prevention; Chinese Academy of Medical Sciences |
| 9. | Comirnaty (tozinameran or BNT162b2) | mRNA‐based vaccine | Pfizer BIONTECH |
| 10. | CVnCoV | mRNA‐based vaccine | CureVac (Phase II/III) |
| 11. | AG0302‐COVID19 | DNA‐based vaccine | TaKaRa (Phase II) |
| 12. | ZyCoV‐D | DNA‐based vaccine | Zydus (Phase III) |
| 13. | INO‐4800 | DNA‐based vaccine against Spike protein | INOVIO |
| 14. | Ad5‐nCoV | Recombinant adenovirus type 5 vector | CanSino Biologics |
| 15. | AZD1222 | Spike‐specific antibody | University of Oxford, AstrzZeneca, CEPI |
| 16. | BBIBP‐CorV | Inactivated SARS‐CoV‐2 | Sinopharm, Beijing Institute of Biological Products |
| 17. | BBV152 (Covaxin) | Inactivated SARS‐CoV‐2 | Bharat Biotech Indian Council of Medical Research |
| 18. | CoronaVac | Inactivated SARS‐CoV‐2 | Sinovac |
| 19. | Sputnik V Gam‐COVID‐Vac | Nonreplicating viral vector (adenovirus) | Gamaleya Research Institute of Epidemiology and Microbiology |
| 20. | Tozinameran | modRNA | BioNTech, Pfizer, Fosun Pharma |
9. Indian Approach in Vaccine Development
More than 150 COVID‐19 immunizations are being developed over the world and expectations are high to put up one for commercialization to the public in record time to facilitate the worldwide emergency. A few endeavors are in progress to help make that conceivable, including the U.S. government's Operation Warp Speed activity, which has promised $10 billion and intends to create and convey 300 million portions of a protected, compelling COVID‐19 immunization by January 2021. The WHO is additionally planning worldwide endeavors to build up a vaccine, with an optimization that conveying 2 billion dosages before the finish of 2021. It can regularly take 10–15 years to offer an immunization for sale to the public; the quickest ever was the vaccine for mumps that took 4 years during the 1960s. Immunizations experience a three‐stage clinical preliminary cycle before they are sent to administrative offices for endorsement, which can be an extensive cycle itself. Even after a vaccine is endorsed, it faces potential barricades with regards to scaling up creation and appropriation, which likewise incorporates choosing which populaces ought to get it first and at what cost. Numerous antibodies additionally remain in what's called stage four, a ceaseless phase of customary investigation [66].
Six Indian organizations are taking a shot at an immunization for COVID‐19, joining worldwide endeavors to locate a brisk preventive for the lethal disease spreading quickly over the world, says a top Indian researcher. While Zydus Cadila is dealing with two immunizations, Serum Institute, Biological E, Bharat Biotech, Indian Immunologicals, and Mynvax are creating one antibody each [67].
The primary methodology manages improvement of a DNA antibody against the major viral surface protein liable for the passage of the novel SARS‐CoV‐2. The plasmid DNA would be brought into the host cells, where it would be deciphered into the viral protein and evoke a solid insusceptible reaction interceded by the cell and humoral arms of the human safe framework, which assume a fundamental part in security from sickness just as viral freedom. The secondary methodology manages advancement of a live weakened recombinant measles infection vectored immunization against COVID‐19. The recombinant measles vaccine created by invert hereditary qualities would communicate codon‐improved proteins of the novel SARS‐CoV‐2 and will induce long‐term specific neutralizing antibodies, which will provide protection from the infection [68].
The Serum Institute of India developed Novavax. The vaccine also seemed to generate T‐cells, the type of immune cells that also help protect the body from infection, in the 16 volunteers who were randomly selected and tested for T‐cell response [69].
India‐based drug organization Biological E has consented to an arrangement with Johnson and Johnson (J&J) unit Janssen Pharmaceutica to produce J&J's COVID‐19 immunization up‐and‐comer, Ad26.COV2.S. Under the arrangement, Biological E will support creation capacities with regards to medicate substance and medication result of the antibody competitor presently in Phase I/IIa clinical preliminaries [70].
COVAXIN is India's first indigenous COVID‐19 antibody by Bharat Biotech and is created in a joint effort with the ICMR–NIV. The indigenous, inactivated antibody is created and fabricated in Bharat Biotech's BSL‐3 (Bio‐Safety Level 3) high regulation office. The immunization got DCGI endorsement for Phase I and II Human Clinical Trials and the preliminaries will start across India from July, 2021 [71, 72].
Hyderabad‐based human and animal vaccination producer Indian Immunologicals (IIL) presented COVID‐19 antibody in a joint effort with Australia Griffith University. IIL and Griffith University will be working to develop lessened SARS‐CoV‐2 antibody utilizing most recent codon deoptimization technology [73, 74].
Mynvax is another startup that tried to prevent COVID‐19 and initiate vaccine development in collaboration with IISc [75].
10. Conclusive Significance and Future Perspective
COVID‐19 is a serious global challenge and it is very important and primary requisite for every scientific committee to frequently develop its cure. Despite much less virulent characteristic of SARS‐CoV‐2 as compared with SARS‐CoV and MERS‐CoV, it is associated with devastated mortality among susceptible individuals with severe comorbidities. The new version of the virus has challenged public, medical, as well as economic sector of all affected countries. It has infected almost 10 lac people across world and has taken many lives. Majorly affected countries include China, its neighbor countries, Italy, Spain, India, Brazil, and the United States of America. Mortality rates of Italy, Spain, and the United States of America are much higher than mortality rate of China. The impact on under developing countries would be more devastated as being the second most populated country after China and its under poverty line population. Social distancing and personal hygiene are some of the curative procedures and a valuable one which had applied in most of the countries. Although the infection rate of the disease is much higher, it will be ended soon with harboring all essential preventive measures. This review highlights essential facts about COVID‐19 disease in India, which are important to be discussed and points out some crucial parameters that need to be done or under process for making its cure on the primary basis.
The authors declare no conflict of interest.
11. Acknowledgements
The authors acknowledge financial support from the Department of Science and Technology‐SERB, Council of Scientific and Industrial Research‐Institute of Genomics and Integrative Biology under the research project GAP0145 (SERB‐DST Grant no: EEQ/2016/000514).
References
- 1. Bogoch, A. , Watts, A. , Thomas‐Bachli, C. , Bogoch, I. I., Watts, A., Thomas‐Bachli, A., Huber, C., Kraemer, M., and Khan, K. (2020) Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J. Trav. Med. 13, taaa008. https://pubmed.ncbi.nlm.nih.gov/31943059/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu, H. , Stratton, C. W. , Tang, Y. W. , Lu, H., Stratton, C. W., and Tang, Y. W. (2020) Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 92, 401–402. https://pubmed.ncbi.nlm.nih.gov/31950516/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao, S. , Lin, Q. , Ran, J. , Zhao, S., Lin, Q., Ran, J., Musa, S. S., Yang, G., Wang, W., Lou, Y., Gao, D., Yang, L., He, D., and Wang, M. H. (2020) Preliminary estimation of the basic reproduction number of novel coronavirus (2019‐nCoV) in China, from 2019 to 2020: A data‐driven analysis in the early phase of the outbreak. Int. J. Infect. 92, 214–217. https://pubmed.ncbi.nlm.nih.gov/32007643/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hussain, S. , Pan, J. , Chen, Y. , Hussain, S., Pan, J., Chen, Y., Yang, Y., Xu, J., Peng, Y., Wu, Y., Li, Z., Zhu, Y., Tien, P., and Guo, D. , (2005) Identification of novel subgenomic RNAs and noncanonical transcription initiation signals of severe acute respiratory syndrome coronavirus. J. Virol. 79(9), 5288–5295. https://pubmed.ncbi.nlm.nih.gov/15827143/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang, L. F. , Shi, Z. , Zhang, S. , Wang, L. F., Shi, Z., Zhang, S., Field, H., Daszak, P., and Eaton, B. T. (2006) Review of bats and SARS. Emerg. Infect. Dis. 12(12), 1834–1840. https://pubmed.ncbi.nlm.nih.gov/17326933/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edelstein, M. and Heymann, D. L . (2015) What needs to be done to control the spread of Middle East respiratory syndrome coronavirus?. Future Virol. 5, 497–505. https://pubmed.ncbi.nlm.nih.gov/32201495/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothan, H. A. and Byrareddy, S. N . (2020). J. Autoimm, 109, 102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corman, V. M. , Ithete, N. L. , Richards, L. R. , Corman, V. M., Ithete, N. L., Richards, L. R., Schoeman, M. C., Preiser, W., Drosten, C., and Drexler, J. F. (2014) The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J. Virol. 88, 11297–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou, P. , Yang, X. L. , Wang, X. G. , Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , Si, H. R. , Zhu, Y. , Li, B. , Huang, C. L. , Chen, H. D. , Chen, J. , Luo, Y. , Guo, H. , Jiang, R. D. , Liu, M. Q. , Chen, Y. , Shen, X. R. , Wang, X. , Zheng, X. S. , Zhao, K. , Chen, Q. J. , Deng, F. , Liu, L. L. , Yan, B. , Zhan, F. X. , Wang, Y. Y. , Xiao, G. F. , Shi, Z. L. (2020); A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273. https://pubmed.ncbi.nlm.nih.gov/32015507/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahmed, S. F. , Quadeer, A. A. , and McKay, M. R . (2020) Viruses 12, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song, P. and Karak, P. (2020). Biosci. Trends , 14, 1–2. [DOI] [PubMed] [Google Scholar]
- 12. Zaki, A. M. , Van Boheemen, S. , Bestebroer, T. M. , Osterhaus, A. D., and Fouchier, R. A. N. Engl. J. Med. (2012);367, 1814–1820. [DOI] [PubMed] [Google Scholar]
- 13. Chan‐Yeung, M. , Xu, R. H , Salzberger, B., Buder, F., Lampl, B., Ehrenstein, B., Hitzenbichler, F., and Hanses, F . (2003). Respirology;8, S9–14.15018127 [Google Scholar]
- 14. Holshue, M. L. , DeBolt, C. , Lindquist, S. , et al. (2020) N. Engl. J. Med. 382, 929–936.2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang, Y. , Lu, Q.‐B. , Liu, M.‐J. , et al. (2020). medRxiv. 10.1101/2020.02.10.20021675. [DOI] [Google Scholar]
- 16. WHO. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019‐nCoV). January 30, 2020. https://www.who.int/newsroom/detail/30-01-2020-statement-on-thesecond-meeting-of-the-international-healthregulations-(2005)-emergency-committeeregarding-the-outbreak-of-novel-coronavirus(2019-ncov) (accessed June 16, 2020).
- 17. Jiang, X. , Rayner, S. , and Luo M, H . (2020) J. Med. Virol. 92, 476–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO report of COVID‐19 , Retrieved 6 January 2021.
- 19. Knvul, S. and Caryn, R. R . The Coronavirus: What Scientists Have Learned So Far. The New York Times. Retrieved 24 March 2020. [Google Scholar]
- 20. Daily COVID‐19 Bulletin . PIB India (@PIB_India) on Twitter. Twitter. Retrieved 3 June 2020. [Google Scholar]
- 21. Infections over 3 Lakh, Five Cities with Half the Cases: India's Coronavirus Story so Far. The Week. Retrieved 15 June 2020. [Google Scholar]
- 22. Regan, H. ; Mitra, E. ;, and Gupta, S. (2020) India Places Millions Under Lockdown to Fight Coronavirus . Retrieved 23 March 2020.
- 23. Reid, D. (2020) India Confirms its First Coronavirus Case. CNBC. Retrieved 30 January 2020. [Google Scholar]
- 24. India's First Coronavirus Death is Confirmed in Karnataka. Hindustan Times. Retrieved 27 March 2020. [Google Scholar]
- 25. Coronavirus: All International Arrivals to India to share Travel History at Airports. The Economic Times. Retrieved 4 March 2020. [Google Scholar]
- 26. SAD, Congress Leaders Trade Barbs as over 600 Nanded Pilgrims Contract Coronavirus. The Times of India. Press Trust of India. Retrieved 4 May 2020. [Google Scholar]
- 27. Coronavirus: Indian Airports to Now Screen Passengers From Four More Countries Including Nepal. Outlook. Retrieved 23 February 2020. [Google Scholar]
- 28. Sharma, N. C. and C. (2020) 30% covid‐19 cases in India linked to Tablighi Jamaat event: Govt. Livemint. Retrieved 5 June 2020. [Google Scholar]
- 29. Coronavirus Outbreak: Govt Working on a Containment Plan. The Economic Times. Retrieved 12 March 2020. [Google Scholar]
- 30. Aarogya Setu app , India has shown the way, says World Bank | India News ‐ Times of India.
- 31. National Institute of Virology develops 1st indigenous Elisa test kit for Covid‐19: Harsh Vardhan. The Times of India. Retrieved 11 May 2020. [Google Scholar]
- 32. Coronavirus Testing Strategy Revised in India Ambit Widened. NDTV.com. Retrieved 10 April 2020. [Google Scholar]
- 33. No Approved, Definitive Therapies for COVID‐19; Convalescent Plasma one of Several Emerging Therapies: ICMR. ANI News. Retrieved 28 April 2020. [Google Scholar]
- 34. India Offers $10 mn for Coronavirus Emergency Fund: PM Modi to SAARC Leaders. Livemint. Retrieved 15 March 2020. [Google Scholar]
- 35. Sharma, S. (2020) How Conference Hall at Health Ministry Emerged as Coronavirus‐Control War‐Room. Hindustan Times. Archived from the original on 7 March 2020. Retrieved 7 March 2020. [Google Scholar]
- 36. Nagaraja, G. (2020) COVID‐19 Threat: Underground Operations at Singareni Coal Mines Come to a Halt. The Wire. Retrieved 2 April 2020. [Google Scholar]
- 37. Madabhavi, I. , Sarkar, M. , and Kadako, N . (2020) Monaldi Arch. Chest Dis. 90, 1298 [DOI] [PubMed] [Google Scholar]
- 38. Swaroop, S. S. , Sahoo, S. S., Sahu, D. P., and Kankaria, A. (2020) Monaldi Arch. Chest Dis. 90, 1405. [DOI] [PubMed] [Google Scholar]
- 39. Yadav, S. R. , Yadav, S. R., Kumar, R., Gupta, N., Ish, P., Chakrabarti, S., and Kumar, A. (2020) Monaldi Arch. Chest Dis. 90, 1338. [Google Scholar]
- 40. Killerby, M. E. , Biggs, H. M. , Haynes, A. , Dahl, R. M. , et al. (2018) J. Clin. Virol. 101, 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang , Zhang, T., Wu, Q., and Zhang, Z. (2020) Curr. Biol., 30, 1346–1351.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elfiky, A. A .(2020) Life Sci., 248, 117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen, Y. , Liu, Q. , and Guo, D .(2020) J. Med. Virol. ; 92, 418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eckerle, L. D. , Becker, M. M. , Halpin, R. A. , Eckerle, L. D., Becker, M. M., Halpin, R. A., Li, K., Venter, E., Lu, X., Scherbakova, S., Graham, R. L., Baric, R. S., Stockwell, T. B., Spiro, D. J., and Denison, M. R. (2010) PLoS Pathog. 6(5): e1000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ogando, N. S. , Ferron, F. , Decroly, E. , Canard, B. , Posthuma, C. C. , and Snijder, E. J . (2019) Front. Microbiol. 10, 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smith, E. C. , Blanc, H. , Vignuzzi, M. , and Denison, M. R . (2013) PLoS Pathog. 9(8): e1003565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ren, L. L. , Wang, Y. M. , Wu, Z. Q. , Ren, L. L., Wang, Y. M., Wu, Z. Q., Xiang, Z. C., Guo, L., Xu, T., Jiang, Y. Z., Xiong, Y., Li, Y. J., Li, X. W., Li, H., Fan, G. H., Gu, X. Y., Xiao, Y., Gao, H., Xu, J. Y., Yang, F., Wang, X. M., Wu, C., Chen, L., … Wang, J. W. (2020) Chin. Med. J. [Google Scholar]
- 48. Schett, G. , Sticherling, M. , Schett, G., Sticherling, M., and Neurath, M. F. (2020) Nat. Rev. Immunol. 20, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang, C. , Wang, Y. , Li, X. , Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., Cheng, Z., Yu, T., Xia, J., Wei, Y., Wu, W., Xie, X., Yin, W., Li, H., Liu, M., Xiao, Y., … Cao, B. (2020) China Lancet. 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lei, J. , Li X., Qi, X. , Lei, J., Li, J., Li, X., and Qi, X. (2020) Radiology ; 200236. [Google Scholar]
- 51. Li1, W. , Zhang, C. , Sui, J. , Li, W., Zhang, C., Sui, J., Kuhn, J. H., Moore, M. J., Luo, S., Wong, S. K., Huang, I. C., Xu, K., Vasilieva, N., Murakami, A., He, Y., Marasco, W. A., Guan, Y., Choe, H., and Farzan, M. (2005) EMBO J. 24, 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wrapp, D. , Wang, N. , Jory, A. , Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., Abiona, O., Graham, B. S., and McLellan, J. S. (2020) Science 367, 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Silvio, s. (2020) Lancet, 8, e18. [Google Scholar]
- 54. Singh, S. P. , Pritam, M. , Thakur, B. P. and Yadav, P. (2020) J. Med. Virol. 1–25. [Google Scholar]
- 55. Vashist, S. K . (2020) Diagnostics (Basel). 10(4):E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Corman, V. M. , Landt, O. , Kaiser, M. , Corman, V. M., Landt, O., Kaiser, M., Molenkamp, R., Meijer, A., Chu, D. K., Bleicker, T., Brünink, S., Schneider, J., Schmidt, M. L., Mulders, D. G., Haagmans, B. L., van der Veer, B., van den Brink, S., Wijsman, L., Goderski, G., Romette, J. L., Ellis, J., Zambon, M., Peiris, M., … Drosten, C. (2020) Euro Surveill. 25(3): 2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vincent, J. L. and Taccone, F. S. (2020) Lancet, 8, 430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Belouzard, S. , Chu, V. C. , and Whittaker, G. R . (2009) Proc. Natl. Acad. Sci. USA; 106, 5871–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. CDC test for COVID 19 . https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html. Accessed 25th September 2020
- 60. Haiou, L.i , Zhou, Y. , Zhang, M. , Li, H., Zhou, Y., Zhang, M., Wang, H., Zhao, Q., and Liu, J. (2020) Antimicrob. Agents Chemother., 64, e00483–e00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Balachandar, V. , Mahalaxmi, I. , Balachandar, V., Mahalaxmi, I., Kaavya, J., Vivekanandhan, G., Ajithkumar, S., Arul, N., Singaravelu, G., Senthil Kumar, N., and Mohana Dev, S. (2020) Eur. Rev. Med. Pharmacol. Sci. 24, 3422–3425. [DOI] [PubMed] [Google Scholar]
- 62. Balamurugan, S. , Konlavat, S. , Kittikhun, W. , and Waranyoo, P. (2020) Asian Pac. J. Allergy Immunol. ; 38, 10–18.32134278 [Google Scholar]
- 63. Ambrosino, I . (2020) Monaldi Arch. Chest Disease; 90, 1389. [DOI] [PubMed] [Google Scholar]
- 64. Pinto, D. , Park, Y. J. , Beltramello, M , Pinto, D., Park, Y. J., Beltramello, M., Walls, A. C., Tortorici, M. A., Bianchi, S., Jaconi, S., Culap, K., Zatta, F., De Marco, A., Peter, A., Guarino, B., Spreafico, R., Cameroni, E., Case, J. B., Chen, R. E., Havenar‐Daughton, C., Snell, G., Telenti, A., Virgin, H. W., … Corti, D. (2020) Nature 583, 290–295. [DOI] [PubMed] [Google Scholar]
- 65. Thomas, K. , LaFraniere, S. , Weiland, N. , Goodnough, A. , and Haberman, M. (2020) F.D.A. Clears Pfizer Vaccine, and Millions of Doses Will Be Shipped Right Away. The New York Times. Retrieved 12 December 2020. [Google Scholar]
- 66. https://www.nationalgeographic.com/science/health-and-human-body/human-diseases/coronavirus-vaccine-tracker-how-they-work-latest-developments-cvd/, Accessed 5th January 2021.
- 67. https://www.thehindubusinessline.com/economy/covid-19-vaccines-for-india-but-at-what-cost/article32708600.ece, Accessed 5th January 2021.
- 68. https://www.zyduscadila.com/public/pdf/pressrelease/Zydus_Cadila_launches_a_fast_tracked_programme_to_develop_vaccine_for_the_novel_coronavirus_2019-nCoVCOVID-19).pdf, Accessed 5th January 2021.
- 69. https://www.republicworld.com/india-news/general-news/sii-to-produce-an-additional-100-million-covid-19-vaccine-dosages-for.html, Accessed 5th January 2021.
- 70. https://www.pharmaceutical-technology.com/news/biological-e-covid-vaccine-deals/, Accessed 5th January 2021.
- 71. https://www.bharatbiotech.com/covaxin.html, Accessed 5th January 2021.
- 72. https://timesofindia.indiatimes.com/business/india-business/indian-immunologicals-joins-hands-with-australias-griffith-university-to-develop-covid-19-vaccine/articleshow/75027609.cms, Accessed 5th January 2021.
- 73. https://economictimes.indiatimes.com/small-biz/startups/newsbuzz/mynvax-iisc-incubated-startup-looks-at-covid-19-vaccine-in-18-months/articleshow/75873139.cms. Accessed 5th January 2021
- 74. https://www.who.int/docs/default-source/coronaviruse/protocol-v2-1.pdf?sfvrsn=a9ef618c_2, Accessed 5th January 2021.
- 75. Gohl, D. M. and Garbe, J. , (2020) Biorxiv. 10.1101/2020.05.11.088724. [DOI] [Google Scholar]


