Abstract
The literature on COVID‐19‐related thyroid complications has accumulated over the past year or so as the pandemic has accelerated throughout the world. In particular, several recent case reports have been published describing a possible correlation between COVID‐19 disease and subacute thyroiditis (SAT). In this review, we briefly present one of our own patients and review the current published literature in this area up to January 2021, including analyses of major series of thyroid function tests in patients with significant COVID‐19 infection. We conclude that while the great majority of patients with severe COVID‐19 infection may show manifestations of the sick euthyroid syndrome, clinicians should be aware of the possibility of SAT, especially in the early weeks and months following even mild COVID‐19 infection.
Keywords: COVID‐19, Thyroiditis, Thyrotoxicosis, Viral thyroiditis
1. INTRODUCTION
The COVID‐19 pandemic has affected all aspects of our lives worldwide, causing a tremendous strain on healthcare services, affecting directly and indirectly the course and treatment of many common illnesses. Each medical specialty continues supporting their patients with COVID‐19‐ related illnesses and their consequences, 1 , 2 , 3 , 4 , 5 , 6 , 7 and several recent authoritative reviews have been published in specific areas of endocrinology. 1 , 3 , 4 , 6 , 7 , 8 , 9 , 10
At the beginning of this year, most authors were trying to translate experience from the SARS‐CoV pandemic from 2002 to COVID‐19, as few data were available on COVID‐19 itself. In terms of thyroid function and COVID‐19, management strategies for thyroid disease have been published 11 and we have reviewed earlier data based on publications up to March 2020. 10 However, over recent months the number of COVID‐19‐related papers has greatly increased, principally based on case reports as well as retrospective analyses. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 We now review the current situation, beginning with an illustrative case history of one of our patients, and emphasizing the apparent relationship between COVID‐19 infection and subacute thyroiditis (SAT).
1.1. Case summary
A 57‐year‐old Caucasian lady presented in July 2020 for a remote thyroid review to one of us (DD): Her referral was triaged before accepting for a remote review (confirming that a face‐to‐face consultation was not required), and her case was found to be safe for a remote consultation.
In February 2020, she went skiing having previously been healthy. It was highly probable that she contracted COVID‐19 at the beginning of March 2020, as she met with a friend who suffered from COVID‐19 disease, and subsequent to that she herself developed problems with a loss of smell and taste and also the new onset of a severe dry cough. At this time, routine COVID‐19 testing was not available in the UK, but this does appear in retrospect to be the likely diagnosis.
In May 2020, she had noticed pain in her anterior neck, in the region of the thyroid, radiating to the jaw and to the head. She also noticed a neck swelling, and she was referred to a local hospital by her GP for an urgent review. A thyroid ultrasound was arranged for her: It suggested possible thyroiditis on a background of a multinodular goitre (excluding thyroid cancer), and she was discharged back to her GP.
She continued to have neck pain accompanied by a dry cough and a feeling as if she was needing to continually clear her throat. She started to feel tired and gain weight, but simultaneously felt hot, sweaty and constipated, had mood swings, was irritable, and had tremors and palpitations. All of these symptoms were absolutely new to her. Her GP arranged for assessment of thyroid function tests, which were consistent with thyroid over‐activity. For the severe neck pain, she was treated with Ibuprofen 200mg three times per day and paracetamol 1g three times per day.
We confirmed biochemical thyrotoxicosis, with positive anti‐TPO and anti‐Tg antibodies, while her TSH‐R antibodies were negative. Her COVID‐19 antibodies were positive.
Her thyroid ultrasound (Figure 1) showed a normal‐sized thyroid with patchy areas of variably reduced parenchymal echogenicity bilaterally, with typical ultrasound features of a subacute (‘de Quervain's’) thyroiditis, which was more extensive on the left than the right. The thyroid was tender during the examination. There were some spared areas with normal echogenicity, and two benign looking (U2) hyperechoic nodules measuring 6mm inferiorly on the right and 7mm superiorly on the left. Reactive left paratracheal lymph nodes were noted without pathological nodes elsewhere in the neck.
FIGURE 1.
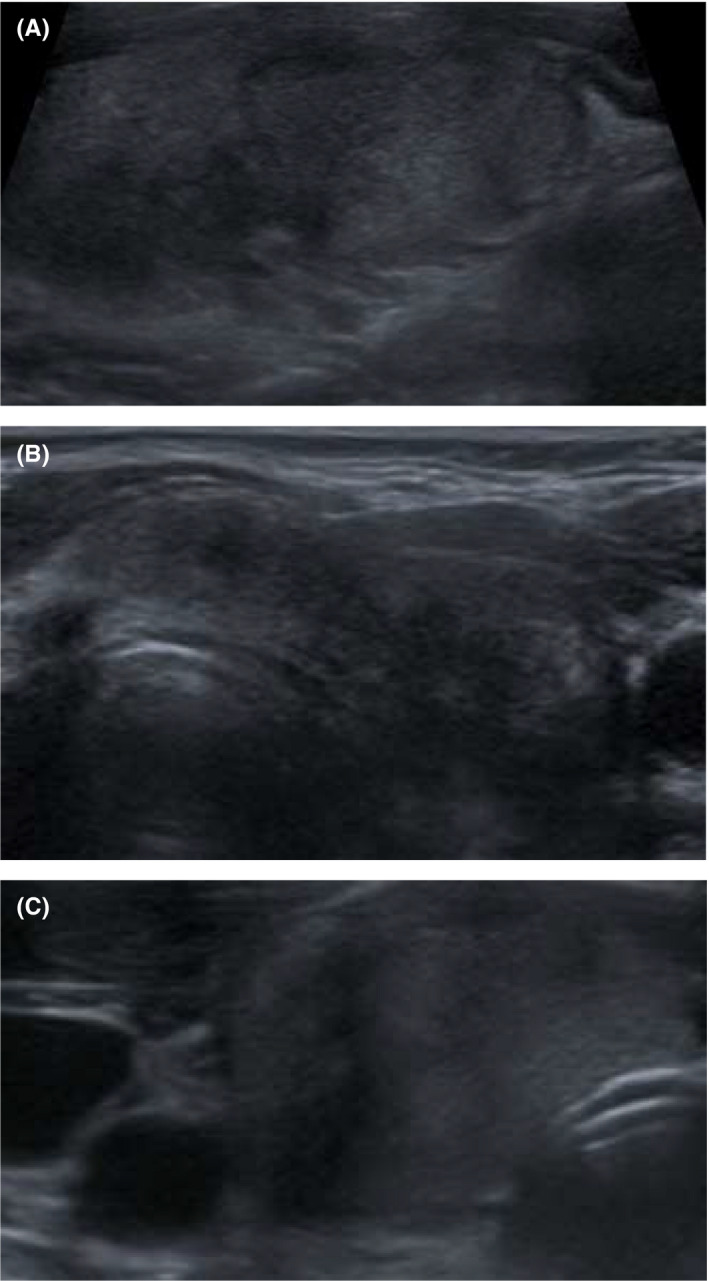
Thyroid ultrasonography, showing 3 images. The thyroid showed patchy areas of variable reduced echogenicity in the parenchyma bilaterally, with typical ultrasound features of a subacute (de Quervain's) thyroiditis. The thyroid was tender during examination
Her technetium pertechnetate radionuclide thyroid scan (Figure 2) showed poor tracer uptake within the neck and thyroid bed. The thyroid gland was not clearly visualized. Background activity was increased. The uptake function was reported at 0.1% (normal range 0.4%‐4%) and was compatible with subacute thyroiditis (SAT).
FIGURE 2.

Technetium pertechnetate radionuclide thyroid scan, showing 4 images (2(A) anterior view, 2(B) anterior view with a marker, 2(C) right anterior, 2(D) left anterior view). Poor tracer uptake within the neck and thyroid bed. The thyroid gland not clearly visualised. Background activity increased. The uptake function reported at 0.1% (normal range 0.4%‐4%) compatible with subacute thyroiditis (SAT)
We considered corticosteroid (‘steroid’) therapy, but she was not keen to start them due to the COVID‐19 pandemic and preferred to manage her symptoms with simple analgesics and propranolol. Her thyroid function tests settled over a period of six weeks and she returned to the care of her GP. During follow‐up in primary care, she developed subclinical hypothyroidism, and low‐dose thyroxine treatment was started with 25ug daily.
1.2. COVID‐19 and thyroiditis
In this case report, we demonstrate that SAT seemed to follow infection with COVID‐19, and as expected was self‐limiting. It also illustrates how the delivery of health care has changed during the current pandemic (remote vs. face‐to‐face), possibly in a manner which will out‐live the current situation.
At present, the effect of acute COVID‐19 on thyroid function in a population with no pre‐existing thyroid disease is yet to be fully determined, as the reports are conflicting between thyrotoxicosis, euthyroidism and suppression of thyroid function. 12 , 13 , 14 , 15
Recently, several case reports and series have been published on COVID‐19‐related SAT. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Other papers provide some insight into thyroid complications in or followed by COVID‐19 disease including Graves’ disease, 25 , 26 Hashimoto's disease, 27 atypical thyroiditis 28 and ‘painless thyroiditis’, 29 as well as post‐partum thyroiditis following COVID‐19. 30
Lania et al conducted a single‐centre retrospective study in 287 consecutive patients (193 males, median age: 66 years, range: 27‐92y) hospitalized for COVID‐19 in non‐intensive care units. They reported that 58 patients (20.2%) were found with thyrotoxicosis (overt in 31 cases), 15 (5.2%) with hypothyroidism (overt in only 2 cases), and 214 (74.6%) with normal thyroid function. Subsequently, they concluded that COVID‐19 may be associated with a high risk of thyrotoxicosis in relation to systemic immune activation induced by the SARS‐CoV‐2 infection. 14 By contrast, Khoo et al 12 conducted an observational study and concluded that most patients with COVID‐19 present with euthyroidism. They observed mild reductions in TSH and fT4 in keeping with a non‐thyroidal illness syndrome. In that study, in COVID‐19 survivor thyroid function tests at follow‐up returned to baseline. This cohort observational study included adult patients admitted to hospital with suspected COVID‐19, excluding not only those with pre‐existing thyroid disease but also those missing either free thyroxine (fT4) or TSH measurements. Of 456 patients, 334 had COVID‐19 and 122 did not. Most patients (86.6%) presenting with COVID‐19 were euthyroid, with none presenting with overt thyrotoxicosis. Patients with COVID‐19 had a lower admission TSH and fT4 compared to those without COVID‐19. In the COVID‐19 patients with matching baseline thyroid function tests from 2019 (n = 185 for TSH and 104 for fT4), both TSH and fT4 were reduced on admission compared to baseline. In a complete‐cases analysis of COVID‐19 patients with TSH measurements at follow‐up, admission and baseline (n = 55), TSH was seen to recover to baseline at follow‐up. 12
Chen et al retrospectively evaluated thyroid function in patients with COVID‐19 and also suggested a clinical picture similar to the sick euthyroid syndrome. They analysed 50 patients with COVID‐19 who had no previous thyroid problems prior to COVID‐19 infection: thyroid function tests were assessed during the active infection and following recovery. They found a transient decease in TSH and fT3 whose severity corresponded to the severity of illness; however, the tests normalized after recovery. In particular, they found that TSH below the normal range was present in 56% (28/50) of the patients with COVID‐19. The levels of TSH and serum total triiodothyronine (TT3) of the patients with COVID‐19 were significantly lower than those of the healthy control group and non‐COVID‐19 pneumonia patients. The more severe the COVID‐19, the lower were the levels of TSH and TT3 (P <.001). The total thyroxine (TT4) level of the patients with COVID‐19 was not significantly different from the control group. None of the patients received thyroid hormone replacement therapy. On recovery, no significant differences in TSH, TT3, TT4, free triiodothyronine (fT3) or free thyroxine (fT4) levels were found between the COVID‐19 and control groups. 13
The fact that the serum TSH levels of the patients with COVID‐19 were significantly lower in the severe and critical group compared with non‐COVID‐19 pneumonia patients with a similar degree of severity indicates that there might be a unique effect of COVID‐19 on TSH‐secreting cells. Therefore, Chen et al suggested two possible mechanisms that might account for these changes, similar to hypotheses suggested before regarding SARS‐CoV infection. 10 , 13 One is a direct viral effect on the pituitary cells, another is an indirect effect of various systemic changes mediated by activation of various pro‐inflammatory cytokines caused by the virus infection. 13 , 31 It is worth mentioning that in the Chen et al study the thyroid hormones were tested while most patients (31/50) were receiving glucocorticoids. Therefore, excluding the effect of hormonal changes in the pituitary–endocrine axis feedback loops may be difficult. 13
Looking at individual case studies (summarized in Table 1), Brancatella et al presented 5 cases of SAT in relation to COVID‐19. 16 , 17 The main symptoms included neck pain, fever and fatigue. In these patients, all had ultrasonographic features of SAT. One patient underwent a radionuclide technetium scan which also confirmed SAT, nearly all presented with thyrotoxicosis on a background of no previous thyroid issues, except for one patient. All patients were treated with either corticosteroids or anti‐inflammatory agents and returned to full health. One case needed levothyroxine replacement therapy. In these cases, neck pain presented 15‐36 days after COVID‐19 symptom resolution. 16 , 17
TABLE 1.
Biochemistry. Patient presented with mild biochemical thyrotoxicosis on the background of positive anti‐TPO and anti‐Tg antibodies but negative TSH‐R antibodies. CRP and ESR were mildly elevated but her full blood count (FBC) and liver function tests (LFTs), as well as the renal profile were within normal limits. COVID‐19 IgG antibodies were positive
|
11.06.2020 a |
07.07.2020 | 17.07.2020 | |
|---|---|---|---|
| TSH |
0.1miu/L (normal range 0.27‐4.2) |
<0.01mIU/L (normal range 0.35‐4.94) |
<0.01mIU/L (normal range 0.35‐4.94) |
| fT4 |
21.2pmol/L (normal range 12‐22) |
23.4pmol/L (normal range 9‐19) |
20.8pmol/L (normal range 9‐19) |
| fT3 | ‐ |
8.5pmol/L (normal range 2.9‐4.9) |
5.6pmol/L (normal range 2.9‐4.9) |
| Anti‐Tg Abs | ‐ |
6.61 IU/mL (normal range 0‐4.11) |
‐ |
| Anti‐TPO Abs | ‐ |
71.80 IU/mL (normal range 0‐5.61) |
‐ |
| TSH‐R Abs | ‐ |
<0.80 IU/L (normal range 1.51‐3) |
‐ |
| ESR |
22mm/hr (normal range 0‐19) |
24mm/hr (normal range 0‐19) |
|
| CRP |
16mg/L (normal range 0‐5) |
15.3mg/L (normal range 0‐5) |
5mg/L (normal range 0‐5) |
| LFTs | normal | normal | ‐ |
| Renal profile | normal | ||
| FBC | normal | normal | normal |
| COVID‐19 IgG (Abbott) | ‐ | positive | ‐ |
Abbreviations: thyroid‐stimulating hormone (TSH), free thyroxine (fT4), free triiodothyronine (fT3), thyroid peroxidase antibodies (Anti‐TPO Abs), thyroglobulin antibodies (Anti‐Tg Abs), TSH receptor antibodies (TSH‐R Abs), C‐reactive protein (CRP), full blood count (FBC), and liver function tests (LFT).
Result from GP at the time of the referral (please note some differences between normal ranges between laboratories where the blood tests were conducted).
Mattar et al reported a case of a hospitalized patient with acute COVID‐19 who as an in‐patient developed SAT and presented with tachycardia, anterior neck pain and thyroid function tests revealing hyperthyroidism, together with consistent ultrasonographic evidence suggesting SAT. He did not have any previous history of thyroid disease but had a positive family history for thyroid disease. Biochemically, he presented with elevated both fT4 and fT3 on a background of negative TPO/Tg and TSH‐R antibodies. Treatment with corticosteroids (20mg prednisolone daily, jointly with atenolol 25mg od) resulted in rapid clinical resolution. He was reviewed at 10 weeks after discharge when his corticosteroids were stopped as he was now found to have normal thyroid function tests and was asymptomatic. 18
Ippolito et al presented a case of COVID‐19‐related SAT that developed on a background of a longstanding non‐toxic nodular goitre with a dominant benign nodule. In that case, initial treatment consisted of methimazole which was changed to corticosteroids following the results of diagnostic imaging (ultrasound and technetium scanning) confirming SAT. 21
The diagnostic dilemmas in our SAT case related to the fact that our patient developed COVID‐19‐related SAT on a background of not only autoimmune‐thyroiditis (AIT) but also a multinodular goitre with a dominant nodule. Both of her TPO and Tg antibodies were positive, confirming AIT, whereas her TSH‐R antibodies were negative, possibly suggesting Hashitoxicosis as a background process leading to thyroid over‐activity.
Thyroid ultrasound can be very helpful in confirming a diagnosis. 32 Scans invariably show densely hypoechoic patchy areas of parenchyma with ill‐defined margins interspersed between normal areas of spared thyroid parenchyma. The thyroid may be enlarged and there is peri‐thyroid oedema in the overlying strap muscles. The patient may experience tenderness over the thyroid during the scan. The appearances are usually bilateral and asymmetric, but the extent of parenchymal changes frequently correlates well with the main sites of tenderness. Extensive SAT can be confused with established AIT on ultrasound, although in the former the presence of neck tenderness can be helpful in directing towards the correct diagnosis. Additionally, patchy SAT can be mistaken for multifocal malignancy due to the irregular hypoechoic nature of the parenchymal changes. 32 , 33 , 34 , 35
The most common treatment in COVID‐19‐induced SAT included short‐term corticosteroids, β‐adrenoceptor blockers as well as analgesics. 16 , 17 , 18 In one case combination treatment of hydroxychloroquine and steroids was reported, 20 although hydroxychloroquine is not currently a recommended therapy. Steroids were used by other authors reporting COVID‐19‐related SAT for different reasons and timelines. In some cases, they were used from the disease onset, in some following revision of the diagnosis. In some cases (ours included), steroids were not used as the disease was mild and the patient declined this therapy.
Subacute thyroiditis (SAT) is a clinical diagnosis of an inflammatory thyroid disorder thought to be of viral origin, generally preceded by an upper respiratory tract infection. Since the disorder is self‐limiting, it is frequently under‐diagnosed. However, the disease should not be overlooked since the associated thyrotoxicosis may worsen the clinical course of concomitant disorders (eg, respiratory distress) and long‐term sequelae, such as autoimmune hypothyroidism. 19 Evidence for viral infection in SAT has been linked to mumps virus, coxsackievirus, adenovirus, Epstein‐Barr virus, rubella and cytomegalovirus, though a specific viral cause is not always found. 18 , 36 It would appear that SARS‐CoV‐2 should be added to the list of viruses causing SAT. As this is still relatively new disease, it is too early to definitively say if COVID‐19 related SAT differs from other viral‐induced SAT in terms of severity, recovery and longer‐term sequelae. Our own experience though indicates a possible overlap between this type of post‐viral thyroiditis and autoimmune thyroid disease particularly with Grave's disease.
Very recently, a single‐blind, randomized controlled study addressing short‐term or 6‐week prednisolone use in the treatment of non‐COVID‐19‐related SAT has been published (patients enrolled to the trial developed SAT between 2013 and 2014). The study showed fewer side effects of glucocorticoids but similar efficacy and recurrence rates with short‐term prednisone compared with the 6‐week treatment for SAT. The authors concluded that short‐term prednisone with a better safety profile may be an alternative strategy for ameliorating moderate‐to‐severe symptoms of SAT. 37 However, in our experience we would generally recommend steroids in the treatment of COVID‐19‐related SAT when appropriate and safe, after discussing with the patient the risks and benefits of this therapy. However, we would start with non‐steroidal anti‐inflammatory drugs and possibly propranolol, and reserve steroids for patients with severe persisting pain.
From a pathophysiological standpoint, previous studies examining the pathology of the thyroid in SARS proposed several mechanisms of thyroid organ damage that include host immune over‐reaction, immune deficiency related to infection, destruction of lymphocytes, inhibition of the innate immune response and direct cellular destruction with apoptosis playing a key role (summarized by Mattar et al). 18 A recent study also suggested a possible genetic predisposition to SAT in association with not only HLA‐B*35 but also HLA‐B*18:01, HLA‐DRB1*01 and HLA‐C*04:01. 38 Much has been discussed regarding ACE‐2, which is key to the mechanism of SARS‐CoV‐2 infection with the virus using it as a host cell receptor to invade human cells. Studies based on SARS‐CoV‐2 in 2020 have shown that ACE‐2 expression levels are highest in the thyroid compared to other organs, such as the small intestine, kidneys, heart and adipose tissue, which does suggest a plausible mechanism for the pathophysiology of thyroiditis in COVID‐19 (summarized by Mattar et al). 18
The main challenge with SAT during the COVID‐19 pandemic is a potential need to use corticosteroids; however, in nearly all case reports SAT occurred some months after COVID‐19 illness. As noted above, retrospective studies actually showed that thyroid function tests during active COVID‐19 disease remain either normal or similar to the sick euthyroid syndrome.
A new manner of care delivery via remote consultations has become extremely popular (over the telephone or video) after initial endorsing (when appropriate) by the UK General Medical Council (https://www.gmc‐uk.org/ethical‐guidance/ethical‐hub/remote‐consultations) (Figure 3) as well as the European Society for Endocrinology. 1 , 5 , 6 , 10 Our patient was initially referred within the ‘two‐week cancer pathway’ but once cancer was excluded, she was referred and accepted for the remote consultation, which seemed safe in that case scenario. Nevertheless, investigations such as ultrasound and radionuclide scanning do require a patient visit, with implications for personal protective equipment and infection control in service departments during a period of reduced access to radiology (secondary to redeployment and staff absences). 39 , 40
FIGURE 3.
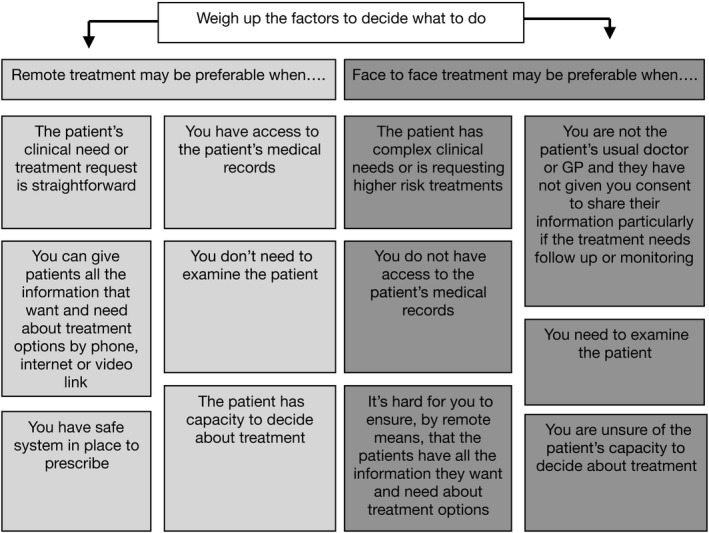
UK General Medical Council guidelines for remote consultations (adapted) (https://www.gmc‐uk.org/ethical‐guidance/ethical‐hub/remote‐consultations)
2. SUMMARY
A series of case reports of COVID‐19‐related subacute thyroiditis suggest that SAT associated with viruses such as SARS‐CoV‐2 should be recognized as a complication of COVID‐19 and should be considered as a differential diagnosis when acutely infected patients present with tachycardia without evidence of progression of COVID‐19 illness. 18 It should also be considered if patients with a previous medical history of COVID‐19 present with neck pain and new onset of thyroid function alterations, regardless of positive or negative history of previous problems or family history of thyroid issues. However, routine thyroid function tests screening in a population of COVID‐19 patients in an acute phase of the disease may not be necessary, as such patients generally show a severe variant of the sick euthyroid syndrome. The incidence of SAT following COVID‐19 remains unclear, and clearly, there is selective reporting, although many patients may remain undiagnosed. The current review suggests that clinicians should be alert to this self‐limiting condition, which in general simply requires consideration of 03B2‐adrenoceptor blockade as well as non‐steroidal anti‐inflammatory agents, and prednisolone for more severe or persisting pain, but future studies are needed to develop more precise clinical guidelines.
CONFLICT OF INTEREST
Nothing to declare.
3.
TABLE 2.
Summary of case reports on subacute thyroiditis (SAT) and COVID‐19
| Age Gender Origin | Thyroid‐related biochemistry (TFTs and antibodies) | Timeline from COVID‐19 disease | Imaging (thyroid US and NM Tc 99‐m scan) | Symptoms of SAT | Treatment of SAT and subsequent follow‐up | PMH of thyroid issues | |
|---|---|---|---|---|---|---|---|
| (Brancatella, Ricci, Viola, et al, 2020) | 18 Caucasian female | Thyrotoxicosis TgAb (‐)TPOAb (‐)TRAb (‐) | 15 days after a PCR + swab | Thyroid US: bilateral and diffuse hypoechoic areas. | Fever, neck pain radiated to the jaw, palpitations | Symptoms improved within 1 week after steroid initiation, and thyroid function and biochemistry normalized in 40 days. | none |
| (Campos‐Barrera, Alvarez‐Cisneros, & Davalos‐Fuentes, 2020) | 37 Mexican female | thyrotoxicosis TgAb (‐)TPOAb (‐)TRAb (‐) | A month after initial COVID‐19 presentation and PCR + swab | NM Tc 99‐m scan: no uptake in the thyroid | Severe neck pain radiating to the jaw and ear, fatigue | Patient was treated with steroids. During her follow‐up visit one month after the SAT diagnosis, patient has remained asymptomatic, but her lab tests were still altered with anaemia, thrombocytopenia, high ESR, and low TSH. | none |
| (Ippolito, Dentali, & Tanda, 2020) | 69 Caucasian female | thyrotoxicosis TgAb (‐)TPOAb (‐)TRAb (‐) | Five days after the diagnosis of COVID‐19 related pneumonia | Thyroid US: enlarged hypoechoic thyroid with decreased vascularity. NM Tc 99‐m scan: no uptake in the thyroid | Palpitations, insomnia, agitation | Patient was initially given methimazole, but the thyrotoxicosis worsened. Subsequently she was given steroids, and after 10 days, all laboratory findings and symptoms improved. | non‐toxic MNG with a dominant benign nodule |
| (Ruggeri, Campenni, Siracusa, Frazzetto, & Gullo, 2020) | 43 Caucasian female | thyrotoxicosis TgAb (‐)TPOAb (‐)TRAb (‐) | Six weeks after the onset of the upper respiratory tract infection | Thyroid US: diffusely enlarged and hypoechogenic thyroid gland. NM Tc 99‐m scan: markedly reduced thyroid uptake | Anterior neck pain, fatigue, tremors, palpitations | Patient was managed with oral steroids, which were gradually tapered. A progressive resolution of symptoms was observed and within 4 weeks, TFTs and inflammatory markers normalized. | none |
| (Chong, Shkolnik, Saha, & Beegle, 2020) | 37 Asian male | ThyrotoxicosisTgAb (‐)TPOAb (‐)TSI (‐) | One month after COVID‐19 illness | Thyroid US: diffusely heterogeneous echotexture, thyroiditis | Anterior neck pain, fatigue, chills, palpitation, heat intolerance, anorexia, unintentional weight loss | Patient was managed initially with steroids, aspirin and propranolol. Once patient developed hypothyroidism, levothyroxine was started. Aspirin and propranolol were discontinued due to the resolution of neck pain and palpitations. | none |
| (Ruano, Zorzano‐Martinez, Campos, Rius, & Hernandez, 2020) | 28 Caucasian female | Thyrotoxicosis TgAb (‐)TPOAb (‐)TSI (‐) | One month after initial COVID‐19 symptoms | NM Tc 99‐m scan: no uptake in the thyroid | Fever, neck pain irradiated to the jaw, sore throat, palpitations, severe asthenia | Patient was treated with aspirin and propranolol. Symptoms improved within 24 hours, with a total relief in 2 weeks. | none |
| (Mattar, Koh, Rama Chandran, & Cherng, 2020) | 34 Asian male | Thyrotoxicosis TgAb (‐)TPOAb (‐)TRAb (‐) | on day 9‐10 of COVID‐19 illness | Thyroid US: enlarged thyroid with heterogeneous echotexture. Both lobes with hypoechoic areas with ill‐defined margins corresponding to the hard regions palpable. Colour flow Doppler showed reduced blood flow in both lobes. | Tachycardia, anterior neck pain | Treatment with corticosteroids and beta‐blockers resulted in rapid clinical resolution. Patient was reviewed after 10 weeks in the outpatient clinic after completion of tapering course of steroids and was clinically well with no symptoms and normal TFTs. | none |
| (Asfuroglu Kalkan & Ates, 2020) | 41 Caucasian women | Thyrotoxicosis TgAb (+)TPOAb (+)TRAb (‐) | During active COVID‐19 | Thyroid US: a relative diffuse decrease of vascularity and parenchyma heterogeneity | Feverneck pain | Prednisolone daily with significant improvement. Patient was discharged on prednisolone tapering dose for 4 weeks with outpatient follow‐up. | none |
| (Brancatella, Ricci, Cappellani, et al, 2020) | four Caucasian females aged 29‐46 years | TFTs available in three cases suggestive of destructive thyroiditis | 16 to 36 days after resolution of COVID‐19 | Thyroid US: enlarged thyroid with diffuse and bilateral hypoechoic areas and (in three patients) absent vascularization at colour Doppler.NM Tc 99‐m scan: absent thyroid uptake (completed in one patient) | Neck pain radiated to the jaw, palpitations, fever, asthenia, atrial fibrillation | Symptoms disappeared a few days after commencement of treatment (prednisone in three patients and ibuprofen in one). Six weeks after the onset of SAT all patients were asymptomatic and inflammatory markers had turned back to the normal range. Two patients were euthyroid while two were diagnosed with subclinical hypothyroidism. |
three patients none one patient non‐toxic goitre |
Abbreviations: thyroid ultrasonography (thyroid US), nuclear medicine thyroid uptake scan using Tc 99‐m (NM Tc 99‐m scan), thyroid function tests (TFTs), previous medical history of thyroid issues (PMH of thyroid issues), polymerase chain reaction (PCR).
Dworakowska D, Morley S, Mulholland N, Grossman AB. COVID‐19‐related thyroiditis: A novel disease entity?. Clin Endocrinol (Oxf). 2021;95:369–377. 10.1111/cen.14453
Funding information
No financial support was provided for this study.
REFERENCES
- 1. Puig M, Marazuela M, Giustina A. COVID‐19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68(1):2‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID‐19. 2020. [DOI] [PMC free article] [PubMed]
- 3. Bozkurt B, Kovacs R, Harrington B. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID‐19. 2020. [DOI] [PMC free article] [PubMed]
- 4. de Simone G. European Society of Cardiology . Position statement of the ESC Council on Hypertension on ACE‐inhibitors and angiotensin receptor blockers. 2020.
- 5. Dworakowska D, Grossman AB. Renin‐angiotensin system inhibitors in management of hypertension during the COVID‐19 pandemic. J Physiol Pathol. 2020;71(2):173‐178. [DOI] [PubMed] [Google Scholar]
- 6. Filetti S. Editorial: The COVID‐19 pandemic requires a unified global response. Endocrine. 2020;68(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaiser UB, Mirmira RG, Stewart PM. Our Response to COVID‐19 as Endocrinologists and Diabetologists. JCEM. 2020;105(5):1299‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pal R, Bhadada SK. Managing common endocrine disorders amid COVID‐19 pandemic. Diabetes Metab Syndr. 2020;14(5):767‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thangaratinam S, Cooray SD, Sukumar N, et al. Endocrinology in the time of COVID‐19: Diagnosis and Management of Gestational Diabetes Mellitus. Eur J Endocrinol. 2020;183(2):G49‐G56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dworakowska D, Grossman AB. Thyroid disease in the time of COVID‐19. Endocrine. 2020;68(3):471‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boelaert K, Visser WE, Taylor PN, Moran C, Leger J, Persani L. Endocrinology in the time of COVID‐19: management of hyperthyroidism and hypothyroidism. Eur J Endocrinol. 2020;183(1):G33‐G39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khoo B, Tan T, Clarke SA, et al. Thyroid function before, during and after COVID‐19. J Clin Endocrinol Metab. 2020;106(2):e803‐e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen M, Zhou W, Xu W. Thyroid Function Analysis in 50 Patients with COVID‐19: a Retrospective Study. Thyroid. 2020;31(1):8‐11. [DOI] [PubMed] [Google Scholar]
- 14. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID‐19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou R, Wu C, Zhang S, et al. Euthyroid sick syndrome in patients with COVID‐19. Front Endocrinol (Lausanne). 2020;11:566439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brancatella A, Ricci D, Cappellani D, et al. Is Subacute Thyroiditis an underestimated manifestation of SARS‐CoV‐2 infection? insights from a case series. J Clin Endocrinol Metab. 2020;105(10):e3742‐e3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute Thyroiditis After Sars‐COV‐2 Infection. J Clin Endocrinol Metab. 2020;105(7):2367‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute thyroiditis associated with COVID‐19. BMJ Case Rep. 2020;13(8):e237336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruggeri RM, Campenni A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS‐COV‐2: an endocrine complication linked to the COVID‐19 pandemic. Hormones (Athens). 2020;20(1):219‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid‐19 infection. J Endocrinol Invest. 2020;43(8):1173‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ippolito S, Dentali F, Tanda ML. SARS‐CoV‐2: a potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Invest. 2020;43(8):1171‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campos‐Barrera E, Alvarez‐Cisneros T, Davalos‐Fuentes M, Usui T. Subacute Thyroiditis Associated with COVID‐19. Case Rep Endocrinol. 2020;2020:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chong WH, Shkolnik B, Saha B, Beegle S. Subacute Thyroiditis in the Setting of Coronavirus Disease 2019. Am J Med Sci. 2020:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruano R, Zorzano‐Martinez M, Campos A, Rius F, Hernandez M. Subacute thyroiditis might be a complication triggered by SARS‐CoV‐2. Endocrinol Diabetes Nutr. 2020:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ippolito S, Piantanida E, Tanda ML, Caturegli P. Graves' disease insights from a review of the Johns Hopkins surgical pathology archive. J Endocrinol Invest. 2020;43(10):1519‐1522. [DOI] [PubMed] [Google Scholar]
- 26. Mateu‐Salat M, Urgell E, Chico A. SARS‐COV‐2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID‐19. J Endocrinol Invest. 2020;43(10):1527‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tee LY, Harjanto S, Rosario BH. COVID‐19 complicated by Hashimoto's thyroiditis. Singapore Med J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muller I, Cannavaro D, Dazzi D, et al. SARS‐CoV‐2‐related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8(9):739‐741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barahona San Millan R, Tantinya Daura M, Hurtado Ganoza A, Recasens Sala M, Painless thyroiditis in SARS‐CoV‐2 infection. Endocrinol Diabetes Nutr. 2020:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizuno S, Inaba H, Kobayashi K‐I, et al. A case of postpartum thyroiditis following SARS‐CoV‐2 infection. Endocr J. 2020.1‐4. [DOI] [PubMed] [Google Scholar]
- 31. Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015;3(10):816‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee YJ, Kim DW. Sonographic Characteristics and Interval Changes of Subacute Thyroiditis. J Ultrasound Med. 2016;35(8):1653‐1659. [DOI] [PubMed] [Google Scholar]
- 33. Nishihara E, Kudo T, Ito M, et al. Papillary thyroid carcinomas are highly obscured by inflammatory hypoechoic regions caused by subacute thyroiditis: a longitudinal evaluation of 710 patients using ultrasonography. Endocr J. 2020;67(5):569‐574. [DOI] [PubMed] [Google Scholar]
- 34. Sencar ME, Calapkulu M, Sakiz D, et al. The contribution of ultrasonographic findings to the prognosis of subacute thyroiditis. Arch Endocrinol Metab. 2020;64(3):306‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stasiak M, Michalak R, Lewinski A. Thyroid primary and metastatic malignant tumours of poor prognosis may mimic subacute thyroiditis ‐ time to change the diagnostic criteria: case reports and a review of the literature. BMC Endocr Disord. 2019;19(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chiaruzzi M, Arcani R, Mazodier K, et al. A Case of Subacute Thyroiditis Associated with Erythrovirus B19 Infection. Am J Med. 2020;133(6):e296‐e297. [DOI] [PubMed] [Google Scholar]
- 37. Duan L, Feng X, Zhang R, et al. Short‐Term Versus 6‐Week Prednisone in the Treatment of Subacute Thyroiditis: a Randomized Controlled Trial. Endocr Pract. 2020:Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 38. Stasiak M, Tymoniuk B, Michalak R, Stasiak B, Kowalski ML, Lewinski A. Subacute Thyroiditis is Associated with HLA‐B*18:01, ‐DRB1*01 and ‐C*04:01‐The Significance of the New Molecular Background. J Clin Med. 2020;9(2):534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giannoula E, Vrachimis A, Giovanella L, Chatzipavlidou V, Iakovou I. Nuclear thyroidology in pandemic times: the paradigm shift of COVID‐19. Hell J Nucl Med. 2020;23(Suppl):41‐50. [PubMed] [Google Scholar]
- 40. Koukouraki S, Kapsoritakis N, Bourogianni O. COVID‐19 pandemic: Implications for radionuclide therapy in Nuclear Medicine departments. Hell J Nucl Med. 2020;23(Suppl):31‐34. [PubMed] [Google Scholar]


