Dear Editor,
The SARS‐CoV‐2‐pneumonia is a potentially life‐threatening disease, that can lead to a high mortality rate. 1 Nevertheless, most of SARS‐CoV‐2‐cases are characterized by mild symptoms and do not require nor hospitalization nor treatment. The duration and the timing of the treatment approved for the moderate‐severe disease (antivirals and dexamethasone) are not yet fully elucidated, and the need for other treatment are yet under investigation. 2 Furthermore, some cases of COVID‐19 recurrence or relapse after a short‐term symptom free interval have been described, but few data are so far available on long‐term clinical outcome and disease progression. 3
We have recently reevaluated, 6 months after Hospital discharge, 99 patients out of a series of 272 consecutively admitted to Esine Hospital for COVID19 pneumonia between March 5th and April 5th, 2020. 4 Considering patients who died in the meanwhile, 5 these represent the 54% of all the evaluable cohort. Notably, 6 months after discharge, 53% of these patients reported persistent dyspnea or cough. At high‐resolution computed tomography (HRCT), 22% of patients showed ground glass, 26% parenchymal bands, and 7% both of these abnormalities. Fibrosis (3% honey combing, 4% septal thickening) was observed in 7% of patients. Finally, 3% of them developed a new need for long term oxygen treatment.
To discuss possible clinical scenarios, we report here the experience of three patients with nasopharyngeal swab positive for SARS‐CoV‐2 and pneumonia, admitted during the first epidemic wave, who, after initial recovery, had a clinical and radiological worsening of the lung disease. Their radiological findings are described in Figure 1. Patients 1 and 2 were included in our original descriptions of Esine cohorts. 4 , 5
Figure 1.
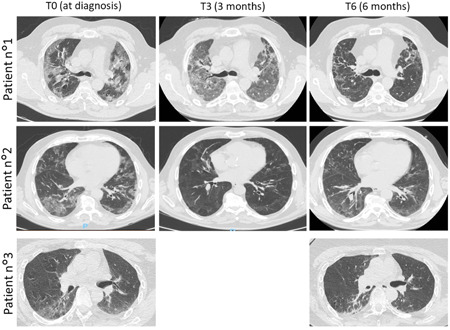
Radiological findings of the three patients at the moment of the COVID‐19 diagnosis and after 3 (T3) and 6 months (T6) of follow‐up. The chest high‐resolution computed tomography (HRCT) showed an evolution of pneumonia. Patient 1, T0: presence of 40% ground glass pneumonia, T3: presence of 70% ground glass pneumonia and septal thickening, T6: presence of honey combing; Patient 2, T0: presence of 35% ground glass pneumonia, T3: improving of ground glass and parenchyma bands, T6: presence of 35% ground glass pneumonia; Patient 3, T0: presence of 25% ground glass pneumonia, T3: not available, T6: presence of 20% ground glass pneumonia and parenchymal bands
The first patient is a 48‐year‐old healthy man, admitted (16th March) for dyspnea, cough and fever, who developed SARS‐CoV‐2‐pneumonia. He was treated with antivirals (darunavir/ritonavir), hydroxychloroquine, high‐flow oxygen in continuous positive airway pressure (C‐PAP) mode. After a quick clinical worsening, the patient was transferred in intensive care unit (ICU) for orotracheal intubation and treated with dexamethasone (20 mg/day for 10 days) and enoxaparin (anticoagulant dose). After 52 days of hospitalization, he was restored from orotracheal intubation and transferred in subintensive unit. For persistent dyspnea, prednisone was started (25 mg/day, slowly tapered to 12.5 mg/day). He was discharged on 3rd July. The symptoms ameliorated till the 31st July, when he was readmitted in ICU for fever and respiratory failure. Multi‐Resistant‐Staphylococcus‐Aureus (MRSA) was found in bronchoalveolar lavage (BAL) and antibiotic treatment was administered. Six months after the initial diagnosis, New York Heart Association (NYHA) Class II dyspnoea persisted with worsening of radiological findings. For nocturnal desaturation, C‐PAP was prescribed, together with prednisone (7.5 mg/day).
The second patient is an 80‐year‐old man, suffering from several comorbidities (Type 2 diabetes mellitus, arterial hypertension, dyslipidemia and chronic obstructive lung disease), who was admitted for respiratory failure in SARS‐CoV‐2‐pneumonia (19th March). Low‐flow oxygen mask, and off‐label treatment with colchicine (1 mg/day) and hydroxychloroquine were administered, together with prednisone (25 mg/day). Clinical conditions improved, since to no‐need for oxygen. Patient was discharged after 6 days with a short‐term mild dosage prednisone (12.5 mg/day) and colchicine prescription (1 month and 14 days far away). After 3 months clinical conditions were stable, only mild dyspnoea was reported, and HRCT showed a significant amelioration of pneumonia. At 6 months, relapse of II class NYHA dyspnoea occurred and patient was treated with prednisone 25 mg/day.
The third patient is a 43‐year‐old woman suffering from genetic encephalic leukodystrophy who was admitted for SARS‐CoV‐2‐pneumonia (6th May). Patient was discharged without treatment after 1 week because clinical condition did not identify a critical state nor clinical negative prognostic factors. After 5 months, she was admitted for respiratory failure, epilepsy and worsening of the previous radiological features and treated with low‐flow oxygen, broad‐spectrum antibiotics and methylprednisolone 40 mg/day, with amelioration of the symptoms.
In this letter, we report three different cases of COVID‐19 pneumonia, in which a long‐term persistence of chronic lung damage was observed, despite different clinical approaches. Moreover, our observations suggest that clinical and radiological signs of long‐term damage may be observed not infrequently after COVID‐19 pneumonia.
Different possible mechanisms of long‐term lung damage may be hypothesized. First, it might be driven by persistent presence of SARS‐CoV‐2 infection. The recurrence of SARS‐CoV‐2 has been described shortly after two consecutive negative swabs, in patients with previous SARS‐CoV‐2‐pneumonia. 3 Although this observation raised the doubt of reinfection, in most similar cases false negative tests are the most likely explanation. 3 Due to the highest sensitivity performed within all the specimens tested, BAL fluid was proposed as the best one for COVID‐19 diagnosis. 6 However, a high concordance between upper respiratory tract swabs and BAL was further observed in two different multicenter Italian studies. 6 , 7 Although in our patients, real‐time PCR on BAL was not performed, we remark that nasopharyngeal swabs were long‐term continuously negative for SARS‐CoV‐2, suggesting that reinfection was unlike.
Second, there might be a role for immune system in the maintenance of a chronic phase of the pulmonary disease, as observed in connective tissue diseases‐related‐ and some other idiopathic interstitial lung diseases (ILD) characterized by chronic progression. 8 The worsening of ILD after COVID‐19 here described might be similar to the progressive fibrosing clinical phenotype observed in many forms of ILD after phases of acute exacerbations. 8
Short‐term steroid treatment was followed by disease progression in Patients 1 and 2. In some other ILD, corticosteroids and/or other immunosuppressive treatment showed some benefit in preventing lung fibrosis progression. 8 Nevertheless, chronic use of corticosteroids may be associated to serious infections (Patient 1). 9 Interestingly, the MRSA coinfection in Patient 1 might be a possible trigger for the polyclonal activation of immune system by the superantigens complex, explaining the reactivation of inflammatory process initially induced by SARS‐CoV‐2. The paradoxical use of immunosuppressive treatment for an infective disease was suggested by different studies, performed in the first pandemic spread, without relevant side effects. 10 Notably, coinfections appeared more likely related to corticosteroids treatment than to other immune‐suppressant agents. 9
The final point of discussion is whether to treat or not pauci‐symptomatic patients despite the fact that the guidelines do not indicate any treatment in these patients. 2 However, in some cases, as in Patient 3, lung damage might progress, raising the doubt that a more aggressive strategy could be useful also in these subsets of patients. From this point of view, a prompt and a well tolerable treatment should be provided at the COVID‐19 diagnosis. Although based on a retrospective study including just nine patients, the successful use of colchicine at the beginning of the disease in the setting of community healthcare patients might an example of a possible approach. 11 Other similar approach with antiinflammatory drugs was described in literature during the pandemic. Antimalarials, initially described as potential prophylaxis or treatment for COVID‐19, were not confirmed to be efficacious in reducing mortality in comparison with the SoC in randomized trials. 12 N‐acetylcysteine, a precursor of reduced glutathione, and baricitinib, an orally administered, selective inhibitor of Janus kinase 1 and 2, are under investigation as an add‐on therapy in COVID‐19 together with antiviral agents. 13 , 14
In conclusion, the reported cases illustrate the possible arise of chronic lung damage after COVID‐19 pneumonia. The use of numerous off‐label treatments in these patients, which was almost inevitable during the dramatic first wave of the pandemic, might represent a limitation in our observation. Moreover, we cannot rule out the possible role of other etiologic factors (similar to MRSA in Patient 1) in the re‐exacerbation of pulmonary disease.
The frequency of this chronic lung damage after COVID‐19 and its underlined mechanisms have yet to be clarified; clinical trial might be warranted to investigate the timing and the endurance of treatments to prevent it.
REFERENCES
- 1. Grasselli G, Greco M Zanella A, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345‐1355. 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. https://www.covid19treatmentguidelines.nih.gov
- 3. Lafaie L, Célarier T, Goethals L, et al. Recurrence or relapse of COVID‐19 in older patients: a description of three cases. J Am Geriatr Soc. 2020;68:2179‐2183. 10.1111/jgs.16728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scarsi M, Piantoni S, Colombo E, et al. Association between treatment with colchicine and improved survival in a single‐centre cohort of adult hospitalised patients with COVID‐19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79:1286‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piantoni S, Andreoli L, Colombo E, et al. Response to: ‘Correspondence on “Association between treatment with colchicine and improved survival in a single‐centre cohort of adult hospitalised patients with COVID‐19 pneumonia and acute respiratory distress syndrome” by Kawada [published online ahead of print January 27, 2021]. Ann Rheum Dis. 2021. 10.1136/annrheumdis-2020-219787 [DOI] [PubMed] [Google Scholar]
- 6. Patrucco F, Albera C, Bellocchia M, et al. SARS‐CoV‐2 detection on bronchoalveolar lavage: an Italian multicenter experience. Respiration. 2020;99:970‐978. 10.1159/000511964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geri P, Salton F, Zuccatosta L, et al. Limited role for bronchoalveolar lavage to exclude COVID‐19 after negative upper respiratory tract swabs: a multicentre study. Eur Respir J. 2020;56(4):2001733. 10.1183/13993003.01733-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spagnolo P, Distler O, Ryerson CJ, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)‐associated interstitial lung diseases (ILDs). Ann Rheum Dis. 2020;80:143‐150. 10.1136/annrheumdis-2020-217230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157‐176. 10.1016/j.rdc.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. 10.1016/j.autrev.2020.102568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Della‐Torre E, Della‐Torre F, Kusanovic M, et al. Treating COVID‐19 with colchicine in community healthcare setting. Clin Immunol. 2020;217:108490. 10.1016/j.clim.2020.108490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with covid‐19. N Engl J Med. 2020;383(21):2030‐2040. 10.1056/NEJMoa2022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Flora S, Balansky R, La Maestra S. Rationale for the use of N‐acetylcysteine in both prevention and adjuvant therapy of COVID‐19. FASEB J. 2020;34(10):13185‐13193. 10.1096/fj.202001807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with covid‐19 [published online ahead of print December 11, 2020]. N Engl J Med. 2020:NEJMoa2031994. 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]


