Abstract
The Covid‐19 pandemia has many other undesirable consequences apart of virus infection. Less people is hospitalized due to acute coronary syndrome and the delay to seek medical attention has increased. Patients with ST segment elevation myocardial infarction arrive at the hospital too late to be timely treated and we have recently seen mechanical complications that were more frequent in the past decades before the use of reperfusion strategies. In this report we describe the presentation, evolution and detailed imaging evaluation of two patients with unusual presentations of cardiac rupture: left ventricular pseudoaneurysm and left ventricular intramyocardial dissecting hematoma.
Keywords: left ventricular pseudoaneurysm, left ventricular thrombi, myocardial infarction, myocardial rupture
1. INTRODUCTION
In the early days while our country was facing the COVID‐19 pandemic, the government established an extensive quarantine to slow down the growing curve of this infectious disease. Meanwhile, we were observing a lower incidence of people being hospitalized due to acute coronary syndrome together with an increased delay in these patients seeking medical attention. This scenario was already described by the first countries struck by the coronavirus outbreak and is perhaps one of the reasons for the significant increase in cardiac arrest taking place outside of hospital enviroments. 1 , 2 Many patients with ST segment elevation myocardial infarction arrive at the hospital too late to be timely treated, and we have recently seen mechanical complications that were more frequent in the past decades before the use of reperfusion strategies. In this report we describe the presentation, evolution and detailed imaging evaluation of two patients with unusual presentations of cardiac rupture.
2. CASE PRESENTATION
2.1. Case 1
A 70‐year‐old female patient with a history of hypertension and hypothyroidism was attended in the emergency department because of palpitations and dyspnea. Physical examination showed inspiratory crackles over the inferior half of the lung fields, jugular venous distention, a 3/6 systolic murmur, and mild orthopnea. Blood pressure was 140/80, heart rate 120 beats/min and respiratory rate 25 breaths/minute. Admission ECG revealed rapid atrial fibrillation with QS complex and negative T waves in anterior and inferior leads that spontaneously converted to sinus rhythm soon after arrival (Figure 1A). Laboratory tests were unremarkable, notably troponin and creatine phosphokinase were normal, and therefore, a recent anterior–inferior (apical) myocardial infarction was diagnosed. When the patient was asked about any symptoms in the previous weeks, she said she had felt moderate intensity pain in her left arm followed by two syncopal episodes 45 days previous; however, she did not request medical attention due to fear of becoming infected with COVID‐19. The following day an echocardiogram revealed a normal sized left ventricle (LV) with extensive akinesis of mid and apical segments, severely depressed LV systolic function (ejection fraction 25%), and a moderate pericardial effusion. There was a big anechogenic cavity 5 cm wide connected to the left ventricular apex by a 1.4 cm sharp neck (Figure 1B, Movie S1). Color and Pulsed wave Doppler depicted low velocity bidirectional flow across the defect (Figure 1C,D, Movie S2). Coronary angiography was performed showing single vessel disease with a totally occluded mid left anterior descending coronary artery (LAD). With the diagnosis of LV rupture contained by a big pseudoaneurysm, the patient was transferred to a tertiary care reference center to perform a surgical repair of the defect. Nevertheless, the operation was deferred because of patient stability and high surgical risk.
FIGURE 1.
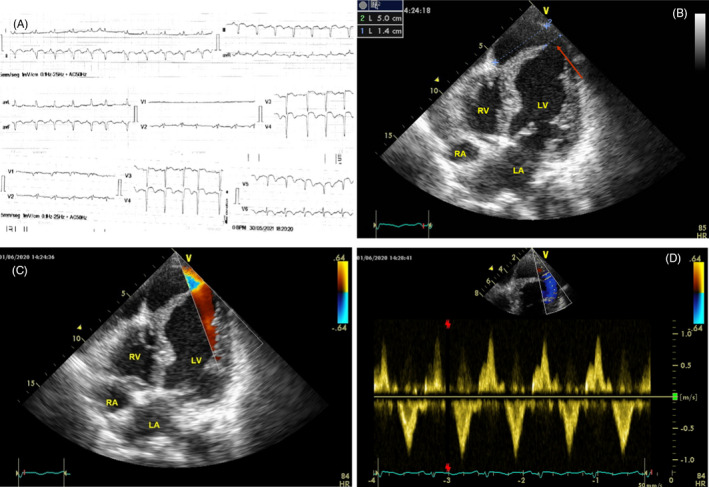
Case 1. Left ventricular pseudoaneurysm. A, ECG showing prominent Q waves. B, Apical four chamber view showing the pseudoaneurysm connected to the LV cavity by a sharp neck (arrow). C, Color Doppler images showing flow across the defect. D, Pulsed Doppler tracings depicts low velocity bidirectional flow across the orifice. LV = left ventricle; RV = right ventricle; LA = left atrium; RA = right atrium
2.2. Case 2
A 61‐year‐old male patient with a history of diabetes, hypertension, and cigarette smoking was brought to the emergency room complaining of substernal chest pain associated with class IV dyspnea lasting more than 48hs. He had experienced frequent anginal symptoms for three weeks before seeking medical attention but had decided to stay home to avoid contact with COVID‐19‐infected patients. At arrival, he had cold extremities, tachypnea, tachycardia, and his blood pressure was 90/50. The ECG depicted prominent Q waves in anterior and inferior leads with 2 mm residual ST segment elevation (Figure 2A). Troponin was markedly increased and a chest X‐ray showed signs of acute pulmonary edema. Evolved ST segment elevation infarction complicated with cardiogenic shock was diagnosed, and a coronary angiography was performed showing LAD occlusion and a 70% obstruction of the left circumflex. LAD recanalization was unsuccessful and a left circumflex angioplasty with a drug eluting stent was completed. Next day echocardiogram showed akinesis of mid and apical segments of the LV with preserved thickening limited to the basal segments and an ejection Fraction of 20%–25%. An apical left ventricular expansion with a notably increased wall thickness (29 mm) of nonhomogeneous appearance was clearly depicted (Figure 2B, C and Movie S3). There was a sharp dyskinetic movement of the endomyocardial border of the structure but no flow signal could be seen over this area (Movie S4). Cardiac Magnetic Resonance (CMR) confirmed the diagnosis of intramyocardial dissecting hematoma showing an apical crescentic mass in T1‐ weighted images with late gadolinium enhancement all around the hematoma, compatible with extensive myocardial fibrosis (Figure 2D, E and Movie S5). The surgical risk was deemed too high, so the patient was treated conservatively, requiring one week of vasopressors and mechanical ventilatory support. A transthoracic echocardiogram was periodically repeated showing progressive decrease in the intramyocardial hematoma volume with no evidence of pericardial effusion. Apical wall thickness decreased to 22 mm in a month and to 16.8 mm on day 45 with an increase in echogenicity consistent with organization (Figure 2F and Movie S6).
FIGURE 2.
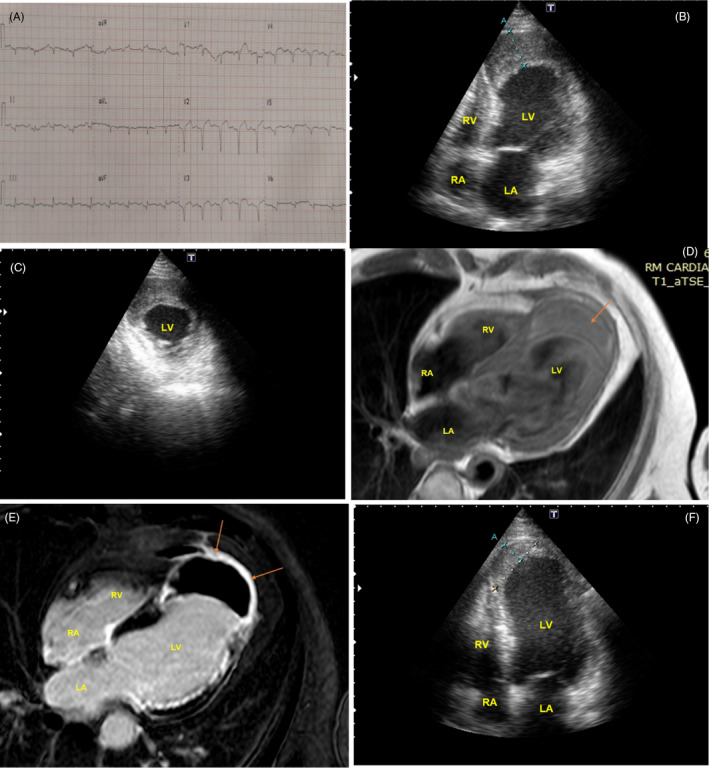
Case 2. Intramyocardial dissecting hematoma. A, Admission ECG with Q waves in multiple leads. B, Echocardiogram apical four chamber view showing a grossly thickened left ventricular free wall (see measure A = 29 mm) compatible with intramyocardial hematoma (arrow). C, Short axis view. The hematoma can be seen in the anteroseptal and anterolateral aspect of LV apex. D, T1‐weighted CMR images showing an apical crescentic mass compatible with hematoma (arrow). E, CMR images showing late gadolinium enhancement around the hematoma compatible with fibrosis (arrows). F, Echocardiogram at 45 days after admission. A clear reduction in hematoma volume is evident (16,8 mm in measure A). LV = left ventricle; RV = right ventricle; LA = left atrium; RA = right atrium
3. DISCUSSION
Left ventricular free wall rupture is usually a fatal event resulting in massive bleeding into the pericardial space followed by cardiac tamponade and electromechanical dissociation. However in very rare cases, the leakage is contained by pericardium and adjacent tissue fibrous adhesions and a pseudoaneurysm develops. Echocardiography is the usual imaging method to establish the diagnosis because of its widespread availability and excellent imaging quality. The usual finding is an anechogenic cavity of variable size adjacent to the myocardial structures communicated to the LV cavity by a smaller neck with systo‐diastolic bidirectional flow. A useful criterion for differential diagnosis with a true aneurysm is the ratio of the orifice width to the maximal parallel internal diameter of the neo‐cavity of less than 0.5 (it was 0.28 in our case). 3 Surgical treatment is recommended when diagnosis is established during the first month of myocardial infarction because risk of rupture is unpredictable. Nevertheless, some chronic cases are incidentally discovered and may be followed and treated conservatively. 4 The second case described in this report represents another unusual presentation of myocardial rupture. Intramyocardial dissecting hematoma has been already described a long time ago in necropsy specimens but with improvements in imaging methods it can be readily displayed in vivo. Characteristic pathologic features are massive infiltration of blood into and through the myocardial wall dissecting between the muscle bundles, sometimes resulting in large channels and lakes. 5 The mechanism might be related to hypoxia‐induced endothelial disruption of small intramyocardial vessels with hemorrhage and swelling of the infarcted area. Incomplete and delayed reperfusion may predispose to this complication, as previous reports described a higher angina‐balloon time, low prevalence of TIMI 3 flow, and a poorer myocardial blush in this group of patients. 6 Optimal treatment in this scenario is still under debate, but short‐term prognosis is usually good when only apical segments are involved. 7 , 8 Echocardiography and CMR can precisely delineate the hematoma, and serial follow‐up testing might demonstrate the reduction in its volume across days or weeks as in our case 2.
In conclusion, we present two different morphological expressions of cardiac rupture in myocardial infarction. Since transmural necrosis is the usual pathologic substrate favoring this complication, the delay in performing angiography and the failure to open the infarct‐related artery might have had a key role in both of our cases. The fear of going out to seek medical attention may result in a collateral damage of the COVID‐19 outbreak precluding a timely diagnosis and treatment. Nowadays, a proper diagnostic approach of these rare complications can be made with noninvasive imaging methods.
CONFLICTS OF INTEREST
None.
Supporting information
Movie S1. Transthoracic echocardiography. Apical four chamber view showing LV mid and apical segments akinesis and the pseudoaneurysm. Note the prominent fluttering of the myocardial flap.
Movie S2. Transthoracic echocardiography. Color Doppler demonstrates low velocity systo‐diastolic flow across the defect.
Movie S3. Transthoracic echocardiography. Apical four chamber view showing a grossly thickened left ventricular apex.
Movie S4. Apical four chambers Color Doppler. No flow signal can be seen inside the apical mass.
Movie S5. CMR images showing akinesis of LV apical segments with an apical hematoma.
Movie S6. Transthoracic echocardiography at 45 days after hospital admission. There is a clear reduction in hematoma volume together with an increase in its echogenicity.
Allende NG, Santos R, Sokn FJ, et al. Unusual presentations of cardiac rupture during COVID‐19 pandemic. Echocardiography. 2021;38:469–472. 10.1111/echo.15006
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. De Filippo O, D’Ascenzo F, Angelini F, et al Reduced rate of hospital admissions for ACS during Covid‐19 outbreak in Northern Italy. N Engl J Med. 2020;383:88‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garcia S, Albaghdadi MS, Meraj PM, et al Reduction in ST‐segment elevation cardiac catheterization laboratory activations in the United States during COVID‐19 pandemic. J Am Coll Cardiol. 2020;75:2871‐2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gatewood RP, Nanda NC. Differentiation of left ventricular pseudoaneurysm from true aneurysm with two‐dimensional echocardiography. Am J Cardiol. 1980;46:869‐878. [DOI] [PubMed] [Google Scholar]
- 4. Frances C, Romero A, Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. 1998;32:557‐561. [DOI] [PubMed] [Google Scholar]
- 5. Lewis AJ, Burchell HB, Titus JL. Clinical and pathologic features of postinfarction cardiac rupture. Am J Cardiol. 1969;23:43‐53. [DOI] [PubMed] [Google Scholar]
- 6. Spinelli L, Stabile E, Giugliano G, et al Intramyocardial dissecting hematoma in anterior wall ST elevation myocardial infarction: impact on left ventricular remodeling and prognosis. Int J Cardiovasc Imaging. 2018;34:201‐210. [DOI] [PubMed] [Google Scholar]
- 7. Rezaei‐Kalantari K, Saedi S, Alizadeasl A, et al Left ventricular intra‐myocardial dissection after myocardial infarction. Echocardiography. 2020;37:2160‐2162. [DOI] [PubMed] [Google Scholar]
- 8. Vargas‐Barrón J, Roldán FJ, Romero‐Cárdenas A, et al Dissecting intramyocardial hematoma: clinical presentation, pathophysiology, outcomes and delineation by echocardiography. Echocardiography. 2009;26:254‐261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Transthoracic echocardiography. Apical four chamber view showing LV mid and apical segments akinesis and the pseudoaneurysm. Note the prominent fluttering of the myocardial flap.
Movie S2. Transthoracic echocardiography. Color Doppler demonstrates low velocity systo‐diastolic flow across the defect.
Movie S3. Transthoracic echocardiography. Apical four chamber view showing a grossly thickened left ventricular apex.
Movie S4. Apical four chambers Color Doppler. No flow signal can be seen inside the apical mass.
Movie S5. CMR images showing akinesis of LV apical segments with an apical hematoma.
Movie S6. Transthoracic echocardiography at 45 days after hospital admission. There is a clear reduction in hematoma volume together with an increase in its echogenicity.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.


