Abstract
The present article aims to analyze epidemiologic aspects of the novel coronavirus pandemic (COVID‐19) over different countries across the globe. While analyzing the overall spread of the disease, clusters of countries could be identified where the population‐adjusted number of cases and mortality rates (MRs) were significantly different from the others. To draw a comparison over the countries at the same stage of infection, the nature and spread of the infection was evaluated at the 90th day of the pandemic for each country. It was observed that the countries with prevalent malarial transmission tended to have lesser population‐adjusted COVID‐19 caseloads. It was further observed that high population coverage of the Bacillus Calmette–Guérin vaccination was negatively associated with population‐adjusted caseloads and MRs due to COVID‐19. The present cross‐sectional study is an attempt to bring in several social, economic, and structural confounders into understanding of the nature and spread of this novel pandemic globally.
Keywords: disease control, endemic infection, epidemiology, SARS coronavirus, vaccines/vaccine strains, virus classification
1. INTRODUCTION
The novel coronaviral disease‐2019 (COVID‐19) is a new human respiratory tract infection that has grown into a pandemic. 1 Despite having a worldwide distribution, there exists significant country‐to‐country and continent‐to‐continent variation of disease spread, extent, and severity. Interestingly the mortality rates (MRs) or case fatality rates are different in different countries at the same stage of contagion, for example, both Spain and Italy experienced higher death statistics compared to China at the same stage of infection. 2 At the time of writing SARS‐CoV‐2 has killed more than 571,000 people, with over 12 million infected, and has engendered a global economic shutdown, perhaps more serious than the great depression of the 1930s. 3 While it is predicted that the virus may remain entrenched in various pockets of populations and may give rise to a multitude of coming epidemics and global health crises, only an effective and safe vaccine can curb the spread of the virus. It has been suggested that till an effective, safe and specific COVID‐19 vaccine arrives, the Bacille Calmette‐Guérin (BCG) vaccine may serve as an interim candidate. 3 On the other hand it has also been suggested that COVID‐19 mortality is markedly higher in countries without a widely practiced BCG vaccination policy. 4 These observations could be of biological value or could be just “curious statistical banalities.” To address this issue, we designed and conducted a cross‐sectional study to test any association of country‐wise COVID‐19 epidemiology with different vaccination policies, endemic infectious diseases, and social confounders like developmental indices and airline travel.
2. MATERIALS AND METHODS
2.1. Number of countries and population‐adjusted COVID‐19 cases and mortality and case fatality rates
In this study, we started with 184 countries across the world and collected data on the following variables (wherever available) for each of them related to COVID‐19: Number of COVID‐19 infected individuals, deaths, recoveries, and the total number of tests performed in the first 90 days starting from the day of detection of the first case (for the countries which have not reached 90 days of infection till June 04, 2020; data were taken till June 04, 2020). In addition to the above information, we collected data on the following additional variables from the latest available population estimate: total population, counts of the numbers of tuberculosis (TB), human immunodeficiency virus (HIV) and malaria cases in a year, population coverage (in %) of the vaccines like BCG, diphtheria, pertussis, and tetanus (DTP1), Haemophilus influenzae type B vaccine (HiB3), measles, polio vaccine 1 (IPV1), and Rubella vaccine (RCV); number of airline passengers carried in a year and the human development index (HDI) as a measure of development for the countries. From the data, we have calculated the MRs and RRs for individual countries as the number of COVID‐19 deaths and recoveries per million population, respectively.
We collected data from freely accessible online data repositories on the following aspects: COVID‐19 related data (number of cases, number of deaths, number of tests) at the 90th day of onset of first reported case in a particular country or on 4th June 2020 (whichever is earlier) 5 ; malaria, TB, and HIV prevalence (number of cases) 6 , 7 , 8 ; percentage population covered by the following vaccines: BCG, DTP1, haemophilus influenzae b vaccine (HiB), measles vaccine, poliovaccine (IPV1), RCV 9 ; population per country and HDI of the countries 10 , 11 , 12 ; and number of airline passengers 13 ; and number of COVID‐19 tests done. 14
2.2. Categorization of countries, for the purpose of comparison of COVID‐19 statistics, was done in the following manner
Countries were categorized into various classes for comparison of COVID‐19 related epidemiology based on the following classification scheme:
-
i.
Vaccination: countries were categorized in a bipartite manner based on <95% or ≥95% coverage of each vaccine studied;
-
ii.
Malaria transmission: malaria‐free countries (no reported indigenous malaria cases), mild‐moderate malaria transmission (≤100,000 cases per 1 million population) and high malaria transmission (>100,000 cases per 1 million population);
-
iii.
TB prevalence: mild (<5 cases per 100,000 population), moderate (5–99 cases per 100,000 population) and high (≥100 cases per 100,000 population);
-
iv.
HIV prevalence: Low prevalence (<1000 per million population), moderate prevalence (≤10,000, ≥1000 per million population), and high prevalence (>10,000 per million population).
Comparison of COVID‐19 cases, MR and RR were compared among various country‐wise categories as described above were done and odd's ratios with confidence intervals were obtained. Next to allow for confounders a multidimensional clustering technique (K‐Means Clustering 4 ) was used to classify the countries into a number of clusters or groups and thereby identifying the countries which are similar in nature. Instead of classifying the countries based on only a single variable, for example, coverage of any particular vaccine, it would be useful to analyze the characteristics of the groups, constructed using the coverage of more than one vaccine. The method helps us to analyze and access the impact of the interaction of several vaccines which is otherwise difficult to measure. All statistical analysis were done using the software R (version 3.6.1.).
3. RESULTS
3.1. Relation of number of COVID‐19 cases (population adjusted) with other endemic infectious diseases
We begin with comparing COVID‐19 cases to previously reported malaria prevalence. There is a negative correlation between number of malaria cases per million and COVID‐19 cases per million (r = –.26, p = .006). In malaria‐free countries (n = 31 countries) Odds of COVID‐19 was 2.122 (95% confidence interval [CI]: 2.117–2.1271) compared to countries with mild‐to‐moderate malaria transmission (n = 52 countries with ≤100,000 malaria cases per million population) and 11.06935 (95% CI: 10.974–11.165) compared to countries with high malaria transmission (n = 30 countries with >100,000 malaria cases per million population). The same odds ratio for countries with mild‐to‐moderate malaria transmission was 5.215 (95% CI: 5.17–5.26) compared to the countries with high malaria transmission.
The odds of having COVID‐19 cases in countries with mild TB prevalence (n = 40 countries with <5 TB cases per 1 lakh population) was 4.616 (95% CI: 4.606–4.625) times more compared countries with moderate TB prevalence (n = 81 countries with 5–99 TB cases per 1 lakh population) and 11.474 (95% CI: 11.443–11.506) times more compared to countries with high TB prevalence (n = 57 countries with ≥100 TB cases per 1 lakh population). The Odd of COVID‐19 infection for low HIV prevalence countries (n = 52 countries with <1000 HIV cases per million population) was 0.647 (95% CI: 0.645–0.648) compared to countries with moderate prevalence (n = 67 countries with 1000–10,000 HIV cases per million population), and 3.058 (95% CI: 3.035–3.081) compared to high prevalence countries (n = 68 countries with >10,000 HIV cases per million population). The odd of COVID‐19 cases for the second category countries was 4.725 (95% CI: 4.691–4.760) compared to the last category.
3.2. Relation of COVID‐19 statistics with vaccine coverage
Relationship of number of COVID‐19 cases (population adjusted) with vaccine coverage statistic is detailed in Table 1. Countries with lower coverage of BCG, measles, and Rubella‐containing vaccine (RCV vaccine) coverage had significantly higher proportion of cases. Regarding the outcome of COVID‐19, the only significant relation was with BCG vaccination coverage which was negatively correlated with MR and positively with recovery rate (RR). The map in Figure 1A is showing the MR due to COVID‐19 for the different countries. The MR is higher for countries like France, Spain, UK, and Italy. Figure 1B is showing the RR from COVID‐19 across different countries. Figure 1C shows the coverage of BCG vaccination for the countries across the globe, which indicates that countries like the United States, Canada, France, Italy, Spain, and the UK are the ones having the least coverage of BCG.
Table 1.
Showing the odds ratio of COVID‐19 cases (population adjusted) and COVID‐19 outcome measures
| Vaccine | Number of cases | MR | RR | ||
|---|---|---|---|---|---|
| OR (95% CI) | Correlation coefficient (r) | p value | Correlation coefficient (r) | p value | |
| BCG | 1.893988 (1.889862, 1.898123) | −0.4580251 | 5.364 × 10−9 | 0.2021302 | .01375 |
| DTP1 | 0.5613104 (0.5598011, 0.5628238) | −0.02443825 | .7598 | −0.08055915 | .3128 |
| HiB3 | 0.5598935 (0.5585527, 0.5612375) | 0.09195491 | .2631 | −0.002450564 | .9762 |
| IPV1 | 0.9915015 (0.9890725, 0.9939365) | 0.07801166 | .3378 | 0.0245322 | .7634 |
| Measles | 1.164201 (1.161405, 1.167005) | −0.04192178 | .6105 | 0.04555095 | .5799 |
| RCV | 1.097302 (1.094514, 1.100098) | 0.0607095 | .4876 | 0.0433463 | .6203 |
Note: The odds ratios are calculated for the countries in which a particular vaccine covers <95% of the population versus the countries where that vaccine covers ≥95% of the population.
Abbreviations: CI, confidence interval; MR, mortality rate per hundred thousand population; OR, odd's ratio; RR, case recovery rate.
Figure 1.
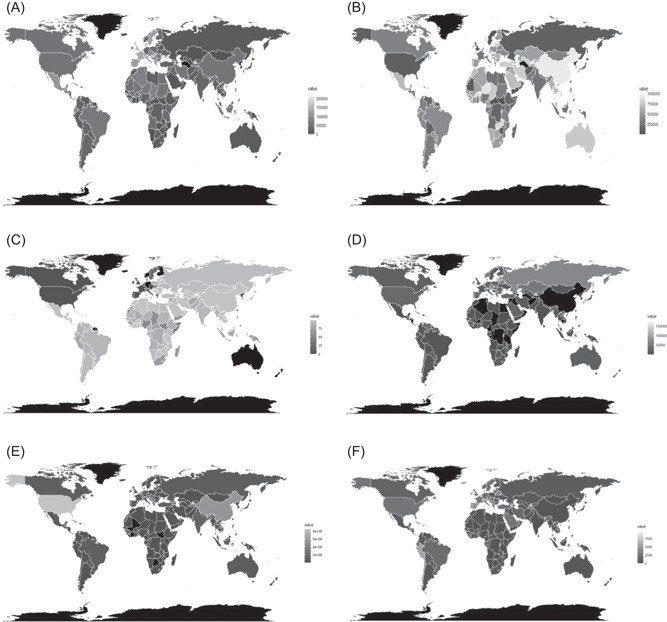
Maps showing the MR (A), RR (B) due to COVID‐19 for different countries. As shown, in (A) France, Spain, UK, Italy, Mexico do have high mortality rates but the recovery rates from COVID‐19 for most of the states are fairly high all over the world as shown in (B). (C,D) Shows the coverage of BCG vaccination (C) and the number of tests per million population (D) for countries across the globe. As shown in (C), most of the Asian, African, and South American countries are covered with BCG vaccination and United States, Canada, France, Italy, Spain, and the UK are the ones having the least coverage of BCG. (D) Shows that United States, Russia, Iceland, Norway, Sweden, Chile are performing well in terms of testing per million. However, for some of the countries, the counts of the tested samples per million population are missing. (E) Shows the number of airline travelers and (F) shows the COVID‐19 cases per million population till June 4, 2020. From the (E) it can be seen that United States, China, UK, France, and India yields significant number of air passengers and as shown in (F) it is evident that the countries with more airline travelers tend to have more COVID‐19 cases per million population
The scatter plot in Figure 2A is showing that the lesser the BCG coverage, the more fatal the disease turns out to be. Figure 2B is showing the relationship between BCG coverage and RR per million infected during the COVID‐19 pandemic.
Figure 2.
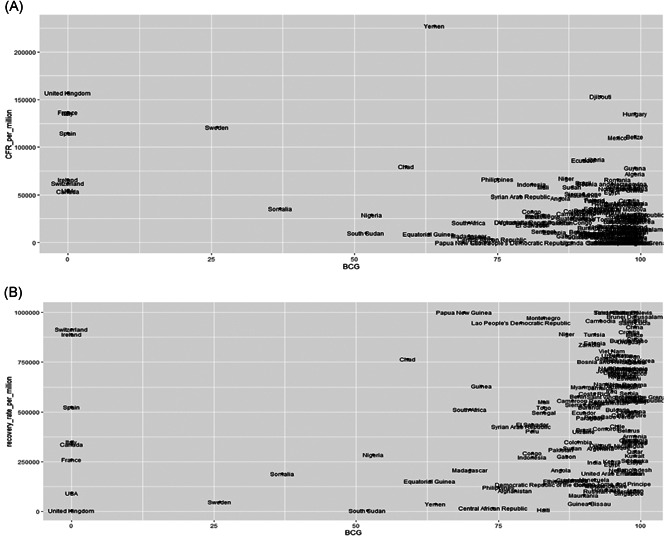
Scatter plot showing the relationship between BCG coverage and MR per million (A) and RR per million (B) due to COVID‐19. As shown in the left panel, the lesser the BCG coverage, the more fatal the disease turns out to be. (B) is showing an insignificant relationship between the two variables
From Table 1, it is obvious that RR hardly depends on the vaccine coverage (other than BCG). This phenomenon is quite similar to that of MR.
3.3. Relation of number of COVID‐19 cases with confounders
Correlation coefficients of COVID‐19 case counts (per million population) of each country with the different confounders were as follows: with number of tests per million population: r = .504, p = 4.154 × 10−10; with human development index (HDI): r = .527, p = 4.062 × 10−11; and with number of airline passengers: r = .219, p = .006831. The map in Figure 1D is showing the distribution of the number of tests per million population.
Figure 1E is showing the number of airline travelers across different countries. United States, China, UK, France, India are some of the countries with a significant number of air passengers. The counts of the airline travelers are obtained from prepandemic data based on availability. The countries with more airline travelers tend to have more COVID‐19 cases as shown in Figure 1F.
To find out the impact of the age distribution of the population of a country on COVID‐19 outbreak and fatalities, we have taken three age groups (0–14, 15–64, and 65 years or above) and have data on the percentage of population in each of the groups for all the countries under study. There was a significant positive correlation between the percentage of population aged ≥65 and COVID‐19 cases per million (r = .187, p = .012) and MR (r = .456, p = 1.761 × 10−10). Correlations between the percentage of population belonging to the age group 0–14 and COVID‐19 cases per million and COVID‐19 MR were both highly significantly negative (r = −.378 and −.345 and p = 2.201 × 10−7 and 2.475 × 10−6 respectively). However, for the age group 15–64 years, both the correlation coefficients were nonsignificant.
For the countries with <15% of the population aged ≥65 and BCG coverage >95%, the odds for COVID‐19 infection is 1.0327 (95% CI: 1.0299–1.0356) times more compared to the countries where the <15% of the population aged ≥65 years and BCG coverage <95%. The odds for COVID‐19 is 4.6612 (95% CI: 4.6399–4.6826) times in countries with ≥15% of the population aged ≥65 years and <95% BCG coverage compared to countries with ≥15% of the population aged 65 years with BCG coverage ≥95%.
3.4. Multidimensional clustering of COVID‐19 statistics
Results from the clustering experiments are shown in Table 2. In the first clustering experiment based on vaccine coverage, two clusters are identified, the first one (26 countries) with higher BCG coverage and lower coverage of all other vaccines and the second one (96 countries) having reverse representation. Previously for univariate analysis, we studied the impacts of each of the vaccine coverage separately. Multidimensional techniques allow us to compare between the impacts of several vaccination coverage together with its impact on COVID‐19 infection. For the 26 countries (which includes Italy, France, Sweden, United Kingdom) in the first cluster with comparatively lower BCG coverage COVID‐19 infection prevalence was higher compared to other countries.
Table 2.
Results of the clustering experiments
| Experiment 1 | Clustering of COVID‐19 cases with vaccine coverage | |||||||
| Cluster | Number of countries | BCG | DTP1 | HiB3 | Measles | IPV1 | RCV | |
| 1 | 26 | 92.57692 | 88.57692 | 78.15385 | 80.50000 | 61.42308 | 79.46154 | |
| 2 | 96 | 89.25000 | 97.03125 | 93.96875 | 93.29167 | 95.52083 | 93.11458 | |
| Experiment 2 | Clustering of COVID‐19 cases based on various infectious diseases prevalence and % of aged population | |||||||
| Cluster | Number of countries | HIV per 100,000 population | Malaria per 100,000 population | COVID‐19 cases per 100,000 population | TB per 100,000 population | % of population with age 65 or above | ||
| 1 | 27 | 2263.1220 | 28,846.9640 | 17.0721 | 231.1852 | 2.7889 | ||
| 2 | 70 | 1333.0150 | 1299.0770 | 334.9131 | 133.7500 | 6.9075 | ||
| Experiment 3 | Clustering of COVID‐19 cases along with various confounders | |||||||
| Cluster | Number of countries | HDI | Airline passenger counts per million population | COVID‐19 cases per million population | COVID‐19 tests performed per million population | |||
| 1 | 2 | 0.9365000 | 26,686,333.0 | 5257.8920 | 125,125.75 | |||
| 2 | 18 | 0.8861111 | 3,900,318.5 | 2863.5187 | 42744.62 | |||
| 3 | 98 | 0.7094388 | 448,151.9 | 787.3707 | 15445.51 | |||
| Experiment 4 | Clustering of COVID‐19 cases with BCG coverage, malaria transmission, and number of airline passengers | |||||||
| Cluster | Number of countries | BCG coverage | Airline Passenger counts | COVID‐19 cases | Malaria cases | |||
| 1 | 13 | 72.30769 | 92,547,922 | 103,276.23 | 615,065.5 | |||
| 2 | 2 | 49.50000 | 700,318,755 | 434,262.50 | 0.0 | |||
| 3 | 79 | 86.92405 | 5,083,230 | 13,136.57 | 2,497,394.0 | |||
Note: The cells represent cluster centers.
In experiment 2, clustering is done considering the prevalence of several endemic infectious diseases (HIV, malaria, COVID‐19, and TB) per 1 lakh population and percentage of aged population. We observe that countries with greater aged population, tend to have more COVID‐19 infections per million, but less HIV, malaria, and TB infections.
In experiment 3, various confounders and COVID‐19 case‐loads are clustered together. Three clusters of countries are obtained, having 2, 18, and 98 countries, respectively. It indicates that lower HDI associates with lesser airline passenger, lesser COVID‐19 tests performed and lesser COVID‐19 case‐load detected.
In experiment 4, BCG vaccine coverage, malaria case numbers, and number of airline passengers are factored along with COVID‐19 cases. Three clusters of countries are obtained, having 13, 2, and 79 countries respectively. Cluster 1 has countries like Brazil, Indonesia, South Korea, India, Thailand, and so on. Cluster 2 has only China and the United States. Cluster 3 has mostly the sub‐Saharan countries and other countries like Afghanistan, Congo, Chad, Gabon, Bhutan, Bangladesh, and so forth. In this cluster, number of malaria cases is directly and BCG vaccine coverage is indirectly proportional to COVID‐19 case load. However, the effects of number of airline passengers still persist.
4. DISCUSSION
We observed that COVID‐19 cases vary significantly from one country to another. Since we allowed 3 months for each country (altogether we have 184 countries with partial/full observations corresponding to all the variables), we may safely assume that a snapshot of the pandemic at similar stages in different countries was taken. COVID‐19 statistics varied with vaccination coverage and distribution of different endemic infections. However, several confounders like number of airline passengers, HDI, and number of tests performed added additional directionalities to the statistics. In the final multidimensional clustering models, we observed that despite the effect of the confounders, number of COVID‐19 cases were the lowest where BCG coverage and malaria endemicity were the highest (detailed in Table 2). Also, we observed that with lower HDI and lower numbers of COVID‐19 tests lead to lower COVID‐19 prevalence estimates. Additionally, higher prevalence of endemic infections like TB, HIV and malaria was associated with lower COVID‐19 prevalence estimates. Unlike the other infectious diseases, it is clear from the second clustering experiment (Table 2) that COVID‐19 is more prevalent in the older population. From experiment 3 (Table 2), it is very clear that countries with lower HDI had the least number of cases, but this could be related to less number of tests performed and lower number of airline passengers. The final clustering experiment shows that increased BCG coverage and malaria prevalence negatively influences COVID‐19 case‐loads. Therefore such unsupervised multidimensional clustering might explain, at least partially, the nature and spread of COVID‐19 among various countries.
BCG (Mycobacterium bovis Bacillus Calmette‐Guérin) is a live attenuated vaccine against TB, given at or right after birth and is effective in reducing incidence of severe forms of TB like TB meningitis or milliary TB. 4 It is said that vaccination with BCG induced a permanent change in the immune system, called “trained immunity” characterized by enhanced protection against unrelated pathogens. 15 In the innate immune cells introduction to BCG induces histone modifications (methylation and acetylation) and other epigenetic influences at promoter sites of several inflammatory cytokine genes especially interleukins 1 (IL‐1), IL‐6, and tumor necrosis factor. 16
Clinically these immune changes has been observed to be translated into various health benefits of the BCG vaccine; like up to 50% reduction of infant MR attributable to protection against unrelated infectious agents, especially respiratory tract infections and neonatal sepsis, 17 reduced incidence of respiratory syncytial virus infection, 18 prevention of respiratory tract infections in older individuals, 19 and almost 70% reduction of overall respiratory tract infections 20 to cite a few.
On the other hand, endemic infections especially malaria, induces well‐described and diverse changes in the host immune system. For example, T cells isolated from naïve adults, cultured in vitro with Plasmodium falciparum antigens, respond in a classical MHC Class II‐restricted manner by proliferating and secreting cytokines. Apart from developing into malaria parasite responsive TCRαβ+ cells and TCRγδ+ cells, they also express a memory phenotype, 21 These malaria‐reactive memory Th1 cells, however, are capable of responding to a wide range of antigens including Toxoplasma gondii, tetanus toxoid, adenovirus, mycobacterial, streptococcal, and fungal antigens. 22 The most famous example of malaria‐primed immunity was the advent of “malariatherapy” of patients suffering from neurosyphilis in 1917 by von Wagner‐Jauregg that fetched him the Nobel Prize in 1927. Brown and Brown later argued that repeated infections achieved a diverse repertoire of antigenic memory. 23 Malaria‐induced immune changes include both adaptive and innate immune changes and specifically diverse B cell repertoire changes like polyclonal activation via TLR9‐engagement, hypergammaglobulinemia and diversion of specific antibody responses among others. 24
Such parasite exposure induced stunting of severity of viral infections is well known, for example, Giardia lamblia induced modulation of rotaviral infection, 25 and protection against Chikungunya virus by infection with Plasmodium spp. 26 , 27 Various immune mechanisms for such cross‐protection are hypothesized, like immunomodulation, suppression of dendritic cell responses, activation of basophils and phagocytic macrophages and modulation the complement system, among others. 28
We observed in the present cross‐sectional study, that malaria endemicity is associated with reduced COVID‐19 cases and BCG vaccination is associated with reduced mortality. As shown in Figure 1C, for the countries like Italy, France, Spain, Sweden, a very lower BCG coverage has been observed on the other hand, Figure 1A suggests that the MRs are surprisingly high for those countries. This leads us to believe that the MRs of these countries are likely to be higher due to vaccine coverage. We refined the results by including multiple confounders like other commonly used vaccines, several other endemic infections, age structure of the population and societal confounders like airline passengers, HDI and number of tests performed to understand whether the correlations and associations that we have obtained are just spurious or it has a strong causality behind.
However, the inherent limitations of these kinds of studies persist, like proxy‐based measurement, lack of individual level data, potential for systematic differences between areas in recording disease frequencies and measurement of exposures. Also, along with the clustering technique, some other multivariate learning algorithms can be implemented here for better classification. The present study does not consider the current scenario (after the pandemic hits the countries) of the airline passengers and its impact on the infection counts. This is important as many countries closed their airspace which should also be relevant information for the present study. Also, the result of the present analysis is applicable for the dynamics of the first three months of the pandemic only. Nonetheless, we provide evidence that BCG and malaria exposure might be beneficial against this current pandemic. This study provides credence to the hypothesis that training of immunity by BCG vaccination and endemic parasitic exposure may be particularly beneficial against such viral pathogens and vaccine efforts should envisage using such trained immunity as an important tool against emerging pathogens.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Rudra P. Goswami is responsible for conceptualizing the theme of the paper and the overall interpretation of the results. Bhaswati Ganguli is responsible for conceptualizing the statistical analysis and the interpretation of the results and Moumita Chatterjee is responsible for conceptualizing and executing the statistical analysis and the interpretation of the results.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26875
Goswami RP, Ganguli B, Chatterjee M. Endemic infections, vaccinations, and variability of SARS‐COV2 worldwide epidemiology: A cross‐sectional study. J Med Virol. 2021;93:3105–3112. 10.1002/jmv.26875
DATA AVAILABILITY STATEMENT
The data that primarily support the findings of this study are openly available in worldometer at https://www.worldometers.info/coronavirus/, reference number (accessed on 4th June, 2020). Other details are included within the Reference section.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferrari R, Maggioni AP, Tavazzi L, Rapezzi C. The battle against COVID‐19: mortality in Italy. Eur Heart J. 2020;41(22):2050‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Neill LAJ, Netea MG. BCG‐induced trained immunity: can it offer protection against COVID‐19? Nat Rev Immunol. 2020;20:335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyasaka M. Is BCG vaccination causally related to reduced COVID‐19 mortality? EMBO Mol Med. 2020;12(6):e12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronavirus data dashboard of Worldometer. https://www.worldometers.info/coronavirus/. Accessed on 4th June, 2020.
- 6. World Health Organisation . TB statistics. https://www.who.int/tb/country/data/download/en/. Accessed on 6th May, 2020.
- 7. World Health Organisation. HIV statistics . https://www.who.int/hiv/data/en/. Accessed on 6th May, 2020.
- 8. World Health Organisation . 2020. World malaria report. https://www.who.int/malaria/publications/world-malaria-report-2019/en/. Accessed on 6th May.
- 9. World Health Organisation . 2020. Global vaccine monitoring summary. https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragebcg.html, Accessed on 6th May, 2020.
- 10. World Bank Open Data . 2020. https://data.worldbank.org/indicator/SP.POP.TOTL. Accessed on 6th May, 2020.
- 11. United Nations Development Programme . 2020. http://hdr.undp.org/en/content/2019-human-development-index-ranking. Accessed on 6th May, 2020.
- 12. United Nations Development Programme . 2020. http://hdr.undp.org/en/composite/HDI. Accessed on 6th May, 2020.
- 13.Wikipedia link of the list of countries with air passengers (available for the year 2017) around the globe. https://en.wikipedia.org/wiki/List_of_countries_by_airline_passengers
- 14.Number of COVID‐19 tests are obtained from the following two websites and accessed on 4th June, 2020. https://ourworldindata.org/coronavirus-testing https://www.worldometers.info/coronavirus/covid-19-testing/
- 15. Hirve S, Bavdekar A, Juvekar S, Benn CS, Nielsen J, Aaby P. Non‐specific and sex‐differential effects of vaccinations on child survival in rural western India. Vaccine. 2012;30(50):7300‐7308. [DOI] [PubMed] [Google Scholar]
- 16. Netea MG, Domínguez‐Andrés J, Barreiro LB. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;4:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low‐birth‐weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204:245‐252. [DOI] [PubMed] [Google Scholar]
- 18. Stensballe LG, Nante E, Jensen IP, et al. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea‐Bissau: a beneficial effect of BCG vaccination for girls community based case‐control study. Vaccine. 2005;23:1251‐1257. [DOI] [PubMed] [Google Scholar]
- 19. Wardhana DE, Sultana A, Mandang VV, Jim E, et al. The efficacy of Bacillus Calmette‐Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med Indones. 2011;45(3):185‐190. [PubMed] [Google Scholar]
- 20. Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N Engl J Med. 2018;379:138‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fell AH, Currier J, Good MF. Inhibition of Plasmodium falciparum growth in vitro by CD4+ and CD8+ T cells from non‐exposed donors. Parasite Immunol. 1994;16:579‐586. [DOI] [PubMed] [Google Scholar]
- 22. Currier J, Sattabongkot J, Good MF. ‘Natural' T cells responsive to malaria. evidence implicating immunological cross‐reactivity in the maintenance of TCR alpha beta+ malaria‐specific responses from non‐exposed donors. Int Immunol. 1992;4:985‐994. [DOI] [PubMed] [Google Scholar]
- 23. Brown KN, Brown IN. Immunity to malaria: antigenic variation in chronic infections of Plasmodium knowlesi . Nature. 1965;208(5017):1286‐1288. [DOI] [PubMed] [Google Scholar]
- 24. IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. 2013. Malaria and some polyomaviruses (SV40, BK, JC, and Merkel Cell Viruses). Lyon (FR): International Agency for Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 104.) Malaria. https://www.ncbi.nlm.nih.gov/books/NBK294249/ [PMC free article] [PubMed]
- 25. Bilenko N, Levy A, Dagan R, Deckelbaum RJ, El‐On Y, Fraser D. Does co‐infection with Giardia lamblia modulate the clinical characteristics of enteric infections in young children? Eur J Epidemiol. 2004;19:877‐883. [DOI] [PubMed] [Google Scholar]
- 26. Teo TH, Lum FM, Ghaffar K, et al. Plasmodium co‐infection protects against chikungunya virus‐induced pathologies. Nat Commun. 2018;9:3905. 10.1038/s41467-018-06227-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teo TH, Howland SW, Claser C, et al. Co‐infection with chikungunya virus alters trafficking of pathogenic CD8(+) T cells into the brain and prevents Plasmodium‐induced neuropathology. EMBO Mol Med. 2018;10:121‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shen SS, Qu XY, Zhang WZ, Li J, Lv ZY. Infection against infection: parasite antagonism against parasites, viruses and bacteria. Infect Dis Poverty. 2019;8(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that primarily support the findings of this study are openly available in worldometer at https://www.worldometers.info/coronavirus/, reference number (accessed on 4th June, 2020). Other details are included within the Reference section.


