Abstract
Introduction
Studies have shown that iron metabolism is affected by coronavirus disease 19 (COVID‐19), which has spread worldwide and has become a global health problem. Our study aimed to evaluate the relationship between COVID‐19 and serum erythropoietin (EPO), hepcidin, and haptoglobin (Hpt) levels with disease severity, and other biochemical values.
Methods
Fifty nine COVID‐19 patients hospitalized in the intensive care unit (ICU) and wards in our hospital between March and June 2020 and 19 healthy volunteers were included in the study. Participants were divided into mild, severe, and critical disease severity groups. Group mean values were analyzed with SPSS according to disease severity, mortality, and intubation status.
Results
Hemoglobin (Hb) levels were significantly lower in the critical patient group (P < .0001) and deceased group (P < .0001). The red blood cell distribution width‐coefficient of variation (RDW‐CV) and ferritin values were significantly higher in the intubated (P = .001, P = .005) and deceased (P = .014, P = .003) groups. Ferritin values were positively correlated with disease severity (P < .0001). Serum iron levels were lower in the patient group compared with the reference range. (P < .0001). It was found that the transferrin saturation (TfSat) was lower in the patient group compared with the control group (P < .0001). It was found that the mean EPO of the deceased was lower than the control group and the survived patient group (P = .035). Hepcidin levels were found to be significantly lower in the patient group (P < .0001). Hpt values were found to be significantly lower in the intubated group (P = .004) and the deceased group (P = .042).
Conclusion
In our study, while serum iron and hepcidin levels decreased in patients diagnosed with COVID‐19, we found that EPO and Hpt levels were significantly lower in critical and deceased patient groups. Our study is the first study examining EPO and Hpt levels in patients diagnosed with COVID‐19.
Keywords: anemia, biochemistry, COVID‐19, erythropoietin, iron homeostasis
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), which emerged in Wuhan, China, spread worldwide, became a pandemic, and was declared a global public health emergency by the World Health Organization (WHO). According to the latest WHO epidemiological data, 98.2 million reported cases and million deaths have been recorded worldwide. The number of cases diagnosed is increasing day by day. 1
To date, little information is known about the interaction of SARS‐CoV‐2 with host iron metabolism. The cytokines IL‐1, IL‐6, INF‐γ, and TNF‐α are potent regulators of iron metabolism, taking part in the cytokine storm in COVID‐19. 2 , 3 Iron homeostasis in COVID‐19 has become a crucial part of COVID‐19 disease. COVID‐19 involves the lungs, and hypoxemia is the main problem. Iron is required for erythrocytes to be able to carry oxygen, and therefore, the effect of iron metabolism in the prognosis of the disease is vital. The similarity in the distant amino acid sequence between the SARS‐CoV‐2–spiked glycoprotein cytoplasmic tail 4 and the hepcidin protein and the suggestion of the possibility of SARS‐CoV‐2 attacking the beta chain of hemoglobin in a biological modeling study has drawn attention to SARS‐CoV‐2 and iron metabolism. 5 Indeed, it was determined that hyperferritinemia or hypoferritinemia developed in the studies conducted. 6 However, it has not been fully elucidated how iron metabolism is affected in COVID‐19. This situation makes it challenging to choose a target for treatment. Although many agents that affect iron metabolism (rhEPO, iron chelators, anti‐hepcidin monoclonal antibodies, ferroportin antibodies) are recommended in the treatment, there is no clear evidence. 7
COVID‐19 has a high rate of hospitalization and mortality. Identifying patients with the highest risk of severe disease and enabling earlier aggressive interventions are essential in reducing mortality and managing health resources by reducing the need for intensive care. Therefore, some indicators are needed at an early stage for the severity and prognosis of the disease. In our study, we aimed to evaluate the relationship between COVID‐19 and serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values.
2. METHODS
2.1. Setting
This cross‐sectional study was conducted at the İstanbul Sisli Hamidiye Etfal Training and Research Hospital Health Sciences University after the institutional ethics committee approved the research plan (date: April 22, 2020, No 2727). Written informed consent was obtained from all participants. Consent was obtained from the relatives of the patients who could not give consent.
2.2. Patients
The study included patients who had SARS‐CoV‐2 RNA detected by reverse transcription polymerase chain reaction (RT‐PCR) (Bio‐speedy® SARS‐CoV‐2 Double Gene RT‐qPCR Kit; Bioeksen) in their nasopharyngeal or oropharyngeal swab specimens and hospitalized between March 2020 and June 2020 at the COVID intensive care unit and COVID wards of Health Sciences University Sisli and Sarıyer Hamidiye Etfal Training and Research Hospital. The presence of being under 18 years of age, hematological disease, chronic kidney disease, malignancy, giving no consent, pregnancy, and breastfeeding were determined as exclusion criteria. After completing the patient group, serum samples were collected from healthy volunteers of similar gender and age at the rate of 1/3 of the total number of patients for the control group. Information on gender, age, concomitant diseases, and the treatments given to all patients included in the study was recorded. Patients were grouped according to disease severity, intubation status, and survival status.
The classification used in the 7th edition of the COVID‐19 diagnosis and treatment plan published by the Chinese National Health Committee was used in the classification according to disease severity. 8
According to this classification:
Mild cases are defined as mild pneumonia or fever without pneumonia, other respiratory symptoms.
Severe cases are defined as patients meeting any of the following:
Respiratory rate of ≥ 30/min, hypoxia (SpO2 ≤ 93%),
PaO2/FiO2 ratio < 300.
Lung infiltration over 50% within 24‐48 hours.
Critical cases are defined as presence of any of the conditions of respiratory failure, septic shock, and/or multiple organ failure requiring mechanical ventilation.
2.3. Data acquisition
Venous blood samples were obtained in the morning after 12 hours of overnight fasting from all subjects who participated in the study. For routine biochemical parameters, venous blood samples collected in gel vacuum tubes (BD) were kept at room temperature for 30 minutes and then centrifuged at 3220×g for 10 minutes, and their serums were separated. In all serum samples, routine biochemical parameters were studied on the Beckman Coulter AU680 device on the day the samples were obtained. Total iron‐binding capacity (TIBC) was calculated by summing serum iron level and UIBC levels. Transferrin saturation was calculated with the formula of serum iron/TIBCx100. Serum samples for ferritin were studied on the Beckman Coulter DXI800 device. A whole blood sample taken into K2 EDTA tubes (BD) for hemogram analysis was studied on the Mindray BC6800 device on the same day the samples were obtained. The samples to be studied utilizing ELISA were kept at room temperature for 20 minutes, and thereafter, serums were separated by centrifugation at +4°C for 20 minutes at 1000×g. They were stored in Eppendorf tubes at −80°C until the day of analysis. Before the test analysis, they were kept at −20°C for 12 hours and at +4°C for 12 hours, respectively. The sera were thawed at room temperature and homogenized by vortexing and used in the ELISA study on the test analysis day. Bioassay Technology Laboratory brand Human Erythropoietin ELISA Kit (Cat. No: E1029Hu) was used for EPO analysis, Bioassay Technology Laboratory brand Human Haptoglobulin ELISA Kit (Cat. No: E1117Hu) was used for Hpt analysis, Bioassay Technology Laboratory brand Human Hepcidin ELISA Kit (Cat. No: E1019Hu) was used for hepcidin analysis. Washings were done on a BIO‐TEK ELx50 washer, and readings were performed on a BIO‐TEK ELx500 reader.
2.4. Statistical analysis
SPSS (Statistical Package for the Social Sciences) 21.0 for Windows program was used for statistical analysis. Statistical alpha significance level was considered as P < .05. If the data showed normal distribution, the Student t test was used for independent 2 groups, and one‐way ANOVA tests were used for independent 3 groups to compare group averages. Post hoc analyses were performed for the subgroup analysis of the groups for whom the one‐way ANOVA test was performed. If the data did not show normal distribution, the Kruskal‐Wallis test was used for independent 3‐groups analysis, and the Mann‐Whitney U test or Wilcoxon's W test was used for subgroup analysis and comparison of independent 2 groups. Diagnostic values of valuable parameters for different severity cases of COVID‐19 patients were assessed by the receiver operating characteristic (ROC) and area under the ROC curve (AUC).
3. RESULTS
3.1. Demographic and clinical characteristics of the participants
In the study, the results of 78 participants, including 59 patients with COVID diagnosis and 19 healthy volunteers, were analyzed. Participants consisted of 34 patients hospitalized in the intensive care unit, 25 patients treated in the ward, and 19 healthy volunteers. Patients were grouped according to disease severity, intubation status, and survival status.
The numerical distribution, mean age, and gender distribution of the patients by disease severity are presented in Table 1.
TABLE 1.
Mean values of gender and age of the groups according to the disease severity
| Disease severity | n | Gender | Mean | St± | Min | Max | |
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Control group | 19 | 13 | 6 | 63.47 | 11.467 | 42 | 86 |
| Mild | 18 | 13 | 5 | 60.89 | 12.409 | 42 | 88 |
| Severe | 19 | 12 | 7 | 64.11 | 10.718 | 47 | 85 |
| Critical | 22 | 16 | 6 | 65.59 | 11.253 | 48 | 87 |
| Total | 78 | 54 | 24 | 63.63 | 11.360 | 42 | 88 |
3.2. Results
No significant difference was found between the patient groups in terms of mean age, both according to disease severity (P = .636) and survival status (P = .168). No significant difference was also found between the patient groups in terms of gender distribution according to disease severity (P = .916) and survival (P = .482). While 22 patients were intubated, 37 patients were not intubated. Twenty‐three patients died, and 36 patients were discharged.
A significant difference was observed between the critical patient group and all other groups in terms of mean Hb values (P < .0001). While there was no significant difference between the control group and severe cases (P = .198), Hb values were significantly lower in the deceased patients compared with the survivors (P < .0001). Hb values were lower in intubated patients compared with nonintubated patients (P < .0001). When RDW‐CV values were examined, it was observed that there was a significant difference between the critical patient group and the control group, and mild and severe patient groups (P < .01). RDW‐CV levels were significantly higher in those who died compared with survivors (P = .014).
It was observed that as the severity of the disease increased, the ferritin value increased, and there was a significant increase between the groups (P < .0001). Serum ferritin levels were higher in the deceased patients than in the survivors (P = .003) and higher in intubated patients than in nonintubated patients (P = .005). When serum iron levels were studied according to disease severity, a difference was found between the control group and all groups (P < .001). However, there was no significant difference in serum iron levels in terms of survival (P = .176).
When the TIBC and UIBC levels were evaluated, a significant difference was observed between all disease severities (P < .0001). According to disease severity, when UIBC levels were evaluated, no statistical difference was found between the control group and the mild disease group (P = .236). TIBC and UIBC were negatively correlated with disease severity. Both TIBC and UIBC levels were significantly lower in the deceased than survivors (P < .0001). Both TIBC and UIBC levels were significantly lower in those who were intubated compared with those who were not intubated (P < .0001). It was found that transferrin saturation was significantly lower in patients diagnosed with COVID‐19 compared to the control group (p|<0.0001). However, when the subgroup analysis was performed, it was observed that this difference was present in the mild‐severe patient group (P < .0001), and the means of the critical patient group did not show a statistically significant difference compared with the control group (P = .123). No significant difference was observed in TfSat levels in terms of survival (P = .222). Intubated patients had significantly higher TfSat levels compared with nonintubated patients (P = .026).
A significant difference was found between the groups by the disease severity in terms of the mean EPO (P = .007). It was observed that this difference was only between critical patients, the control group (P < .0001), and mild cases (P = .015). EPO values were significantly lower in the deceased patients compared with the survivors (P < .035). A statistically significant difference was observed in hepcidin levels between critical patients and the control group (P < .001). Hepcidin levels were significantly lower in the intubated group than those who were not intubated (P = .005). However, no significant difference was found in hepcidin levels between those who died and those who survived (P = .111). A significant inter‐group difference was observed in haptoglobin values according to disease severity (P < .0001). Haptoglobin levels were significantly lower in the deceased patients than in the survivors (P = .042) and significantly lower in those who were intubated than those who were not intubated (P = .004).
Mean values of the measured parameters are grouped according to the severity of the disease in Table 2, according to survival in Table 3 and according to intubation status in Table 4.
TABLE 2.
Mean values of groups according to the disease severity, for malea and for femaleb
| Disease severity |
Ferritin ug/L |
Iron ug/L |
TIBC ug/L |
TfSat % |
UIBC ug/L |
Hb g/L |
RDW‐CV % |
EPO mIU/ml |
Hepcidin pg/mL |
Haptoglobin ug/mL |
|---|---|---|---|---|---|---|---|---|---|---|
| Reference range |
11‐306.8a 23.9‐336.2b |
700‐1800 | 1910‐3910 | 1180‐3180 |
130‐175a 115‐155b |
11.2‐15 | ||||
| Control group | ||||||||||
| Mean | 68.84 | 784.10 | 3284.73 | 24.31 | 2500.63 | 138.21 | 13.25 | 102.42 | 992.76 | 73.22 |
| St ± | 43.64 | 225.60 | 476.66 | 7.18 | 539.75 | 11.88 | 0.96 | 56.18 | 230.65 | 24.75 |
| Mild | ||||||||||
| Mean | 367.51 | 437.66 | 2695.88 | 16.67 | 2258.22 | 138.27 | 13.42 | 91.39 | 894.67 | 56.83 |
| St ± | 196.49 | 294.60 | 508.63 | 11.67 | 564.27 | 16.07 | 1.12 | 52.00 | 374.66 | 31.02 |
| Severe | ||||||||||
| Mean | 811.21 | 270.68 | 2055.10 | 13.75 | 1784.42 | 125.52 | 13.48 | 119.75 | 1004.69 | 75.62 |
| St ± | 847.84 | 116.07 | 613.99 | 5.94 | 587.62 | 16.09 | 1.04 | 106.19 | 626.91 | 91.04 |
| Critical | ||||||||||
| Mean | 1205.77 | 335.36 | 1669.59 | 21.29 | 1334.22 | 98.45 | 14.81 | 64.75 | 603.26 | 32.30 |
| St ± | 853.47 | 206.71 | 549.42 | 12.99 | 570.65 | 23.94 | 1.72 | 21.09 | 244.37 | 20.56 |
| Total | ||||||||||
| Mean | 639.27 | 452.52 | 2393.76 | 19.12 | 1941.24 | 123.92 | 13.78 | 93.47 | 863.17 | 58.48 |
| St ± | 756.44 | 291.76 | 822.21 | 10.63 | 721.09 | 24.31 | 1.41 | 67.22 | 424.51 | 52.26 |
| P value | .0001 | .0001 | .0001 | .0001 | .0001 | .0001 | .002 | .007 | .0001 | .0001 |
Abbreviations: EPO, erythropoietin; Hb, hemoglobin; RDW‐CV, coefficient of variation of red cell distribution width; TfSat, transferrin saturation; TIBC, total iron‐binding capacity; UIBC, unsaturated iron‐binding capacity.
TABLE 3.
Mean values of groups according to the survival status
| Survival |
Ferritin ug/L |
Iron ug/L |
TIBC ug/L |
TfSat % |
UIBC ug/L |
Hb g/L |
RDW‐CV % |
EPO mIU/mL |
Hepcidin pg/mL |
Haptoglobin ug/mL |
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors | ||||||||||
| Mean | 592.92 | 369.25 | 2388.38 | 16.06 | 2019.13 | 129.25 | 13.53 | 98.91 | 880.87 | 62.08 |
| St ± | 658.93 | 235.31 | 651.47 | 9.71 | 640.57 | 19.69 | 1.10 | 76.67 | 493.46 | 68.61 |
| Exitus | ||||||||||
| Mean | 1183.04 | 308.95 | 1666.17 | 19.63 | 1357.21 | 103.78 | 14.62 | 77.57 | 728.42 | 40.67 |
| St ± | 846.61 | 200.88 | 515.70 | 12.82 | 540.36 | 26.28 | 1.78 | 59.17 | 407.20 | 32.08 |
| P value | .003 | .176 | .0001 | .222 | .0001 | .0001 | .014 | .035 | .111 | .042 |
Abbreviations: EPO, erythropoietin; Hb, hemoglobin; RDW‐CV, coefficient of variation of red cell distribution width; TfSat, transferrin saturation; TIBC, total iron‐binding capacity; UIBC, unsaturated iron‐binding capacity.
TABLE 4.
Mean values of groups according to the intubation status
| Intubation status |
Ferritin ug/L |
Iron ug/L |
TIBC ug/L |
TfSat % |
UIBC ug/L |
Hb g/L |
RDW‐CV % |
EPO mIU/mL |
Hepcidin pg/mL |
Haptoglobin ug/mL |
|---|---|---|---|---|---|---|---|---|---|---|
| Not intubated | ||||||||||
| Mean | 595.35 | 351.91 | 2366.83 | 15.17 | 2014.91 | 131.72 | 13.45 | 105.95 | 951.17 | 66.48 |
| St ± | 654.36 | 234.26 | 645.04 | 9.17 | 616.96 | 17.12 | 1.07 | 84.39 | 515.66 | 68.48 |
| Intubated | ||||||||||
| Mean | 1205.77 | 335.36 | 1669.59 | 21.29 | 1334.22 | 98.45 | 14.81 | 64.75 | 603.26 | 32.30 |
| St ± | 853.47 | 206.71 | 549.42 | 12.99 | 570.65 | 23.94 | 1.72 | 21.09 | 244.37 | 20.56 |
| P value | .005 | .882 | .0001 | .026 | .0001 | .0001 | .001 | .018 | .005 | .004 |
Abbreviations: EPO, erythropoietin; Hb, hemoglobin; RDW‐CV, coefficient of variation of red cell distribution width; TfSat, transferrin saturation; TIBC, total iron‐binding capacity; UIBC, unsaturated iron‐binding capacity.
The statistical significance of the measured values according to the severity of the patients is given in Table 5.
TABLE 5.
Significance levels of the mean values of the patients between the groups according to the disease severity (P value)
| Mild | Severe | Critical | Difference between all groups | |
|---|---|---|---|---|
| Gender | ||||
| Control | 0.803 | 0.736 | 0.765 | 0.912 |
| Mild | 0.561 | 0.972 | ||
| Severe | 0.517 | |||
| Age | ||||
| Control | 0.925 | 0.999 | 0.950 | 0.636 |
| Mild | 0.866 | 0.646 | ||
| Severe | 0.982 | |||
| Ferritin | ||||
| Control | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Mild | 0.274 | 0.001 | ||
| Severe | 0.117 | |||
| Iron | ||||
| Control | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Mild | 0.036 | 0.328 | ||
| Severe | 0.488 | |||
| TIBC | ||||
| Control | 0.002 | 0.0001 | 0.0001 | 0.0001 |
| Mild | 0.001 | 0.0001 | ||
| Severe | 0.025 | |||
| TfSat | ||||
| Control | 0.006 | 0.0001 | 0.123 | 0.0001 |
| Mild | 0.693 | 0.157 | ||
| Severe | 0.019 | |||
| UIBC | ||||
| Control | 0.236 | 0.001 | 0.0001 | 0.0001 |
| Mild | 0.009 | 0.0001 | ||
| Severe | 0.006 | |||
| Hb | ||||
| Control | 1.000 | 0.198 | 0.0001 | 0.0001 |
| Mild | 0.204 | 0.0001 | ||
| Severe | 0.0001 | |||
| RDW‐CV | ||||
| Control | 0.703 | 0.430 | 0.0001 | 0.002 |
| Mild | 0.855 | 0.007 | ||
| Severe | 0.006 | |||
| EPO | ||||
| Control | 0.086 | 0.559 | 0.0001 | 0.007 |
| Mild | 0.879 | 0.016 | ||
| Severe | 0.105 | |||
| Hepcidin | ||||
| Control | 0.114 | 0.129 | 0.0001 | 0.0001 |
| Mild | 0.903 | 0.008 | ||
| Severe | 0.033 | |||
| Haptoglobin | ||||
| Control | 0.002 | 0.033 | 0.0001 | 0.0001 |
| Mild | 0.808 | 0.004 | ||
| Severe | 0.041 | |||
3.3. ROC Curve
According to the survival status, when the patient data were evaluated with the ROC curve, the area under the curve was mostly found in the disease severity, ferritin, intubation status, and RDW‐CV data (Figure 1).
FIGURE 1.
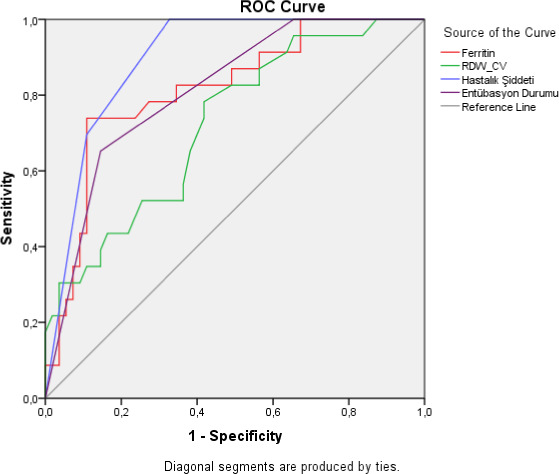
Evaluation of the relationship of the survival status of the patients with the measured parameters and the sensitivity and specificity of these values, by ROC curve
In order to evaluate survival, serum ferritin value was calculated as at 631 ug/L cutoff level, sensitivity as 73.9%, and specificity as 89.1%. For patients in the critical stage as disease stage, mortality prediction could be predicted with 69.6% sensitivity and 89.1% specificity.
While intubated patients died with 65.2% sensitivity and 85.5% specificity, sensitivity was measured as 65.2% and specificity as 61.8% for RDW‐CV cutoff value of 13.55.
4. DISCUSSION
In our study, Hb levels decreased with disease severity, and this decrease was even more remarkable in the intubated and deceased patient group. In a study conducted with 117 patients with a diagnosis of COVID‐19, it was found that the Hb values of the patients were relatively lower than the normal reference value and that it was more pronounced in severe and critical patients with mild anemia in most cases. 9 In a meta‐analysis conducted by Lippi et al with 1210 patients with COVID‐19, hemoglobin values were significantly lower in severe cases than in mild cases. A progressive decrease in hemoglobin values indicated that the disease would be a harbinger of poor clinical progress. 10 In the study conducted by Wang L et al on 339 patients hospitalized due to COVID‐19 diagnosis, it was stated that most of the patients' hemoglobin levels were below the normal range, but no significant difference was found in hemoglobin levels between the deceased and the survivors. 11 The reason we found different results in terms of survival may be the age range of the patients. The patient group's age range in our study was between 42 and 88 years, while the patients in the study conducted by Wang et al were over 65 years. Inclusion of the patient population on a broader age range in our study is likely to affect the difference in Hb value in survival. The reason for the Hb decrease in COVID‐19 patients remains unclear. In the current state of research, there is no explanation for the decrease in hemoglobin levels in COVID‐19. 12 The rapid change in red blood cells may be thought to be due to decreased production or hemolysis. However, it is not known whether the SARS‐CoV‐2 virus causes hemolysis. CD147, the new receptor of the spike protein of the SARS‐CoV‐2 virus, is an adhesion molecule expressed at all stages during the differentiation and maturation of erythrocytes. 12 The reason for the development of anemia in patients may be the relationship between the CD147 molecule of hematopoietic progenitor cells (HPC) and the spike protein. It is also possible that the SARS‐CoV‐2 virus invades erythrocytes via CD147, causing hemolysis. Erythrocyte lysis may occur in COVID‐19 due to the high oxidative environment. Whatever the cause, it is obvious that anemia will exacerbate hypoxia and worsen these patients' prognosis. The lower levels of Hb in intubated patients indicate this condition.
Red blood cell distribution width measures the variation in the volumes of red blood cells (RBC). Since erythrocytes characteristically decrease in cellular volume throughout their lifetime, the persistence of these older, smaller cells increases the volume variance and causes an increase in RDW. 13 , 14 A high RDW may reflect a clinical situation in which RBC production is slowed in inflammation, as well as indicative of hemolysis. In a cohort study of Foy et al on 1198 COVID‐19–diagnosed patients, a significant increase in mortality was found to be associated with the increased RDW values at admission and during hospitalization. An RDW of over 14.5% at admission for SARS‐CoV‐2 infection was associated with a 2.5‐fold increased risk of mortality. 15 Our findings support the literature that RDW is an indicator of risk and prognosis. The binding of SARS‐CoV‐2 to CD147 and CD26 in erythroblasts piques its effects on hematopoiesis. 16 Cavezzi et al 7 pointed out that SARS‐CoV‐2 can cause sideroblastic anemia with myelodysplastic properties as increased RDW is a reliable marker of myelodysplasia.
Although the primary modulator of ferritin levels is the presence of iron, its synthesis can also be regulated by different inflammatory cytokines such as IL‐1 and IL‐6. 17 , 18 Production of serum ferritin is affected by the presence of iron, pro‐inflammatory cytokines, and hepcidin. 19 Our findings show that serum ferritin level increases and hypoferremia develops in COVID‐19 disease. In a meta‐analysis by Henry et al, serum ferritin level was found to be significantly higher in the survivors and the severe group compared with the nonsevere group. 20 In the study conducted by Zhao et al, serum iron levels were found to be significantly decreased, and abnormally low serum iron levels were found in 45 (90%) of 50 patients. There was no statistically significant difference in pretreatment serum iron levels in the deceased patients and survivors. Also, serum iron levels were found to be decreased in survivors and exitus patients compared with the normal range. 21 Protective ventilation may be the reason for higher serum iron levels in critically ill patients. All patients in the critical patient group are intubated. Protective ventilation has a regulating effect on iron metabolism. 22 The storage of iron as ferritin in macrophages in inflammation explains the decrease in serum iron. The decrease in serum iron level prevents the use of iron for erythrocyte production and contributes to the development of anemia. We think that ferritin level is a crucial parameter in terms of showing prognosis and mortality.
Hepcidin is the primary regulator of iron metabolism, and body iron level is positively correlated with plasma hepcidin concentration. 23 , 24 Hepcidin binds to ferroportin and facilitates its degradation, thus reducing the absorption of iron. 25 Upregulation of hepcidin is common after a viral infection, so there is a decrease in iron uptake and an increase in iron storage in macrophages. 26 Hepcidin synthesis is increased with IL‐6 and an increasing amount of iron in the body. Hepcidin synthesis is reduced with erythropoietin, hypoxia, and anemia. In our study, hepcidin level was found to be lower in the critically ill group compared with the control group. Banchini et al emphasized the need to study hepcidin levels in COVID‐19 patients, recommending the use of iron chelators for downregulation of hepcidin as a possible way to improve symptoms in COVID‐19 patients. 27 In the study conducted by Hippchen et al, 28 IL‐6 and hepcidin levels increased significantly in COVID‐19 patients, and a significant correlation was observed between these two parameters. As COVID‐19 disease is a viral infection and inflammation upregulates hepcidin, it is thought that the level of hepcidin in COVID‐19 patients will increase, but there is no study other than the study conducted by Hippchen et al to support this in the literature. Regarding iron dysmetabolism in COVID‐19, Ehsani found a structural similarity between a SARS‐CoV‐2–spiked glycoprotein cytoplasmic tail and hepcidin protein. 4 Simulating hepcidin's action, SARS‐CoV‐2 can significantly increase circulating and tissue ferritin while inducing serum iron deficiency and hemoglobin deficiency. While showing all the effects of hepcidin, it can suppress hepcidin synthesis from the liver. This situation may explain why we find hepcidin levels lower. Also, the hypoxic condition may have suppressed the hepcidin. Therefore, further studies are needed on hepcidin levels in COVID‐19 patients.
Erythropoietin is a hormone that regulates erythropoiesis and tissue hypoxia stimulates erythropoietin production. It has been shown that erythropoietin and TNF‐α have an inverse dependence factor, and exogenous EPO administration can decrease TNF‐α levels. 29 A low T‐cell count is crucial in its ability to combat SARS‐CoV‐2 infection. 30 SARS‐CoV‐2 glycoproteins bind to human CD26 and possibly reduce CD26 concentration, 31 and CD26 is known to be involved in T‐cell activation. 32 Exogenous EPO releases immature and mature B and T cells from the bone marrow within the first 24 hours after administration, thereby increasing T cells' number. 33 EPO acts by modulating the immune response, 34 increasing T‐cell count, 33 and decreasing inflammatory cytokines such as TNF‐α. 29 EPO has anti‐inflammatory and anti‐apoptotic effects for many cell types and increases live RBC and hemoglobin production, helping restore oxygenation. 22 , 35 Besides, EPO has been shown to have protective effects in ALI/ARDS by reducing pulmonary edema, triggering pulmonary angiogenesis, and protecting the integrity of the pulmonary epithelial‐vascular endothelial cell. 36 Due to all these positive effects, it becomes the miraculous drug of COVID‐19 treatment. We found that the mean EPOs were significantly decreased in the critical patient group. This result suggests that rhEPO therapy may be effective in critically ill patients. In an 80‐year‐old COVID‐19 case published by Hadadi et al, it was reported that both the severity of anemia and the patient's symptoms were significantly reduced within 8 days after 9 days of rhEPO treatment. 37 There are opinions that rhEPO treatment will be an effective treatment in COVID‐19 patients. 38 However, there is no study on the EPO level in COVID‐19 patients. We tried to explain the relationship between the severity of the disease and EPO levels with this study.
Total iron‐binding capacity (TIBC) is an important test used to diagnose iron‐deficiency anemias and other iron metabolism disorders. The percentage of transferrin saturation (TfSat) is calculated by dividing the serum iron by TIBC and multiplying the result by 100. In cases with iron deficiency, the relative transferrin content increases compared with the iron content, and therefore, TIBC values are high. The opposite happens in iron‐overloaded states of the body; the amount of free transferrin in the blood decreases, and as a result, TIBC values are low. Transferrin saturation normal range is 25%‐35%. 39 In our study, disease severity was negatively correlated with both TIBC and UIBC levels. In the study conducted by Hippchen et al, 28 although transferrin level and transferrin saturation decreased compared with the normal range in patients with COVID‐19, it was significant in the hospitalized group and whose condition worsened. Our results support Hippchen et al regarding the transferrin level as it shows the transferrin level of TIBC. On the other hand, TfSat showed a significant decrease in the mild and severe patient group, while there was no significant difference in the critical patient group. In the study conducted by Bolondi et al, 40 the gradual physiological restoration of TfSat after 1 week of infection in COVID‐19 and irregular cytokine response suggested that it could contribute to the TfSat increase. It is known that serum iron and TfSat decrease in the early stages of infections, thus preventing the pathogen from reaching iron, but returning to almost normal values within 7‐10 days. 41 , 42 The fact that we did not standardize the patients' length of stay in our study may have affected the TfSat results. The fact that TfSat in our study had a higher percentage of TfSat compared with those who were not intubated suggests the regulating effect of ventilation on iron metabolism. The fact that all critical patients are intubated may be the reason why no significant difference was observed in critical patients.
Haptoglobin (Hpt) is a plasma protein with hemoglobin binding capacity. While it increases in inflammation, its level decreases in hemolytic anemias. Since haptoglobin is an acute phase reactant, the hemoglobins released by the disease‐causing hemolysis may decrease the Hpt level while expecting to increase in COVID‐19. There is no study in the literature about COVID‐19 and Hpt level yet. In our study, Hpt levels were found to be significantly lower in critical, intubated, and the deceased patients groups. The low values in the critical patient group suggest the possibility of Hb binding with Hpt as a result of hemolysis caused by the disease. It is possible that the SARS‐CoV‐2 virus invades erythrocytes via CD147 or the high oxidative environment causes hemolysis. However, further studies are needed on this subject to elucidate the relationship between COVID‐19 and Hpt.
There are some limitations to our study. First of all, the relatively small sample size and the fact that the patients' baseline values are not known may cause a deficiency in our evaluation. Also, the nonstandardization of the patients' hospitalization periods may not reflect the changes in iron parameters during the disease process.
5. CONCLUSION
Many parameters of iron metabolism in patients with COVID‐19 were examined in the same study of ours. Iron metabolism is affected by COVID‐19. It was determined that the decrease in Hb values in patients diagnosed with COVID‐19 correlated with disease severity and posed a risk for mortality. We determined that RDW values were a good indicator of risk and prognosis and that ferritin level is an essential parameter in showing prognosis and mortality. Unlike the literature, hepcidin levels were low in patients diagnosed with COVID‐19. EPO values were found to be significantly lower in critical patients and patients who died. Due to EPO's potential positive effects, EPO treatment may be considered in the critical patient group. Besides, we think that our study is the first study evaluating EPO and haptoglobin levels in patients with COVID‐19. In the treatment of COVID‐19, there are recommendations for applying rhEPO by targeting iron metabolisms, such as the use of anti‐hepcidin monoclonal antibodies and iron chelators. However, there is no clear evidence about their effects on treatment. The fact that the relationship between COVID‐19 and iron metabolism has not been fully elucidated yet causes its place in treatment not to be determined. Further studies are needed on this subject.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest in this article.
AUTHOR CONTRIBUTION
Erdinç Serin and Sema Yağcı designed the research study. Özlem Acicbe and Mustafa İsmet Zeren provided patient selection, grouping and sample supply. Sema Yağcı and Merve Sena Odabaşı performed the ELISA assays. Sema Yağcı and Merve Sena Odabaşı analyzed and interpreted data. Erdinç Serin and Sema Yağcı drafted and revised the manuscript. Erdinç Serin and Sema Yağcı edited the manuscript. All the authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We would like to thank the staff of the Department of Clinical Laboratory, Department of Infectious Diseases and Intensive Care Unit.
Yağci S, Serin E, Acicbe Ö, Zeren Mİ, Odabaşi MS. The relationship between serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values in patients with COVID‐19. Int J Lab Hematol. 2021;43:142–151. 10.1111/ijlh.13479
Funding information
The authors received no financial support for the research, authorship, and/or publication of this article
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. WHO . Weekly epidemiological update. 2021. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐‐‐27‐january‐2021. Accessed January 30, 2021.
- 2. Henry BM. COVID‐19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020;8(4):e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet (London, England). 2020;395(10229):1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ehsani S. COVID‐19 and iron dysregulation: distant sequence similarity between hepcidin and the novel coronavirus spike glycoprotein. Biology Direct. 2020;15(1): 10.1186/s13062-020-00275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu W, Li H. COVID‐19 disease: ORF8 and surface glycoprotein inhibit heme metabolism by binding to Porphyrin 1 introduction. chemRxiv. 2020; 10.32388/67WH9K [DOI] [Google Scholar]
- 6. Taneri PE, Gómez‐Ochoa SA, Llanaj E, et al. Anemia and iron metabolism in COVID‐19: a systematic review and meta‐analysis. European Journal of Epidemiology. 2020;35(8):763‐773. 10.1007/s10654-020-00678-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cavezzi A, Troiani E, Corrao S. COVID‐19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2):1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 9. Yuan X, Huang W, Ye B, et al. Changes of hematological and immunological parameters in COVID‐19 patients. Int J Hematol. 2020;112(4):553‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42(2):116‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80(6):639‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hopp M‐T, Domingo‐Fernandez D, Gadiya Y, et al. Unravelling the debate on heme effects in COVID‐19 infections. bioRxiv. 2020. 10.1101/2020.06.09.142125. Published online. [DOI] [Google Scholar]
- 13. Higgins JM, Mahadevan L. Physiological and pathological population dynamics of circulating human red blood cells. Proc Natl Acad Sci. 2010;107(47):20587‐20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel HH, Patel HR, Higgins JM. Modulation of red blood cell population dynamics is a fundamental homeostatic response to disease. Am J Hematol. 2015;90(5):422‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foy BH, Carlson JCT, Reinertsen E, et al. Elevated RDW is associated with increased mortality risk in COVID‐19. medRxiv. 2020. 10.1101/2020.05.05.20091702. Published online. [DOI] [Google Scholar]
- 16. Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26, and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020;75 (11):2829‐2845. 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li R, Luo C, Mines M, Zhang J, Fan G‐H. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 2006;281(49):37616‐37627. [DOI] [PubMed] [Google Scholar]
- 18. Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: past, present and future. Biochim Biophys Acta (BBA)‐General Subj. 2010;1800(8):760‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shoenfeld Y. Corona (COVID‐19) time musings: our involvement in COVID‐19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henry BM, De Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 21. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Serum iron level as a potential predictor of COVID‐19 severity and mortality: a retrospective study. Open Forum Infect Dis. 2020;7(7):ofaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lavranos G. The role of blood disorders in the manifestation of ARDS in COVID 19 and EPO as a potential therapeutic agent. Qeios. 2020. 10.32388/67WH9K. Published online. [DOI] [Google Scholar]
- 23. Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci. 2001;98(15):8780‐8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver‐specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811‐7819. [DOI] [PubMed] [Google Scholar]
- 25. Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science (80‐ ). 2004;306(5704):2090‐2093. [DOI] [PubMed] [Google Scholar]
- 26. Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541‐552. [DOI] [PubMed] [Google Scholar]
- 27. Banchini DF, Vallisa D, Maniscalco P, Capelli P. Iron overload and hepcidin overexpression could play a key role in COVID infection, and may explain vulnerability in elderly, diabetics, and obese patients. Acta Biomed. 2020;91(3):1‐8. 10.23750/abm.v91i3.9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hippchen T, Altamura S, Muckenthaler MU, Merle U. Hypoferremia Predicts Hospitalization and Oxygen Demand in COVID‐19 Patients. SSRN Electronic Journal. 10.2139/ssrn.3608074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson CS, Cook CA, Furmanski P. In vivo suppression of erythropoiesis by tumor necrosis factor‐alpha (TNF‐alpha): reversal with exogenous erythropoietin (EPO). Exp Hematol. 1990;18(2):109‐113. [PubMed] [Google Scholar]
- 30. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morimoto C, Schlossman SF. The structure and function of CD26 in the T‐cell immune response. Immunol Rev. 1998;161(1):55‐70. [DOI] [PubMed] [Google Scholar]
- 32. Vankadari N, Wilce JA. Emerging COVID‐19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ito T, Hamazaki Y, Takaori‐Kondo A, Minato N. Bone marrow endothelial cells induce immature and mature B cell egress in response to erythropoietin. Cell Struct Funct. 2017;42(2):149‐157.Published online. [DOI] [PubMed] [Google Scholar]
- 34. Cantarelli C, Angeletti A, Cravedi P. Erythropoietin, a multifaceted protein with innate and adaptive immune modulatory activity. Am J Transplant. 2019;19(9):2407‐2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng Q. Beyond erythropoiesis: the anti‐inflammatory effects of erythropoietin. Cardiovascul Res. 2006;71(4):615‐617.Published online. [DOI] [PubMed] [Google Scholar]
- 36. Kakavas S, Demestiha T, Vasileiou P, Xanthos T. Erythropoetin as a novel agent with pleiotropic effects against acute lung injury. Eur J Clin Pharmacol. 2011;67(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 37. Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human erythropoietin administration in critically ill COVID‐19 patients have miraculous therapeutic effects? J Med Virol. 2020;92(7):915‐918.Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ehrenreich H, Weissenborn K, Begemann M, Busch M, Vieta E, Miskowiak KW. Erythropoietin as candidate for supportive treatment of severe COVID‐19. Mol Med. 2020;26(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dale JC, Burritt MF, Zinsmeister AR. Diurnal variation of serum iron, iron‐binding capacity, transferrin saturation, and ferritin levels. Am J Clin Pathol. 2002;117(5):802‐808. [DOI] [PubMed] [Google Scholar]
- 40. Bolondi G, Russo E, Gamberini E, et al. Iron metabolism and lymphocyte characterisation during Covid‐19 infection in ICU patients: an observational cohort study. World Journal of Emergency Surgery. 2020;15(1). 10.1186/s13017-020-00323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eskeland B, Baerheim A, Ulvik R, Hunskaar S. Influence of mild infections on iron status parameters in women of reproductive age. Scand J Prim Health Care. 2002;20(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 42. Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood, J Am Soc Hematol. 1997;89(3):1052‐1057. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


