Abstract
Objective
To review the global impact of the COVID‐19 pandemic on stroke care‐metrics and report data from a health system in Houston.
Methods
We performed a meta‐analysis of the published literature reporting stroke admissions, intracerebral hemorrhage (ICH) cases, number of thrombolysis (tPA) and thrombectomy (MT) cases, and time metrics (door to needle, DTN; and door to groin time, DTG) during the pandemic compared to prepandemic period. Within our hospital system, between January–June 2019 and January–June 2020, we compared the proportion of stroke admissions and door to tPA and MT times.
Results
A total of 32,640 stroke admissions from 29 studies were assessed. Compared to prepandemic period, the mean ratio of stroke admissions during the pandemic was 70.78% [95% CI, 65.02%, 76.54%], ICH cases was 83.10% [95% CI, 71.01%, 95.17%], tPA cases was 81.74% [95% CI, 72.33%, 91.16%], and MT cases was 88.63% [95% CI, 74.12%, 103.13%], whereas DTN time was 104.48% [95% CI, 95.52%, 113.44%] and DTG was 104.30% [95% CI, 81.99%, 126.61%].
In Houston, a total of 4808 cases were assessed. There was an initial drop of ~30% in cases at the pandemic onset. Compared to 2019, there was a significant reduction in mild strokes (NIHSS 1‐5) [N (%), 891 (43) vs 635 (40), P = 0.02]. There were similar mean (SD) (mins) DTN [44 (17) vs 42 (17), P = 0.14] but significantly prolonged DTG times [94 (15) vs 85 (20), P = 0.005] in 2020.
Interpretation
The COVID‐19 pandemic led to a global reduction in stroke admissions and treatment interventions and prolonged treatment time metrics.
Introduction
The coronavirus (COVID‐19) pandemic has impacted many facets of healthcare worldwide and disrupted essential services. There are increasing reports on reduced acute stroke evaluations and admissions, prolonged symptom onset to hospital arrivals, and delays in the administration of time‐sensitive treatments for acute ischemic stroke (AIS), including intravenous thrombolysis (tPA), and mechanical thrombectomy (MT). 1 , 2 Multiple factors including fear of acquiring the infection in a healthcare setting and governmental lockdowns to prevent the virus transmission in the community have been postulated as causes of decreasing stroke admissions. 2
In this systematic review and meta‐analysis of the literature, we compare the global impact of the COVID‐19 pandemic on stroke care compared to the prepandemic period, and additionally, we report our experience from a Houston‐based healthcare system encompassing 10 hospitals.
Methods
Systematic review
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analysis guidelines (PRISMA). 3 The authors declare that all supporting data are publicly available and appropriately cited in this article. The study protocol has been published in the International Prospective Register of Ongoing Systematic Reviews PROSPERO (CRD42020218130).
Search strategy and selection criteria
Observational cohort studies (prospective and retrospective) suitable for inclusion in the review were identified through an independent search by the Texas Medical Library (TH) of the databases PubMed and Embase. The following keywords were used in all database searches: “COVID” or “COVID‐19” or “SARS‐CoV‐2” or “coronavirus” or “pandemic” AND “stroke” or “cerebrovascular disease” or “Ischemic Stroke” or “intracerebral hemorrhage” or “intracranial hemorrhage” or “Stroke admissions” or “Stroke epidemiology” or “Stroke care” or “Stroke metrics”, restricted to the English language. The last literature search was performed on November 13, 2020. The complete search algorithm used in the search is available in the supplement (Supplementary Methods). Reference lists of included articles were screened for potential studies missed by the initial search. Case reports and surveys, cross‐sectional studies, and non‐English language articles were excluded from consideration. The search results were screened by independent researchers (SR and NS) in a blinded fashion using Rayyan software for systematic reviews 4 and disagreements resolved via consensus of the two authors. Observational studies reporting impact of the COVID‐19 pandemic on stroke care (AIS and ICH) were considered eligible and were included in the present systematic review.
Quality control and bias assessment
Quality control and bias identification in included studies were performed by two independent reviewers who were involved in the screening (SR and NS) with the use of the “The Joanna Briggs Institute (JBI) Critical Appraisal Checklist for analytical cross‐sectional study”. 5 All conflicts were resolved via consensus agreement between the two authors.
Outcomes
Our predefined primary outcome measure was stroke admission rates during the COVID‐19 pandemic compared to the historical period (either time period immediately preceding the pandemic time frame or a corresponding time period from the previous year). We also assessed the impact of the COVID‐19 pandemic on the number of tPA and MT cases and corresponding time metrics, including door to needle times (DTN) and door to thrombectomy times (DTG) and intracerebral hemorrhage (ICH) cases.
Data synthesis and analysis
Studies with a similar time frame between the study period and comparison period and available daily counts of stroke admissions for both periods were included in the meta‐analysis. We compared six criteria – total admissions, tPA cases, MT cases, time metrics (DTN and DTG), and ICH cases of the study period with those from the comparison prepandemic period. As estimated values of some ratios have exceeded 1, we excluded the estimation of variance for each study and focused on the estimation of average for ratios in these six criteria. Based on each study's ratios, we estimated the weighted sum and variance using the number of centers as weights. As variance does not exist within the study level, homogeneity of variance test was not performed and funnel plots were not displayed.
Methods
Houston data
Study population and variables
The institutional review board approved the study and a waiver of consent was granted. We retrospectively analyzed data obtained from our stroke registry, which captured demographic and quality of care data on all stroke and suspected stroke patients admitted directly or transferred to any of the 10 hospitals, including four comprehensive stroke centers (CSC) within our health system based in the Greater Houston region. We assessed all stroke admissions, including ischemic AIS and ICH cases seen between January and June 2019, and compared with stroke admissions between January and June 2020. Demographic data including age, gender, race/ethnicity, and clinical data including time of last known well (LKW), time of hospital arrival, direct admission versus transfer status, use of tPA or MT, and time metrics associated with treatment (DTN and DTG) and discharge disposition (inpatient rehabilitation, skilled nursing facility, home, and hospice) were assessed.
Statistical analysis
We compared demographic and clinical characteristic of the two group of stroke patients admitted from January to June 2019 and those admitted from January to June in 2020. As part of descriptive analyses, continues variables were summarized using mean and standard deviation (SD) or median and interquartile range (IQR). Categorical variables were summarized using frequency counts and percentages. Formal testing hypotheses were performed to compare the two distributions of the measurements between the two time periods. For normally distributed continuous variables, we used a two‐sample t‐test and Wilcoxon Rank‐sum test when the distribution was not normal. To compare the proportions between the two groups of stroke patients, we used Logistic regression models. All analyses were performed at 5% level of significant using SAS 9.4 (SAS institute Inc., Cary, NC).
Results
Systematic review
A total of 52 studies reporting the impact of the COVID‐19 pandemic on stroke admissions were identified for qualitative synthesis. A total of 32,640 stroke admissions from 29 studies 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 were included in the meta‐analysis (Figure 1) (Supplementary Table S1) based on a similar time frame of the study period and comparison period, of which ratios were derived, depending on each criterion data availability. Compared to the prepandemic period, the mean ratio of stroke admissions during the pandemic was 70.78% [95% CI: (65.02%, 76.54%)] (Figure 2) and the mean ratio of ICH cases was 83.10% [95% CI: (71.01%, 95.17%)] (Supplementary Figure S1). The mean ratio of thrombolysis cases was 81.74% [95% CI: (72.33%, 91.16%)] (Supplementary Figure S2) and the mean ratio of MT cases was 88.63% [95% CI: (74.12%, 103.13%)] (Supplementary Figure S3) compared to the prepandemic period. The mean ratio of tPA metrics (DTN time) was 104.48% [95% CI: (95.52%, 113.44%)] (Supplementary Figure S4) and the mean ratio of MT metrics (DTG) was 104.30% [95% CI: (81.99%, 126.61%)] (Supplementary Figure S5) compared to the prepandemic period. Overall, stroke admissions, tPA cases, MT cases, and ICH cases showed a decrease from the comparison period, whereas tPA metrics and MT metrics showed an increase from the comparison period (Supplementary Figure S6).
Figure 1.
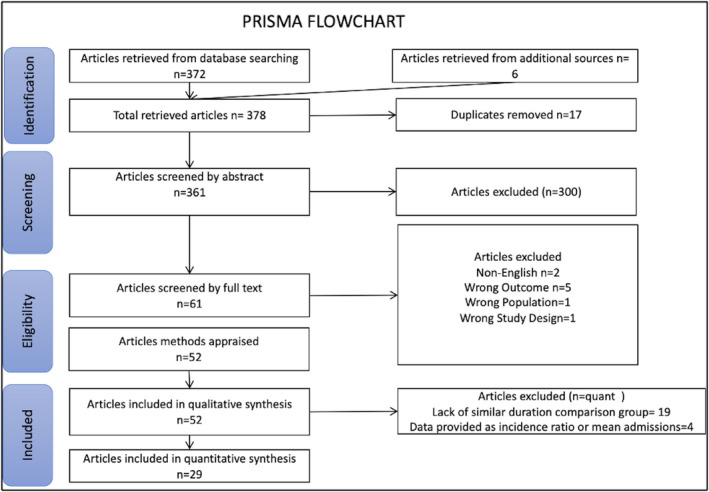
PRISMA Flowchart.
Figure 2.
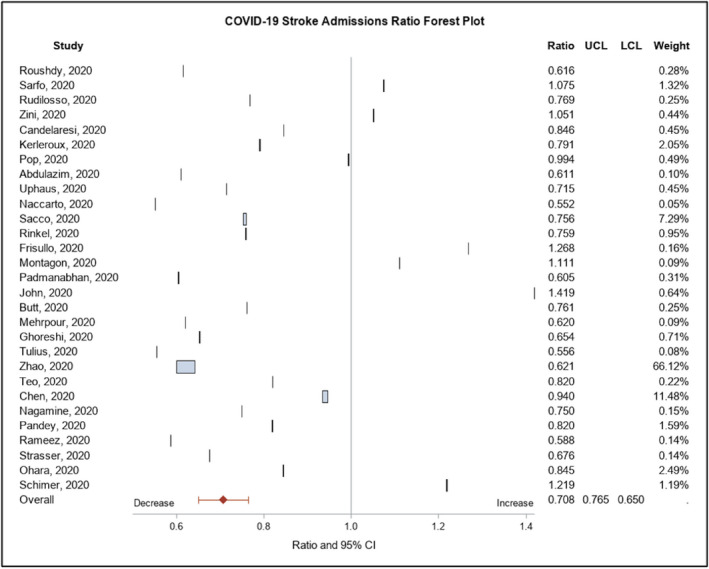
Forest plot of stroke admissions ratio among studies.
Houston data
Baseline characteristics
A total of 4808 cases were assessed, of which 2,596 and 2,212 cases were seen in the first 6 months of 2019 and of 2020, respectively. The mean (SD) age of patients in 2020 was slightly lower compared to 2019 [65 (15) vs 67 (15) years, P = 0.005] and there were fewer patients with mild strokes (NIHSS 1‐5) [N (%), 635 (39) vs 891 (43), P = 0.02] seen in 2020 compared to 2019. The median (IQR) NIHSS in 2019 period was lower than the 2020 study period [4 (1,11) vs 4 (1,13), P = 0.014].
Stroke admissions
After an initial drop of nearly 30% in case volumes at the pandemic onset (Figure 3), when compared to 2019, there was a 14% reduction in overall stroke admissions during the study period in 2020 (Table 1). The reduced volumes were observed irrespective of the hospital's stroke certification status, both at the primary and CSCs. Compared to the 2019 period, a significant decline in patient volumes in the 2020 period was noted in the transferred patients [N (%), 637 (34) vs 829 (36), P = 0.019] and in‐hospital stroke alerts [N (%), 69 (4) vs 111 (5), P = 0.036], whereas the number of direct admissions did not differ significantly [N (%), 1141 (62) vs 1332 (59), P = 0.851] between the two time periods. Furthermore, in terms of the stroke subtype, there were lower proportions of total ischemic strokes [OR (95% CI) = 0.87 (0.77, 0.98), P = 0.03] but no significant differences in the proportions of direct CSC presentations [OR (95% CI) = 0.93 (0.83, 1.04), P = 0.21] and ICH cases [OR (95% CI) = 1.12 (0.96, 1.29), P = 0.15] in 2020 compared to 2019. (Fig. 2).
Figure 3.
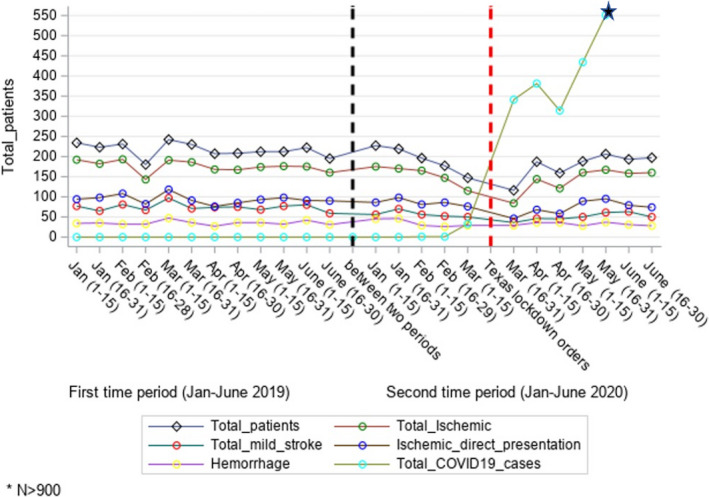
Comparison of stroke admissions between January–June 2019 and January–June 2020 in the Houston cohort.
Table 1.
Comparison of Stroke Care Metrics in the Houston Network between January to June 2020 and January to June 2019.
| Total admissions |
2019 (Jan‐June) Total patients (n) = 2596 |
2020 (Jan‐June) Total patients (n) = 2212 |
Odds Ratio (95% CI) | P‐Value* |
|---|---|---|---|---|
| Age, Mean (SD), year | 67 (15) | 65 (15) | 1.005 (1.002, 1.009) | 0.005** |
| Gender a | ||||
| Men, No. (%) | 1370 (53) | 1182 (53) | 0.974 (0.869, 1.091) | 0.647 |
| Women, No. (%) | 1223 (47) | 1030 (47) | 1.022 (0.912, 1.145) | 0.705 |
| Race b | ||||
| White, No. (%) | 928 (36) | 860 (39) | 0.875 (0.778, 0.983) | 0.025 |
| Unknown, No. (%) | 1045 (40) | 698 (32) | 1.461 (1.297, 1.646) | <0.001 |
| Black or African American, No. (%) | 556 (22) | 565 (25) | 0.794 (0.695, 0.908) | 0.001 |
| Asian, No. (%) | 64 (2) | 86 (4) | 0.625 (0.450, 0.868) | 0.005 |
| American Indian or Alaska Native, No. (%) | 1 (0) | 0 (0) | NA | NA |
| Hospital arrival c | ||||
| In‐hospital patients, No. (%) | 111 (5) | 69 (4) | 1.387 (1.022, 1.884) | 0.036 |
| Transfer from other hospitals, No. (%) | 829 (36) | 637 (34) | 1.160 (1.025, 1.313) | 0.019 |
| Direct presentation to CSC, No. (%) | 1332 (59) | 1141 (62) | 0.989 (0.883, 1.108) | 0.851 |
| Hospital (Region) | ||||
| MHH TMC, No. (%) | 930 (36) | 776 (35) | 1.033 (0.917, 1.163) | 0.592 |
| Other 9 hospitals, No. (%) | 1666 (64) | 1436 (65) | 0.968 (0.860, 1.090) | 0.592 |
| Hospital (Stroke care) | ||||
| 4 Comprehensive stroke centers Ω, No. (%) | 1925 (74) | 1690 (76) | 0.886 (0.777, 1.011) | 0.072 |
| 6 Primary stroke hospitals, No. (%) | 671 (26) | 522 (24) | 1.129 (0.989, 1.287) | 0.072 |
| NIHSS at hospital arrival d | ||||
| NIHSS (0‐42), all patients, Median (IQR) | 4 (1,11) | 4 (1,13) | 0.990 (0.983, 0.998) | 0.014 |
| NIHSS (1‐5), No. (%) | 891 (43) | 635 (39) | 0.854 (0.748, 0.975) | 0.020 |
| Length of stay e, Mean (SD), days | 6 (8) | 6 (6) | 1.008 (1.000, 1.016) | 0.051** |
| Discharge disposition f | ||||
| Acute care Facility, No. (%) | 39 (2) | 29 (1) | 1.147 (0.707, 1.861) | 0.579 |
| Expired, No. (%) | 157 (6) | 159 (7) | 0.831 (0.662, 1.044) | 0.112 |
| Home, No. (%) | 1414 (54) | 1212 (55) | 0.987 (0.881, 1.106) | 0.822 |
| Hospice Healthcare Facility, No. (%) | 61 (2) | 50 (2) | 1.040 (0.713, 1.519) | 0.837 |
| Hospice–Home, No. (%) | 51 (2) | 56 (3) | 0.772 (0.526, 1.132) | 0.185 |
| Left Against Medical Advice, No. (%) | 18 (1) | 12 (1) | 1.280 (0.615, 2.663) | 0.509 |
| Inpatient Rehabilitation, No. (%) | 856 (33) | 684 (31) | 1.099 (0.973, 1.241) | 0.129 |
| Total admission patients | Total patients = 1383 | Total patients = 1130 | ||
| (0 < Hospital arrival‐LKW ≤ 7200 minutes) | ||||
| LKW to Hospital arrival (minutes), Mean (SD) | 716.64 (1088.14) | 636.03 (862.13) | 0.293*** |
CSC Comprehensive stroke center; NIHSS National Institute of Health Stroke Scale; IQR interquartile range; SD standard deviation; LKW last known well; NA not applicable
NA: One patient in group 1 and no patients in group 2, so American Indian or Alaska Native could not be analyzed in the logistic regression
Ω: Comprehensive centers: MHH The Woodlands, MHH Memorial City, MHH Southwest, and MHH TMC
Number of missing data in each of two time periods, respectively: a: 3, 0 b: 2, 3 c: 324, 365 d: 539, 604 e: 1, 9 f: 0, 10.
P‐values for categorical variables are calculated based on logistic regression
P‐values are calculated based on two‐sample t‐test for normally distributed continuous variables and Wilcoxon Rank‐Sum test when the distributions were not normal
p‐values are calculated based on Wilcoxon rank‐sum test for time‐metrics variables
Stroke treatment time metrics
Compared to 2019, no significant differences were observed in the mean (SD) LKW to hospital arrival times in 2020 among the overall stroke admissions [716.64 (1088.14) vs 636.03 (862.13) minutes, P = 0.293] and in the ischemic stroke subtype presenting directly to a CSC [672.07 (1002.85) vs 576.14 (828.87) minutes, P = 0.098]. The number of patients treated with tPA was similar in ischemic strokes presenting directly to CSC in 2019 and 2020 [180 (16.00) vs 166 (17.74), OR (95% CI), 0.883 (0.701, 1.113), P = 0.293] and the number of large vessel occlusions treated with MT was also similar in 2019 and 2020 [101 (8.98) vs 77 (8.23), OR (95% CI) 1.100 (0.807, 1.500), P = 0.547]. Among the ischemic strokes presenting directly at CSCs, there were similar mean (SD) door to tPA [44 (17) vs 42 (18) minutes, P = 0.14] but a significantly prolonged door to thrombectomy times [94 (15) vs 85 (20) minutes, P = 0.005] in 2020 when compared to 2019 (Table 1).
In terms of discharge disposition, differences were noted only in the ICH subgroup between the two time periods. There was a significantly fewer number of ICH patients discharged to an inpatient rehabilitation facility in 2020 compared to 2019 [N (%), 135 (34.01) vs 173 (41.19), P = 0.028], and the in‐hospital mortality rate in the ICH patients was also higher in 2020 compared to 2019 [N (%), 90 (22.67) vs 67 (15.95), P = 0.018].
Discussion
In this systematic review, we summarize published reports of the impact of the COVID‐19 pandemic on stroke admissions and care. Overall, globally, there was ~29% global reduction in stroke admissions compared to the prepandemic period, including ~17% reduction in ICH cases. Moreover, there were fewer treatment interventions, with thrombolysis administration reduced by 18% and thrombectomy interventions by 11%. Additionally, there were prolonged treatment times with an increase in door to needle and groin times by 4%. We added data from our region because Houston became a major global epicenter for COVID‐19 in the time period studied. Our findings of reduced overall stroke admissions across 10 hospitals by ~30% during the pandemic onset and prolonged mechanical thrombectomy treatment times within a large healthcare system in the greater Houston region during the COVID‐19 pandemic are consistent with prior published literature from various stroke centers across the world. There was a transient increase in admissions before the ‘second wave’ of the pandemic in April–May 2020 (Figure 3). Moreover, similar to prior studies, we noticed a significant drop in patients with mild strokes (NIHSS 1‐5), 9 , 30 , 35 but there was no difference in last known well to hospital arrival or the number of cases treated with thrombolysis and thrombectomy in our cohort. 8 , 19
A number of reasons have been postulated to explain the reduced stroke admissions witnessed during the ongoing pandemic. Fear of acquiring the virus through community transmission, particularly in a healthcare setting, likely deters patients with milder strokes from seeking medical attention. 36 Additionally, governmental lockdowns to restrict public movement and community spread hinder access to healthcare systems, and as evidenced by our results, the steepest drop in admissions occurred in the third week of March 2020 when the state of Texas issued lockdown orders. Even though our results do not indicate increased time lapses between last known well and hospital arrival, in the future, when issuing statewide or nationwide mandates, it is crucial to simultaneously incorporate public awareness to encourage patients to seek timely medical care for emergent conditions like stroke and myocardial infarction which are treatable with time‐sensitive treatments. Taking the treatment to the patient with mobile stroke units can be a defining strategy during such crises in the future. Also, telehealth clinics for mild stroke and transient ischemic attacks should be considered.
Our results from Houston show the number of in‐hospital stroke alerts and evaluations were significantly lower during the pandemic than the previous year. It is possible that with increasing COVID‐19 cases admitted to the hospital and the requirement of extensive, time‐consuming donning and doffing of personal protective equipment, hospital staff were not as frequently evaluating patients as they would have otherwise done. Consequently, fewer neurological changes were being detected, and few stroke alerts being called. Additionally, elective surgical procedures across hospitals were suspended during the pandemic. With fewer operative patients, there could have been fewer postoperative complications, particularly cardiovascular procedures which account for most of the in‐hospital stroke alerts. We also noticed a decline in the number of patients transferred to our tertiary referral centers. The likely explanation was that volumes were reduced across the board in referring community hospitals as well, as has been seen elsewhere. 37 Moreover, tertiary centers in Houston were running at capacity, and there were possibly more transfer request denials due to hospital diversions due to lack of beds than the preceding years. Coordination among the hospital leadership and implementation of policies to assign and allocate resources for stroke patients in a future pandemic is vital. 38 , 39
The prolonged door to thrombectomy times in our systematic analyses and in our Houston cohort is of growing concern. 40 The reasons for delay may include delayed recognition of large vessel occlusions in the emergency room due to restructuring of emergency care teams including the endovascular team members (nursing staff and anesthesiologists) to care for the overwhelming number of COVID‐19 patients. Additional back‐up teams can be employed to prevent logistical delays. Whether the delay in treatment affects short‐ and long‐term outcomes in patients treated during the peak of the pandemic remains to be seen.
ICH patients are known to have worse functional outcomes compared to ischemic stroke patients. 41 Expectedly, the length of stay in the ICH cohort in Houston was longer than the ischemic subtype. Moreover, with rehabilitation and nursing facilities being at capacity during the pandemic and requiring negative COVID screening results before accepting hospital discharges, fewer patients were being discharged to inpatient rehabilitation.
Our systematic review has certain limitations. First, the included studies considerably varied in their comparator groups, with some comparing stroke admissions during the pandemic to the corresponding time period from the preceding years, whereas others are comparing admissions with the immediate prepandemic time frame. Moreover, there is considerable variation in the centers' certification status with some reports from primary stroke centers and others from comprehensive stroke centers and hospital systems, leading to potential publication bias. Smaller centers are more likely to run at capacity from COVID‐19 nonstroke admissions and, as a result, have reduced stroke admissions. We have not taken population density into account, which can also affect stroke prevalence rates in a region.
Conclusion
COVID‐19 pandemic has globally impacted stroke care and led to reduced overall stroke admissions (Figure 4), particularly mild stroke admissions and led to delays in stroke treatment. Public health awareness to encourage patients to seek medical attention and restructuring and adequate resource allocation is needed to avoid delays in treatment and subsequent disability. Identifying reasons to mitigate these findings is crucial for the ongoing and future pandemic preparedness.
Figure 4.
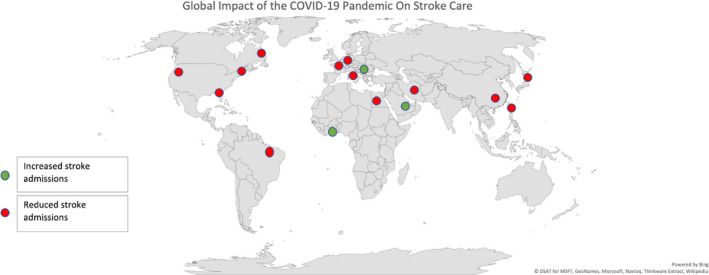
Global Impact of the COVID‐19 Pandemic on Stroke Admissions.
Conflict of Interest
None.
Supporting information
Supplementary Material. Search strategy for systematic review.
Supplementary Table S1. Comparison of 29 studies on 6 criteria (total admissions, thrombolysis (tPA) cases, thrombectomy (MT) cases, tPA metrics (tPA door to needle, DTN time), and MT metrics (door to groin, DTG)) of study period with those from the comparison period.
Supplementary Figure S1. Comparison of intracerebral hemorrhage (ICH) cases between study period and comparison period.
Supplementary Figure S2. Comparison of thrombolysis (tPA) cases between study period and comparison period.
Supplementary Figure S3. Comparison of mechanical thrombectomy (MT) cases between study period and comparison period.
Supplementary Figure S4. Comparison of thrombolysis (tPA) door to needle (DTN) time between study period and comparison period.
Supplementary Figure S5. Comparison of door to groin (DTG) between study period and comparison period.
Supplementary Figure S6. Summary statistics of all ratios from each criterion between study period and comparison period.
Acknowledgment
None.
References
- 1. July J, Pranata R. Impact of the coronavirus disease pandemic on the number of strokes and mechanical thrombectomies: a systematic review and meta‐analysis. J Stroke Cerebrovasc Dis 2020;29(11):105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegler JE, Zha AM, Czap AL, et al. Influence of the COVID‐19 pandemic on treatment times for acute ischemic stroke: the society of vascular and interventional neurology multicenter collaboration. Stroke 2020:Strokeaha120032789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;21(339):b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan‐a web and mobile app for systematic reviews. Syst Rev 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moola S, Munn Z, Sears K, et al. Conducting systematic reviews of association (etiology): the Joanna Briggs Institute's approach. Int J Evid Based Healthcare 2015;13(3):163–169. [DOI] [PubMed] [Google Scholar]
- 6. Roushdy TM, Nahas NME, Aref HM, et al. Stroke in the Time of Coronavirus Disease 2019: experience of Two University Stroke Centers in Egypt. J Stroke 2020;22(2):275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarfo FS, Mensah NO, Opoku FA, et al. COVID‐19 and stroke: experience in a Ghanaian healthcare system. J Neurol Sci 2020;416:117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rudilosso S, Laredo C, Vera V, et al. Acute stroke care is at risk in the era of COVID‐19: experience at a comprehensive stroke center in Barcelona. Stroke 2020;51(7):1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zini A, Romoli M, Gentile M, et al. The stroke mothership model survived during COVID‐19 era: an observational single‐center study in Emilia‐Romagna, Italy. Neurological Sciences 2020;41(12):3395–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Candelaresi P, Manzo V, Servillo G, et al. The impact of covid‐19 lockdown on stroke admissions and treatments in Campania. J Stroke Cerebrovasc Dis 2020;30(1):105448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kerleroux B, Fabacher T, Bricout N, et al. Mechanical thrombectomy for acute ischemic stroke amid the COVID‐19 outbreak: decreased activity, and increased care delays. Stroke 2020;51(7):2012–2017. [DOI] [PubMed] [Google Scholar]
- 12. Pop R, Quenardelle V, Hasiu A, et al. Impact of the COVID‐19 outbreak on acute stroke pathways – insights from the Alsace region in France. Eur J Neurol 2020;27(9):1783–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abdulazim A, Ebert A, Etminan N, et al. Negative Impact of the COVID‐19 Pandemic on Admissions for Intracranial Hemorrhage. Front Neurol 2020;11:584522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uphaus T, Gröschel S, Hayani E, et al. Stroke care within the COVID‐19 pandemic‐increasing awareness of transient and mild stroke symptoms needed. Front Neurol 2020;11:581394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naccarato M, Scali I, Olivo S, et al. Has COVID‐19 played an unexpected "stroke" on the chain of survival? J Neurol Sci 2020;414:116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rinkel LA, Prick JCM, Slot RER, et al. Impact of the COVID‐19 outbreak on acute stroke care. J Neurol 2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frisullo G, Brunetti V, Di Iorio R, et al. Effect of lockdown on the management of ischemic stroke: an Italian experience from a COVID hospital. Neurol Sci. 2020;41(9):2309–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montagnon R, Rouffilange L, Agard G, et al. Impact of the COVID‐19 pandemic on emergency department use: focus on patients requiring urgent revascularization. J Emerg Med 2021;60(2):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Padmanabhan N, Natarajan I, Gunston R, et al. Impact of COVID‐19 on stroke admissions, treatments, and outcomes at a comprehensive stroke centre in the United Kingdom. Neurol Sci 2021;42(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. John S, Hussain SI, Piechowski‐Jozwiak B, et al. Clinical characteristics and admission patterns of stroke patients during the COVID 19 pandemic: a single center retrospective, observational study from the Abu Dhabi, United Arab Emirates. Clin Neurol Neurosurg. 2020;2020(199):106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butt JH, Fosbøl EL, Østergaard L, et al. Effect of COVID‐19 on first‐time acute stroke and transient ischemic attack admission rates and prognosis in denmark: a nationwide cohort study. Circulation 2020;142(12):1227–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehrpour M, Shuaib A, Farahani M, et al. Coronavirus disease 2019 and stroke in Iran: a case series and effects on stroke admissions. Int J Stroke 2020;1747493020937397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghoreishi A, Arsang‐Jang S, Sabaa‐Ayoun Z, et al. Stroke care trends during COVID‐19 Pandemic in Zanjan Province, Iran. From the CASCADE Initiative: Statistical Analysis Plan and Preliminary Results. J Stroke Cerebrovasc Dis 2020;29(12):105321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tulius Silva M, Quintanilha G, Giesel L, et al. The impact of the COVID‐19 pandemic on a stroke center in Latin America. Int J Stroke 2020;15(7):813–814. [DOI] [PubMed] [Google Scholar]
- 25. Zhao J, Li H, Kung D, et al. Impact of the COVID‐19 epidemic on stroke care and potential solutions. Stroke 2020;51(7):1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teo KC, Leung WCY, Wong YK, et al. Delays in stroke onset to hospital arrival time during COVID‐19. Stroke 2020;51(7):2228–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen CH, Liu CH, Chi NF, et al. Maintenance of stroke care quality amid the coronavirus disease 2019 outbreak in Taiwan. J Stroke 2020;22(3):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagamine M, Chow DS, Chang PD, et al. Impact of COVID‐19 on acute stroke presentation at a comprehensive stroke center. Front Neurol 2020;11:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pandey AS, Daou BJ, Tsai JP, et al. Letter: COVID‐19 pandemic‐the bystander effect on stroke care in Michigan. Neurosurgery 2020;87(3):E397–E399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rameez F, McCarthy P, Cheng Y, et al. Impact of a stay‐at‐home order on stroke admission, subtype, and metrics during the COVID‐19 pandemic. Cerebrovasc Dis Extra 2020;10(3):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Strasser S, Miskolczi L, Cunha J, Justynski L. COVID‐19 impact on stroke presentations. J Stroke Cerebrovasc Dis 2020;29(10):105077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohara N, Imamura H, Adachi H, et al. Stroke systems of care during the COVID‐19 epidemic in Kobe City. J Stroke Cerebrovasc Dis 2020;29(12):105343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schirmer CM, Ringer AJ, Arthur AS, et al. Delayed presentation of acute ischemic strokes during the COVID‐19 crisis. J Neurointerv Surg 2020;12(7):639–642. [DOI] [PubMed] [Google Scholar]
- 34. Sacco S, Ricci S, Ornello R, et al. Reduced admissions for cerebrovascular events during COVID‐19 outbreak in Italy. Stroke 2020:Strokeaha120031293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Montaner J, Barragán‐Prieto A, Pérez‐Sánchez S, et al. Break in The Stroke Chain Of Survival Due To COVID‐19. Stroke 2020;51(8):2307–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perry R, Banaras A, Werring DJ, Simister R. What has caused the fall in stroke admissions during the COVID‐19 pandemic? J Neurol 2020;267(12):3457–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siegler JE, Heslin ME, Thau L, et al. Falling stroke rates during COVID‐19 pandemic at a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2020;29(8):104953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wira CR, Goyal M, Southerland AM, et al. Pandemic guidance for stroke centers aiding COVID‐19 treatment teams. Stroke 2020;51(8):2587–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nguyen TN, Abdalkader M, Jovin TG, et al. Mechanical thrombectomy in the era of the COVID‐19 pandemic: emergency preparedness for neuroscience teams: A Guidance Statement From the Society of Vascular and Interventional Neurology. Stroke 2020;51(6):1896–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Al Kasab S, Almallouhi E, Alawieh A, et al. International experience of mechanical thrombectomy during the COVID‐19 pandemic: insights from STAR and ENRG. J Neurointerv Surg 2020;12(11):1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bhalla A, Wang Y, Rudd A, Wolfe CD. Differences in outcome and predictors between ischemic and intracerebral hemorrhage: the South London Stroke Register. Stroke 2013;44(8):2174–2181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material. Search strategy for systematic review.
Supplementary Table S1. Comparison of 29 studies on 6 criteria (total admissions, thrombolysis (tPA) cases, thrombectomy (MT) cases, tPA metrics (tPA door to needle, DTN time), and MT metrics (door to groin, DTG)) of study period with those from the comparison period.
Supplementary Figure S1. Comparison of intracerebral hemorrhage (ICH) cases between study period and comparison period.
Supplementary Figure S2. Comparison of thrombolysis (tPA) cases between study period and comparison period.
Supplementary Figure S3. Comparison of mechanical thrombectomy (MT) cases between study period and comparison period.
Supplementary Figure S4. Comparison of thrombolysis (tPA) door to needle (DTN) time between study period and comparison period.
Supplementary Figure S5. Comparison of door to groin (DTG) between study period and comparison period.
Supplementary Figure S6. Summary statistics of all ratios from each criterion between study period and comparison period.


