Abstract
Severe COVID‐19 patients complicated with aspergillosis are increasingly reported. We present a histopathological proven case of fatal COVID‐19–associated pulmonary aspergillosis (CAPA), due to Aspergillus flavus. This report and existing published literature indicate diagnostic challenges and poor outcomes of CAPA in ICU patients.
Keywords: Aspergillosis, COVID‐19, Immunocompetent
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has been sweeping across the globe. Like severe influenza pneumonia, COVID‐19 is associated with acute respiratory distress syndrome (ARDS), which might be considered a risk of fungal colonisation and infection of the respiratory tract. 1 , 2 Mortality in severe COVID‐19 cases is significant compared with non‐severe infection cases due to the higher co‐infection rate. 3 Unlike bacterial co‐infections, the risk of fungal co‐infections, including oropharyngeal candidiasis, invasive aspergillosis (IA), endemic mycoses, mucormycosis and fusariosis is notable in patients with severe COVID‐19, despite the absence of classical well‐defined host factors. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Possible explanations for the development of fungal co‐infections include immune paralysis caused by COVID‐19 infection–induced ARDS, diffuse alveolar damage with severe inflammatory exudation and lymphopenia. 13 , 14 Preliminary reports showed 19‐33% of severe COVID‐19–associated pulmonary aspergillosis (CAPA) in ICU patients. 15 , 16 Research findings strongly suggest that mechanically ventilated COVID‐19 patients with longer duration of hospital admission should be systematically screened for Aspergillus infections. 15 Here, we describe CAPA in an immunocompetent patient and review the available literature on the subject.
2. CASE REPORT
A 70‐year‐old man with a history of recent hospital admission due to SARS‐CoV‐2 infection with the diagnosis of exacerbation of viral pneumonia that was had been referred to Imam Khomeini Hospital complex Tehran, Iran. Imam Khomeini Hospital complex is the largest referral centre in the country, admitted 25,410 patients in 2020 alone. Time course of the patient is detailed in Figure 1. In the previous hospitalisation, COVID‐19 infection was confirmed by positive nasopharyngeal PCR and with more than 50% field involvement of both lungs on chest CT scan. At the first admission, he had received hydroxychloroquine 200 mg/PO/BID for 5 days, interferon beta‐1 A/SC/every other day for 5 doses and dexamethasone 8 mg/IV/ daily according to the country guideline and had been discharged from the hospital after 12 days with a partial clinical recovery. However, after 3 days he was re‐admitted with exacerbation of respiratory symptoms. On admission, his respiratory rate was 28 /min and Spo2 in room air was 80%. The patient's laboratory data showed lymphopenia (216/mL) and elevated inflammatory markers (ESR: 30 mm/1hr, CRP: 40 mg/L, ferritin: 3,000 ng/mL, lactic acid dehydrogenase: 440 U/L and marked elevated D‐dimer: 2,572 ng/mL). Other laboratory results included WBC: 7,200/mL, PMN: 95.5%, Hb: 16 gr/dL, PLT: 176,000/mL and creatinine: 1.1 mg/dL. SARS‐CoV‐2 PCR test was still positive at the time of his re‐admission. Chest CT scan (Figure 2A) revealed multi‐lobar peripheral ground‐glass opacities compatible with COVID‐19 pneumonia (>50% involvement). Evaluation for heart diseases was negative (normal echocardiography). According to the progression of lung involvement due to the SARS‐CoV‐2 infection, the patient was treated with high‐dose methylprednisolone 250 mg/daily/IV for 3 days followed by dexamethasone 4 mg/IV/TID, atazanavir/ritonavir/PO/daily and supportive care. With the start of treatment, the patient's condition slightly improved, respiratory distress decreased and SpO2 reached 88% in room air, but in the second week of hospitalisation, the recovery process was not significant. In the third week of hospitalisation due to not achieving the desired therapeutic result, especially in the respiratory symptoms and persistence of high inflammatory markers, the patient underwent a new diagnostic evaluation. SARS‐CoV‐2 PCR test was reported positive again. A second CT scan showed reduction in ground‐glass opacities and three new foci of peripheral wedge‐shaped air‐space opacities with reverse halo in the right middle lobe (Figure 2B). Sputum samples for acid‐fast bacilli were negative. Because of likely/plausible fungal infection, voriconazole (6 mg/kg/BID day one followed by 4 mg/kg/BID) was started and the corticosteroid dose (dexamethasone 4 mg/IV/ daily) was reduced. Tissue obtained through CT‐guided biopsy of a peripheral lung lesion showed septate hyphae consistent with Aspergillus. Culture of the biopsy samples showed growth of green, powdery surface colonies suspected for Aspergillus spp. (Figure 3). Molecular identification was performed based on beta‐tubulin gene sequence 17 and identified as Aspergillus flavus. Despite antifungal therapy for 5 days, respiratory failure progressed and he went on non‐invasive ventilation support. Follow‐up CT scan showed that the opacities had evolved into irregular cavities, one of which contained sloughed debris mimicking a fungus ball and two cavities connected with bronchial lumen via bronchial wall defects (Figure 2C). After 48 hours, the patient was intubated on mechanical ventilation due to progressive respiratory failure, while continuing dexamethasone, voriconazole, sofosbuvir/daclatasvir and meropenem therapy. Unfortunately, the patient died after 12 hours with cardiac arrest. An autopsy was not performed.
FIGURE 1.
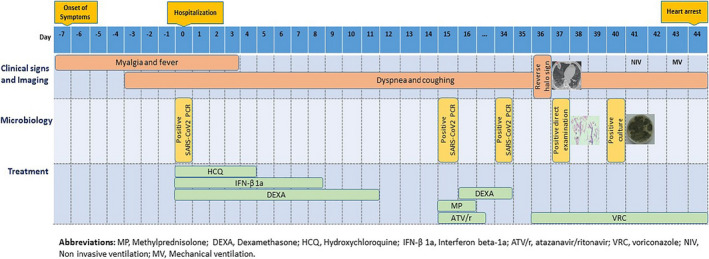
Timeline of the patient with COVID‐19–associated pulmonary aspergillosis
FIGURE 2.
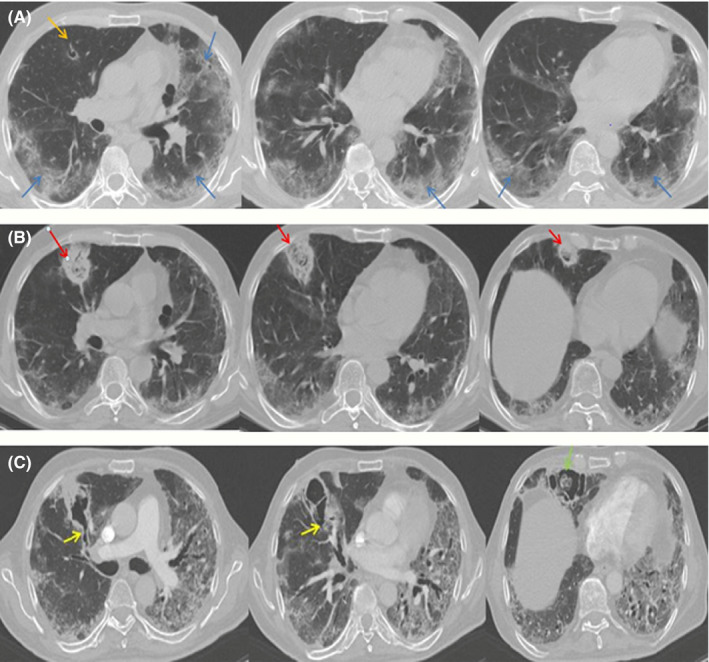
A, Contrast‐enhanced computed tomography chest showing multi‐lobar peripheral ground‐glass opacities; B, the reduced ground‐glass opacities and three new foci of peripheral wedge‐shaped air‐space opacities with reverse halo developed in the right middle lobe; C, the yellow arrows depict the foci of bronchial wall defects. The green arrow shows sloughed debris mimicking invasive aspergillosis
FIGURE 3.
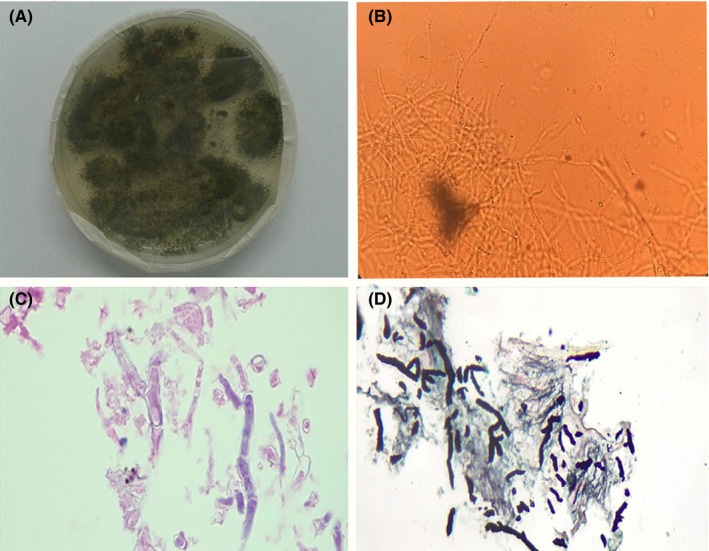
A, Culture on Sabouraud dextrose agar produced green, powdery surface colonies; B, Direct examination of the sample with KOH 10% show hyaline and septated hyphae (×400); C H&E staining show branched and septated hyphae with acute angle hyphae [100X objective]; D, Gomori's methenamine silver (GMS) staining highlights acute angle hyphae [40X objective]
2.1. Literature review
The English literature was reviewed for published CAPA cases using search terms “corona”, “COVID‐19”, “aspergillosis”, “CAPA” and “fungal”. A total of 175 CAPA cases were found and details are presented in Table 1. Although variable case definitions were used, only 7 (4%) cases were classified as proven CAPA.
TABLE 1.
Summary of previously reported cases of Aspergillus infection in COVID‐19 patients
| Authors/ References | Country | Number of patients | Mean age (SD) | Sex Malen (%) | BAL ǀ Serum GM*positive/total (%) | Mechanical ventilationn (%) | Culture / PCRn (%) | Aspergillus species/ Respirator samples (n) | Antifungal therapyn (%) | Outcome (mortality) n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bartoletti et al 6 | Italy | 30 | 63 | 24 (80) | 30/30 (100) ǀ 0/1 (0) | 30 (100) | 19 (63) / 20 (67) | A fumigatus (15), A niger (3), A flavus (1) / ND | VRC 13 (43) | 13 (44%) a |
| White et al 25 | United Kingdom | 25 | ND | ND | 17/19 (89.5) ǀ 1/4 (25) | 18 (72) | 11 (44) / 16 (64) | A fumigatus (10) / NBL (10) | VRC 9 (36), CSP + VRC 2 (8), AMB 2 (12), VRC + AMB 2 (8), FLU 1 (4), VRC + FLU 1 (4), ANI + AMB 1 (4) | 13 (52) |
| Marr et al 35 | USA | 20 | 65.5 | 9 (45) | 1/1 (100) ǀ 4/16 (25) | ND | 17 (85) / ND | A fumigatus (10), A niger (2), A terreus (1), A fumigatus + A niger (2), Aspergillus spp. (2)/ ND | VRC + PSO 1 (5), AMB 1 (5) | 3 (15) |
| Dupont et al 4 | France | 19 | 68.4 | 16 (84.2) | 5/9 (55.6) ǀ ND | 18 (94.7) | 16 (84.2) / ND | A fumigatus (14), A calidoustus (1), A niger (1) / BAL (8), TA (4), BA (6) | VRC 8 (42.1) VRC + CSP 1 (5.3) | 7 (36.8) |
| Falces‐Romero et al 36 | Spain | 10 | 67.1 | 8 (80) | 2/2 (100) ǀ 1/2 (50) | 7 (70) | 10 (100) / ND | A fumigatus (9), A nidulans (1) / BA (10) | VRC 2(20), AMB 1(10), VRC + CSP 1(10), AMB + ISA 1(10), AMB + VRC 1(10), AMB + ANI 1(10), MICA + AMB+ISA + VRC 1(10) | 7 (70) |
| Alanio et al 15 | France | 9 | 62.8 | 6 (66.7) | 1/7 (14.3) ǀ 0/8 (0) | 9 (100) | 7 (77.8) / 4 (44.4) | A fumigatus (7) / BAL (5), TA (2) | VRC 1 (11.1) CSP 1 (11.1) | 4 (44.4) |
| Wang et al 18 | China | 8 | 73 | 8 (100) | ND | 4 (50) | 8 (100) / ND | A fumigatus (8) / BAL (4), Sputum (4) | ND | ND |
| Rutsaert et al 20 | Belgium | 7 | 66.6 | 7 (100) | 5/6 (83.3) ǀ 0/6 (0) | 7 (100) | 6 (85.7) / ND | A fumigatus (5), A flavus (1) / BAL (6), TA (1) | VRC + ISA 2 (28.6), VRC 4 (57.1) | 4 (57.1) |
| Flikweert et al 24 | Netherlands | 7 | 73 | 5 (71.4) | 6/7 (85.7) ǀ ND | 7 (100) | 2 (28.6) / ND | A fumigatus (2) / BAL (2) | VRC + ANI 6 (85.7) | 7 (100) |
| van Arkel et al 37 | Netherlands | 6 | 63.8 | 6 (100) | 3/3 (100) ǀ 0/3 (0) | ND | 5 (83.3) / ND | A fumigatus (4), Aspergillus spp. (1) / TA (2), BAL (3), Sputum (1) | VRC 5 (83.3), AMB 1 (16.7) | 4 (66.7) |
| Koehler et al 16 | Germany | 5 | 62.6 | 3 (60) | 3/3 (100) ǀ 1/5 (20) | 5 (100) | 4 (80) / 4 (80) | A fumigatus (4) / BAL (2), TA (2) | VRC 2 (28.6), AMB 2 (28.6), CSP 2 (28.6), ISA 1(14.3) | 3 (60) |
| Nasir et al 5 | Pakistan | 5 | 69 | 3 (60) | ND ǀ 0/5 (0) | 2 (40) | 5 (100) / ND | A flavus (3), A niger (1), A flavus/A fumigatus (1) / ND | VRC 3 (33.3), AMB 2 (22.2) | 3 (60) |
| Sarrazyn et al 38 | Belgium | 4 | 75 | 3 (75) | 4/4 (100) ǀ ND | 4 (100) | 4 (100) / 2 (50) | Aspergillus spp. (4) / ND | VRC 1 (25), AMB + VRC 2 (50) | ND |
| Mitaka et al 39 | USA | 4 | 78.7 | 4 (100) | ND ǀ1/4 (25) | 4 (100) | 4 (100) / ND | A fumigatus (4) / ND | VRC 3 (75), CSP 1 (25) | 3 (75) |
| Lahmer et al 40 | Germany | 2 | 75 | 2 (100) | 2/2 (100) ǀ 1/2 (50) | 2 (100) | 2 (100) / ND | A fumigatus (2) / BAL (2) | AMB 2 (100) | 2 (100) |
| Lescure et al 41 | France | 1 | 80 | 1 (100) | ND | 1 (100) | 1 (100) / ND | A flavus / TA | VRC, ISA | 1 (100) |
| Blaize et al 42 | France | 1 | 74 | 1 (100) | 0/1 (0) ǀ ND | 1 (100) | 1 (100) / 1 (100) | A fumigatus / TA | ND | 1 (100) |
| Antinori et al 43 | Italy | 1 | 73 | 1 (100) | ND ǀ 1/1 (100) | 1 (100) | 1 (100) / ND | A fumigatus / BAL | AMB | 1 (100) |
| Prattes et al 44 | Austria | 1 | 70 | 1 (100) | ND ǀ 0/1 (0) | 1 (100) | 1 (100) / ND | A fumigatus / TA | VRC | 1 (100) |
| Meijer et al 7 | Netherlands | 1 | 74 | 0 (0) | 1/1 (100) ǀ 0/1 | 1 (100) | 1 (100) / ND | A fumigatus / TA | VRC + CSP | 1 (100) |
| Santana et al 45 | Brazil | 1 | 71 | 1 (100) | 0/1 (0) ǀ 1/1 (100) | 1 (100) | 1 (100) / 1 (100) | A penicillioides / Autopsy | ND | 1 (100) |
| Sharma et al 46 | Australia | 1 | 66 | 0 (0) | ND | 1 (100) | 1 (100) / ND | A fumigatus / TA | VRC | 0 (0) |
| Wu et al 47 | China | 1 | 46 | 1 (100) | ND | ND | 1 (100) / ND | A fumigatus / Sputum | VRC | 0 (0) |
| Schein et al 48 | France | 1 | 87 | 0 (0) | 1/1 (100) ǀ 1/1 (100) | ND | 0 / 1 (100) | ND | VRC | 1 (100) |
| Nasri et al 49 | Iran | 1 | 42 | 0 (0) | ND ǀ 1/1 (100) | 1 (100) | ND / ND | ND | AMB | 1 (100) |
| Mohamed et al 9 | Ireland | 1 | 66 | 1 (100) | ND ǀ 1/1 (100) | 1 (100) | 1 (100) / ND | A fumigatus / TA | AMB | 1 (100) |
| Ghelfenstein et al 50 | France | 1 | 56 | 1 (100) | ND ǀ 0/1 (0) | 1(100) | 1 (100) / ND | A fumigatus / TA | ND | 1 (100) |
| Fernandez et al 51 | Argentina | 1 | 85 | 1 (100) | ND ǀ 1/1 (100) | 1 (100) | 1 (100) / ND | A flavus / TA | VRC | 1 (100) |
| Machado et al 29 | Spain | 8 | 65 | 6 (75) | 2/8 (25) ǀ 4/8 (50) | 8 (100) | 8 (100) / 1 (100) | A fumigatus (5), A fumigatus + A awamori + A terreus(1), A lentulus(1), A citrinoterreus(1) / BA (5), BAL (2), TA (1) | AMB 2 (25), VRC 2 (25), ISA 4 (50) | 8 (100) a |
| Our study | Iran | 1 | 70 | 1 (100) | ND ǀ ND | 1 (100) | 1 (100) / 1 (100) | A flavus / Biopsy | VRC | 1 (100) |
| Total | ‐ | 183 | 68.5 (±9.6) | 120 (65) | 83/105 (79) ǀ 19/73 (26) | 135 (73.7) | 140 (76.5) / 51 (27.8) | A fumigatus(107), A flavus (8), A niger (7), A nidulans (1), A terreus (1), A penicillioides (1), A calidoustus (1), A lentulus (1), A citrinoterreus (1), Aspergillus spp. (7) Mix Aspergillus spp. (4)/ BAL (34), TA (20), BA (21), NBL (10), Sputum (6), Biopsy (1), Autopsy (1) | VRC 60 (32.7), AMB 16 (8.7), CSP 5 (2.6), FLU 1 (0.5), ISA 5 (2.7), Antifungal combination 24 (13.7) | 93 (50.8) |
Abbreviations: AMB, amphotericin B; ANI, anidulafungin; BA, bronchial aspirate; BAL, bronchoalveolar lavage; CSP, caspofungin; FLU, fluconazole; GM, galactomannan; ISA, isavuconazole; MICA, micafungin; NBL: non‐directed bronchial lavage; ND, not determined; PCR, polymerase chain reaction; PSO, posaconazole; SD, standard deviation; TA, tracheal aspirate; VRC, voriconazole.
Authors indicated CAPA‐related mortality.
Galactomannan values interpreted according to EORTC/MSGERC. 52 EORTC/MSGERC denotes European Organization for Research and Treatment of Cancer/ Mycoses Study Group Education and Research Consortium.
3. DISCUSSION
Although secondary bacterial and viral infections are reported at low frequency in COVID‐19 patients, high frequencies of CAPA cases are published in association with COVID‐19 in the ICU. Case series from the Netherlands, Germany and France reported CAPA 19%, 26% and 33% of patients with severe COVID‐19 pneumonia, respectively. 5 Although lower rates were reported from Switzerland (3.8%) and China (7%). 18 , 19 A major challenge remains diagnosing CAPA as the performance of diagnostic Aspergillus biomarkers remains suboptimal. Serum galactomannan (GM) detection is commonly negative even in patients with proven CAPA. 20 In our reviewed cases, serum GM was performed in 73 of 183 CAPA patients (39.8%), while GM was detected in only 19 (26%) patients (Table 1). Bronchoscopy with bronchoalveolar lavage (BAL) remains the preferred diagnostic procedure to diagnose CAPA, and GM was detected in 83 of 105 (79%) CAPA patients who underwent bronchoscopy. 21 However, bronchoscopy with BAL involves an aerosol‐producing procedure with contamination risk for healthcare workers. Although bronchoscopy is recommended to diagnose secondary infection in severe COVID‐19 cases, 22 many centres rely on bronchial aspirate or sputum to diagnose CAPA. 2 However, recovery of Aspergillus from these specimens may reflect upper respiratory tract colonisation rather than invasive infection. 23 Furthermore, there is conflicting evidence that supports both colonisation and invasive infection in Aspergillus‐positive COVID‐19 patients. On the one hand, COVID‐19 patients with evidence for Aspergillus have survived without antifungal therapy, which suggests that a positive culture or GM represents colonisation. 15 Furthermore, post‐mortem CT‐guided lung biopsies showed no evidence of IA, even in patients with positive BAL‐GM. 24 However, on the other hand several case series have shown a higher mortality in ICU patients with CAPA compared to COVID‐19 patients without evidence for Aspergillus. 6 , 25 There was a trend towards lower mortality in CAPA patients receiving antifungal therapy, compared with untreated cases, which suggests that the mortality, at least in part, may be attributable to IA. 6 , 25 To gain more insight into the pathophysiology of CAPA, it is critical to perform histopathological examination of lung tissue samples. However, similar to bronchoscopy, there is strong consensus in the literature that autopsy and thoracic surgery procedures classify as aerosol‐generating procedures, 26 and as a consequence, the number of proven CAPA cases is still very limited. Indeed, our literature review indicated that only in 7 of 175 (4%) CAPA cases the infection was proven, including the current case (Table S1). All classification of proven cases relies on the demonstration of invasive growth of septate hyphae, and the criteria are similar in the various definitions of IA. 27 , 28 , 29 Serum GM was performed in six proven cases, but found negative in four, which underscores the limited diagnostic value of this biomarker. Serum beta‐D‐glucan (BDG) might be a more sensitive marker as it was found to be positive in a higher proportion of patients. 15 However, this marker is not specific for IA and might be detected in patients with candidiasis and Pneumocystis pneumonia.
Various CAPA classification criteria have been used mostly based on those recommended for diagnosing IA in the ICU 28 or those proposed for patients with influenza‐associated pulmonary aspergillosis. 27 The various definitions hamper international CAPA surveillance studies and comparability of studies. The recently published ECMM/ISHAM CAPA consensus definitions might help to further standardise research in this area and support uniform patient classification. 30 Clearly, the utility of certain diagnostic procedures, such as non‐directed bronchial lavage (NBL), and the performance of diagnostic biomarkers on non‐validated samples such as tracheal aspirate and NBL remain to be determined. The difficulty in diagnosing CAPA and the associated risks of diagnostic procedures complicates patient management. A recently published management algorithm recommends to consider a diagnostic fungal work‐up in COVID‐19 patients in the ICU who have a positive aspergillus test performed on upper respiratory tract specimens, such as sputum or tracheal aspirates, and in those who show clinical deterioration or persistent poor respiratory function with no other explanation, or progressive radiology. 31 When the diagnosis of CAPA is confirmed initiation of antifungal therapy should be considered following international treatment guidelines, which recommend triazoles, such as voriconazole or isavuconazole, as first‐line treatment. 23 , 32 In our case, despite voriconazole therapy, the patient died due to concomitant involvement with coronavirus and failure to antifungal therapy. In A fumigatus, triazole resistance should be considered in azole‐treatment failure, but in our case, A flavus was recovered, which is the predominant Aspergillus species causing aspergillosis in Iran. 17 , 33 , 34
In conclusion, recovery of Aspergillus species in a critically‐ill COVID‐19 patient should not be ignored, but requires a diagnostic workup despite suboptimal performance of relevant biomarkers. The new consensus definitions and reporting of proven CAPA cases will help to further understand the pathophysiology of IA in patients with COVID‐19 and help to optimise clinical management.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Mohamad Salehi: Investigation (equal); Project administration (equal); Writing‐review & editing (equal). Nasim Khajavirad: Methodology (equal); Writing‐original draft (equal). Arash Seifi: Investigation (equal); Methodology (equal). Faeze Salahshour: Investigation (equal); Methodology (equal). Behnaz Jahanbin: Investigation (equal); Methodology (equal). Hossein Kazemizadeh: Investigation (equal). jamal Hashemi: Methodology (equal); Validation (equal). Seyed Ali Dehghan Manshadi: Investigation (equal); Methodology (equal). Mohammad Kord: Software (equal); Writing‐original draft (equal). Paul E. Verweij: Writing‐original draft (equal). sadegh Khodavaisy: Data curation (equal); Methodology (equal); Project administration (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
AUTHOR CONTRIBUTIONS
MRS, NK, FS, HK, AS, SJH, SADM and MK conceived the study, treatment, and discussed the case and the implications; S.KH., MK and BJ diagnosed the case; S.KH., MRS and PEV wrote the manuscript. All authors had full access to all data in the study and take responsibility for the integrity of the analysis.
Supporting information
Table S1
ACKNOWLEDGEMENTS
This study has been funded and supported by the Tehran University of Medical Sciences (TUMS), Grant No. 99‐1‐99‐47198.
Salehi M, Khajavirad N, Seifi A, et al. Proven Aspergillus flavus pulmonary aspergillosis in a COVID‐19 patient; A case report and review of the literature. Mycoses. 2021;64:809–816. 10.1111/myc.13255
REFERENCES
- 1. Schauwvlieghe AF, Rijnders BJ, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782‐792. [DOI] [PubMed] [Google Scholar]
- 2. Verweij PE, Gangneux J‐P, Bassetti M, et al. Diagnosing COVID‐19‐associated pulmonary aspergillosis. Lancet Microbe. 2020;1(2):e53‐e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan China. Clin Infect Dis. 2020;71:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dupont D, Menotti J, Turc J, et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 (COVID‐19). Med Mycol. 2020;10:myaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID‐19‐associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID‐19 pneumonia: An observational study from Pakistan. Mycoses. 2020;63(8):766‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID‐19 intubated patients: a prospective study. Clin Infect Dis. 2020;ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meijer EF, Dofferhoff AS, Hoiting O, Buil JB, Meis JF. Azole‐Resistant COVID‐19‐associated pulmonary aspergillosis in an immunocompetent host: a case report. J Fungi(Basel). 2020;6(2):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses. 2020;63(8):771‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohamed A, Rogers TR, Talento AF. COVID‐19 Associated Invasive Pulmonary Aspergillosis: Diagnostic and Therapeutic Challenges. J Fungi(Basel). 2020;6(3):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Messina FA, Marin E, Caceres DH, et al. Coronavirus disease 2019 (COVID‐19) in a patient with disseminated Histoplasmosis and HIV—A case report from Argentina and literature review. J Fungi(Basel). 2020;6(4):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Werthman‐Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID‐19. Am J Emerg Med. 2020, (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID‐19. Clin Microbiol Infect. 2020;26(11):1582‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salehi M, Ahmadikia K, Badali H, Khodavaisy S. Opportunistic Fungal Infections in the Epidemic Area of COVID‐19: A Clinical and Diagnostic Perspective from Iran. Mycopathologia. 2020;185:607‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alanio A, Delliere S, Fodil S, Bretagne S, Megarbane B. High prevalence of putative invasive pulmonary aspergillosis in critically ill COVID‐19 patients. medRxiv.. 2020;8:e48‐e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khodavaisy S, Badali H, Hashemi S, et al. In vitro activities of five antifungal agents against 199 clinical and environmental isolates of Aspergillus flavus, an opportunistic fungal pathogen. J mycol med. 2016;26(2):116‐121. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Yang Q, Zhang P, Sheng J, Zhou J, Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID‐19 in Zhejiang, China: a retrospective case series. Crit Care. 2020;24(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borman AM, Palmer MD, Fraser M, et al. COVID‐19 associated invasive aspergillosis: data from the UK National Mycology Reference Laboratory. J Clin Microbiol. 2020;59(1):e02136–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rutsaert L, Steinfort N, Van Hunsel T, et al. COVID‐19‐associated invasive pulmonary aspergillosis. Annals of Intensive Care. 2020;10(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koehler P, Cornely OA, Kochanek M. Bronchoscopy safety precautions for diagnosing COVID‐19 associated pulmonary aspergillosis‐A simulation study. Mycoses. 2020;64(1):55–59. [DOI] [PubMed] [Google Scholar]
- 22. Wahidi MM, Shojaee S, Lamb CR, et al. The use of bronchoscopy during the COVID‐19 pandemic: CHEST/AABIP guideline and expert panel report. Chest. 2020;158:1268‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ullmann AJ, Aguado JM, Arikan‐Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID‐ECMM‐ERS guideline. Clin Microbiol Infect. 2018;24:e1‐e38. [DOI] [PubMed] [Google Scholar]
- 24. Flikweert AW, Grootenboers MJ, Yick DC, et al. Late histopathologic characteristics of critically ill COVID‐19 patients: Different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020;59:149‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. White L, Dhillon R, Cordey A, et al. A national strategy to diagnose COVID‐19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020;ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson T, Deibert D, Wyatt G, et al. Classification of aerosol‐generating procedures: a rapid systematic review. BMJ Open Respir Res. 2020;7(1):e000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verweij PE, Rijnders BJ, Brüggemann RJ, et al. Review of influenza‐associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46(8):1524‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blot SI, Taccone FS, Van den Abeele A‐M, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 29. Machado M, Valerio M, Álvarez‐Uría A, et al. Invasive pulmonary aspergillosis in the COVID‐19 era: an expected new entity. Mycoses. 2021;64(2):132‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koehler PBM, Chakrabarti A, Chen SCA, et al. Defining and managing COVID‐19 associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2020. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Armstrong‐James D, Youngs J, Bicanic T, et al. Confronting and mitigating the risk of COVID‐19 associated pulmonary aspergillosis. Eur Respiratory Soc. 2020;56:2002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patterson TF, Thompson GR III, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63(4):e1‐e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zanganeh E, Zarrinfar H, Rezaeetalab F, et al. Predominance of non‐fumigatus Aspergillus species among patients suspected to pulmonary aspergillosis in a tropical and subtropical region of the Middle East. Microb Pathog. 2018;116:296‐300. [DOI] [PubMed] [Google Scholar]
- 34. Khodavaisy S, Badali H, Rezaie S, et al. Genotyping of clinical and environmental Aspergillus flavus isolates from Iran using microsatellites. Mycoses. 2016;59(4):220‐225. [DOI] [PubMed] [Google Scholar]
- 35. Marr KA, Platt A, Tornheim JA, et al. Aspergillosis complicating severe coronavirus disease. Emerg Infect Dis. 2021;27(1):18–25. 10.3201/eid2701.202896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Falces‐Romero I, Ruiz‐Bastián M, Díaz‐Pollán B, Maseda E, García‐Rodríguez J, Group SCW . Isolation of Aspergillus spp. in respiratory samples of patients with COVID‐19 in a Spanish tertiary care hospital. Mycoses. 2020;63(11):1144‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Arkel AL, Rijpstra TA, Belderbos HN, Van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID‐19–associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202(1):132‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sarrazyn C, Dhaese S, Demey B, Vandecasteele S, Reynders M, Van Praet JT. Incidence, risk factors, timing and outcome of influenza versus COVID‐19 associated putative invasive aspergillosis. Infect Control Hosp Epidemiol. 2020;1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitaka H, Perlman DC, Javaid W, Salomon N. Putative invasive pulmonary aspergillosis in critically ill patients with COVID‐19: An observational study from New York City. Mycoses. 2020;63(12):1368‐1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lahmer T, Rasch S, Spinner C, Geisler F, Schmid RM, Huber W. Invasive pulmonary aspergillosis in severe COVID‐19 pneumonia. Clin Microbiol Infect. 2020;26(10):1428‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lescure F‐X, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020;20(6):697‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blaize M, Mayaux J, Nabet C, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26(7):1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Antinori S, Rech R, Galimberti L, et al. Invasive pulmonary aspergillosis complicating SARS‐CoV‐2 pneumonia: A diagnostic challenge. Travel Med Infect Dis. 2020;38:101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P. Invasive pulmonary aspergillosis complicating COVID‐19 in the ICU‐A case report. Med Mycol Case Rep. 2020. 10.1016/j.mmcr.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Santana MF, Pivoto G, Alexandre MAA, et al. Confirmed Invasive Pulmonary Aspergillosis and COVID‐19: the value of postmortem findings to support antemortem management. Rev Soc Bras Med Trop. 2020;53:e20200401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharma A, Hofmeyr A, Bansal A, et al. COVID‐19 associated pulmonary aspergillosis (CAPA): An Australian case report. Med Mycol Case Rep. 2020. 10.1016/j.mmcr.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu S, Yang S, Chen R, Chen H, Xu Y, Lin B. Dynamic immune response profiles and recovery of a COVID‐19 patient with coinfection of Aspergillus fumigatus and other baseline diseases: a case report. OMICS. 2020;24(10):615‐618. [DOI] [PubMed] [Google Scholar]
- 48. Schein F, Munoz‐Pons H, Mahinc C, Grange R, Cathébras P, Flori P. Fatal aspergillosis complicating severe SARS‐CoV‐2 infection: a case report. J Mycol Med. 2020;30(4):101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nasri E, Shoaei P, Vakili B, et al. Fatal invasive pulmonary Aspergillosis in COVID‐19 patient with Acute Myeloid Leukemia in Iran. Mycopathologia. 2020;185:1077‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ghelfenstein‐Ferreira T, Saade A, Alanio A, et al. Recovery of a triazole‐resistant Aspergillus fumigatus in respiratory specimen of COVID‐19 patient in ICU–A case report. Med Mycol Case Rep. 2020. 10.1016/j.mmcr.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandez NB, Caceres DH, Beer KD, et al. Ventilator‐associated pneumonia involving Aspergillus flavus in a patient with coronavirus disease 2019 (COVID‐19) from Argentina. Med mycol case rep. (In Press) 2020. 10.1016/j.mmcr.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71(6):1367‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


