Summary
Convalescent plasma can provide passive immunity during viral outbreaks, but the benefit is uncertain for the treatment of novel coronavirus disease 2019 (COVID‐19). Our goal is to assess the efficacy of COVID‐19 convalescent plasma (CCP). In all, 526 hospitalized patients with laboratory‐confirmed SARS‐CoV‐2 at an academic health system were analyzed. Among them, 263 patients received CCP and were compared to 263 matched controls with standard treatment. The primary outcome was 28‐day mortality with a subanalysis at 7 and 14 days. No statistical difference in 28‐day mortality was seen in CCP cases (25·5%) compared to controls (27%, P = 0·06). Seven‐day mortality was statistically better for CCP cases (9·1%) than controls (19·8%, P < 0·001) and continued at 14 days (14·8% vs. 23·6%, P = 0·01). After 72 h, CCP transfusion resulted in transitioning from nasal cannula to room air (median 4 days vs. 1 day, P = 0·02). The length of stay was longer in CCP cases than controls (14·3 days vs. 11·4 days, P < 0·001). Patients with COVID‐19 who received CCP had a decreased risk of death at 7 and 14 days, but not 28 days after transfusion. To date, this is the largest study demonstrating a mortality benefit for the use of CCP in patients with COVID‐19 compared to matched controls.
Keywords: COVID‐19, convalescent plasma, mortality, transfusion, oxygenation
Introduction
The number of confirmed coronavirus disease 2019 (COVID‐19) cases, caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), continues to rise with more than 38·3 million confirmed cases and more than 1 million deaths worldwide as of October 14, 2020. 1 The case fatality rate varies per region but has been estimated to be as high as 15–30%, 2 despite multiple efforts to find a targeted treatment option and control the spread of the disease.
While most patients will have a mild course and convalesce at home, a minority of patients, often with significant comorbidities, will be hospitalized and have a higher incidence of critical or fatal disease. 2 , 3 , 4 , 5 , 6 Complications, including respiratory failure, 7 cardiovascular events (arrhythmias, acute cardiac injury, shock 8 , 9 , 10 ), and thromboembolic events, 11 , 12 , 13 have been described. Elevated inflammatory markers (e.g., D‐dimer, ferritin) and elevated pro‐inflammatory cytokines are associated with severe illness. 14 , 15 Seventy‐five percent of hospitalized patients will require supplemental oxygen, some despite the absence of dyspnoea. Therapeutic modalities to date are focused on reducing inflammation and viral replication and improving oxygenation. 16 , 17 , 18 , 19 , 20
The optimal approach to the treatment of COVID‐19 remains uncertain, 21 and the ‘standard of care’ has changed throughout the pandemic as data emerge. Recently, use of both steroids and remdesivir have demonstrated decreased 28‐day mortality and shorter recovery times. 22 , 23 , 24 , 25 Recent studies suggest reduced mortality after early (< 72h from admission) transfusion of high antibody titre‐containing COVID‐19 convalescent plasma (CCP). 26 In an open‐label study of 103 Chinese patients with severe or life‐threatening COVID‐19, the addition of CCP to standard treatment improved the rate of viral RNA clearance compared to controls. However, there were no statistically significant differences in the mortality at 28 days: 16% vs. 24%; P = 0·30; odds ratio [OR] 0·59; 95% confidence interval (CI) 0·22–1·59. Clinical improvement within 28 days occurred in 51·9% (27/52) of the convalescent plasma group versus 43·1% (22/51) in the control group [difference, 8·8% (95% CI, −10·4% to 28·0%); hazard ratio (HR), 1·40 (95% CI, 0·79–2·49); P = 0·26]. 27
Convalescent plasma passively transfers antibodies capable of neutralizing the virus with the goal of reducing the severity of illness. 28 It is thought to be most effective when administered early in the course of the disease. 28 Recent results from patients treated under the Mayo Expanded Access Program (EAP) show relative risk reduction in 7‐ and 30‐day mortality for patients treated with high‐titre plasma compared to those treated with low‐titre plasma. Additionally, patients transfused ≤3 days from COVID‐19 diagnosis had improved mortality compared to transfusion after four or more days from diagnosis. 29 There is a paucity of data comparing patients with COVID‐19 who receive CCP to those who do not, and these studies are required to prove the efficacy of the treatment. 26 , 30 , 31 While randomized controlled trials are being designed and conducted, the goal of this study is to assess the efficacy of CCP transfusion as a treatment option for hospitalized patients with COVID‐19 using a retrospective analysis of existing electronic health record data.
Methods
This is a retrospective, health system‐based, matched cohort study comparing hospitalized patients with COVID‐19 who received convalescent plasma (CCP cases) to matched controls who had COVID‐19 and received standard treatment but were not transfused with convalescent plasma (controls).
Participants
Both cases and controls included patients aged ≥18 years with laboratory‐confirmed severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and hospitalized at one of eight hospitals in a single academic health system. Patients receiving CCP were considered to have severe or life‐threatening COVID‐19 by criteria defined by the United States Food and Drug Administration (FDA) and were treated under the Mayo EAP. 32 Care across hospitals within the health system was deemed equivocal. Patients were treated with similar clinical protocols and transferred across the health system as bed availability required during the regional surge in COVID‐19 cases.
Procedure
CCP cases were transfused one to two units (approximately 200–500 ml total volume) of ABO‐compatible CCP in accordance with institutional guidelines. The plasma was collected according to FDA guidelines and was obtained from local blood collection centres and national blood suppliers. At the time of this study, antibody titres and neutralizing assays were not available for the transfused CCP. To assess changes in oxygen requirements, the daily highest oxygen delivery device needed was recorded and stratified into five categories by level of support: mechanical ventilation, non‐invasive positive pressure ventilation, non‐rebreather, nasal cannula, or no oxygen requirements. A decrease in support by one category was considered an improvement (Table SI). The length of stay was calculated from the time of arrival at the acute‐care facility. Data from 10% of patients were re‐confirmed by manual review and found to be accurate.
Matching
Matching of controls to CCP cases was executed using a publicly available exact matching macro in SAS (Cary, NC, USA). The control pool was comprised of all hospitalized patients with COVID‐19 throughout the academic health system. Out of 2 013 patients, 263 were matched as controls. Controls were matched by gender, age ±5 years, preceding length of stay (beginning with the arrival time that resulted in their inpatient stay), and oxygen delivery device on the hypothetical transfusion day. All controls had a laboratory‐confirmed SARS‐CoV‐2 by polymerase chain reaction (PCR) within 14 days of the index hospitalization. The control cohort was treated, on average, 29 days prior to the case cohort.
Outcomes
The primary outcome was death from any cause at 28 days with a sub‐analysis of 7‐day and 14‐day adjusted mortality. The secondary outcomes were improvement in oxygenation as defined by a decrease in oxygen delivery device category by one level (Table SI) and the total length of stay with a sub‐analysis of the length of stay for discharged patients.
Statistical analysis
The study was designed to have 80% power to detect a 20% difference in 28‐day mortality between the treatment groups. At least 154 primary outcome events were required for the study to be conclusive in the transfused cohort and 308 in the controls for a goal 2:1 match. Because of the restrictive criteria for an appropriate control, only a 1:1 match of controls to CCP cases was obtained. Baseline characteristics were compared using Pearson’s chi‐square test or Fisher’s exact test for categorical variables when appropriate, and Student’s t‐test for continuous variables. For survival analysis, Kaplan–Meier curves were created to estimate median survival times. The Cox proportional hazard model was used to determine time‐to‐event hazard ratios. The log‐rank test was used to compare the survival curves. Censor time was defined as time to outcome, hospital discharge, or 28 days, whichever occurred first. Analyses were conducted using SAS version 9·4 (Cary, NC, USA), and graphs were created in R.
Study approval
Written informed consent was obtained from the participants receiving CCP or a legally authorized representative before enrollment. The CCP programme was approved on April 13, 2020, by the MedStar institutional review board (IRB) with reliance on the external Mayo IRB, which served as the central IRB for all participating facilities and empanelled an independent Data and Safety Monitoring Board to oversee the safety analysis. A Health
Insurance Portability and Accountability Act (HIPAA) waiver for retrospective analysis was obtained to match the controls for the study. It was approved on June 6, 2020, by the MedStar IRB for secondary research on data or specimens.
Results
Out of 2 013 hospitalized patients with laboratory‐confirmed SARS‐CoV‐2, 526 patients were analyzed across the CCP and control groups. In all, 294 patients received CCP on the Mayo EAP between April 13 and July 7, 2020, and 263 of these patients were matched to controls (Fig 1). Patients used as controls were hospitalized from March 15 to July 19, 2020.
Fig 1.
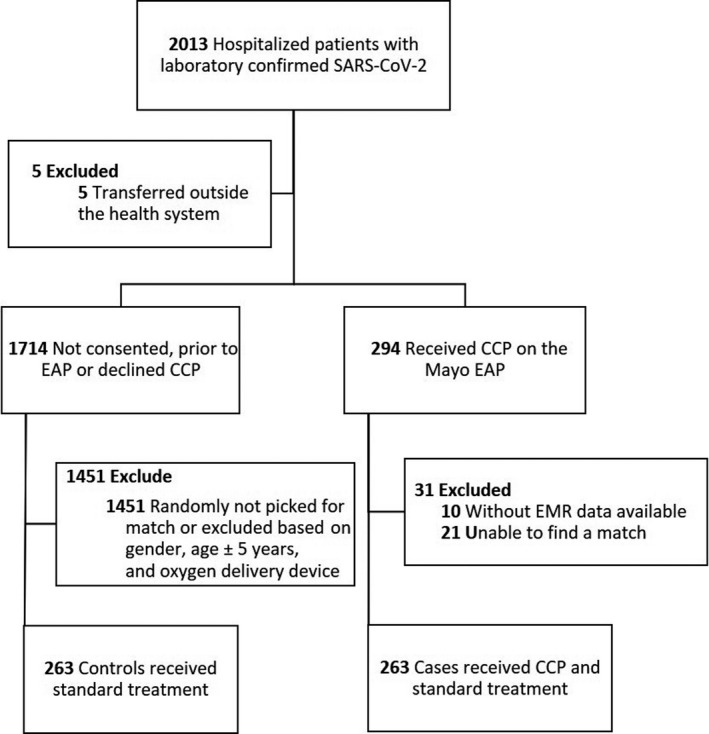
Patient selection and matching. CCP, COVID‐19 convalescent plasma; EAP, Expanded Access Program; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2; EMR, electronic medical record.
Key patient characteristics are presented in Table I. Per design, both groups were balanced in age and gender. The control group had a higher proportion of African Americans (54·28% vs. 42·21%, P = 0·02), and the case group had more Hispanics (17·49% vs. 10·27%, P = 0·01) but were otherwise balanced in race and ethnicity. There was no difference in weight (93·62 kg vs. 93·41 kg, P = 0·92) between controls and CCP cases. Transfused patients received a median of 245·6 ml (SD = 144·40) of CCP.
Table I.
Patient characteristics.
| Variables | Controls (n = 263) | CCP cases (n = 263) | P‐value |
|---|---|---|---|
| Age, mean, (SD), year | 56·1 (14·00) | 55·93 (14·01) | 0·88 |
| Female sex, no. (%) | 96 (36·50) | 96 (36·50) | 1·00 |
| Race, no. (%) | |||
| African American | 144 (54·285) | 111 (42·21) | 0·02 |
| Asian | 5 (1·90) | 6 (2·28) | – |
| Other | 76 (28·90) | 101 (38·40) | – |
| Unknown | 6 (2·28) | 14 (5·32) | – |
| White | 32 (12·17) | 31 (11·79) | – |
| Ethnicity, no. (%) | |||
| Hispanic | 27 (10·27) | 46 (17·49) | 0·01 |
| Non‐Hispanic | 182 (69·20) | 154 (58·56) | – |
| Unknown | 54 (20·53) | 63 (23·95) | – |
| Weight, mean (SD), kg | 93·62 (26·87) | 93·41 (24·23) | 0·92 |
| Medications, no. (%) | |||
| Azithromycin | 177 (67·30) | 157 (59·70) | 0·07 |
| Dexamethasone | 21 (7·98) | 70 (26·62) | <0·001 |
| Hydrocortisone | 25 (9·51) | 33 (12·55) | 0·26 |
| Hydroxychloroquine | 123 (46·77) | 12 (4·56) | <0·001 |
| Methylprednisolone | 32 (12·17) | 85 (32·32) | <0·001 |
| Prednisone | 1 (0·38) | 5 (1·90) | 0·21 |
| Remdesivir | 9 (3·42) | 107 (40·68) | <0·001 |
| Sarilumab | 0 (0·00) | 1 (0·38) | 1 |
| Tocilizumab | 47 (17·87) | 76 (28·90) | 0·002 |
| Transfused plasma volume, mean (SD), ml | – (–) | 245·6 (144·40) | – |
CCP, COVID‐19 convalescent plasma.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The 28‐day mortality rate was 25·48% in CCP cases and 27·00% in controls, (P = 0·06) (Table II). The 7‐day adjusted mortality rate was statistically different between the two groups and was lower in CCP cases (9·13%) compared to controls (19·77%) (P < 0·001) with an absolute reduction of 10·64%. The 14‐day adjusted mortality continued to be statistically significant, with a mortality rate of 14·83% for CCP cases and 23·57% for the controls (P = 0·01) with an 8·74% absolute reduction (Fig 2). Consistent with previous reports, 33 no transfusion reactions occurred in our cohort.
Table II.
Primary and secondary outcomes.
| Variables | Controls (n = 263) | CCP cases (n = 263) | P‐value |
|---|---|---|---|
| Mortality, no. (%) | |||
| 28‐day | 71 (27) | 67 (25·48) | 0·06 |
| 14‐day | 62 (23·57) | 39 (14·83) | 0·01 |
| 7‐day | 52 (19·77) | 24 (9·13) | <0·001 |
| Length of stay, mean (SD), days | |||
| Overall | 10 (10·86) | 15·67 (13·65) | <0·001 |
| Mechanical ventilation | 15·92 (16·03) | 20·97 (16·07) | 0·07 |
| Non‐invasive positive pressure ventilation | 10·17 (9·47) | 15·04 (13·01) | 0·005 |
| Non‐rebreather | 7·28 (6·08) | 16·88 (11·43) | <0·001 |
| Nasal cannula | 5·41 (4·50) | 8·13 (10·41) | 0·10 |
| LOS for discharged patients* | 14·56 (12·18) | 19·18 (14·75) | <0·001 |
| Improvement in oxygen delivery device category, median, (hazard ratio), days | |||
| Overall | 6 (–) | 3 (1·12) | 0·22 |
| Mechanical ventilation | 15 (–) | 11 (1·43) | 0·11 |
| Non‐invasive positive pressure ventilation | 4 (–) | 3 (1·00) | 0·99 |
| Non‐rebreather | 4 (–) | 2 (1·10) | 0·58 |
| Nasal cannula | 3 (–) | 2 (1·42) | 0·06 |
CCP, COVID‐19 convalescent plasma; LOS, length of stay.
Excluding deceased patients.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Fig 2.
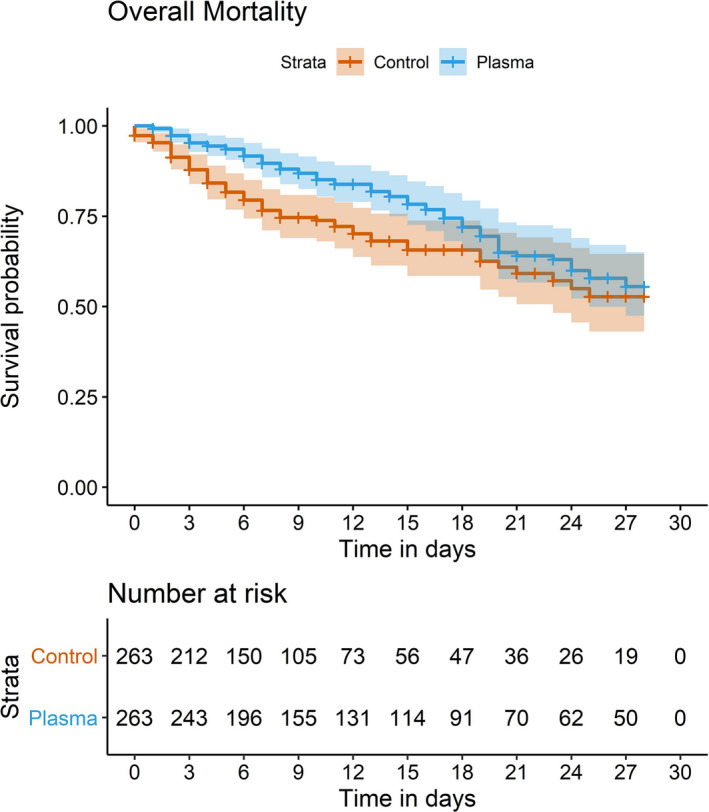
Kaplan–Meier curve showing 28‐day mortality.
The median time to a decrease in the oxygen device category was shorter by three days in the CCP cases compared to the controls (Fig 3). The case cohort had a median of three days to improvement in the oxygen device category compared to six days in the control cohort (P = 0·25); this was not statistically significant when comparing cohorts or subgroups of patients within cohorts. However, a subanalysis of patients transfused within three days of arrival demonstrated a statistical difference (P = 0·02) in oxygen requirement for patients requiring nasal cannula (three days) compared to controls six days; Table SII).
Fig 3.
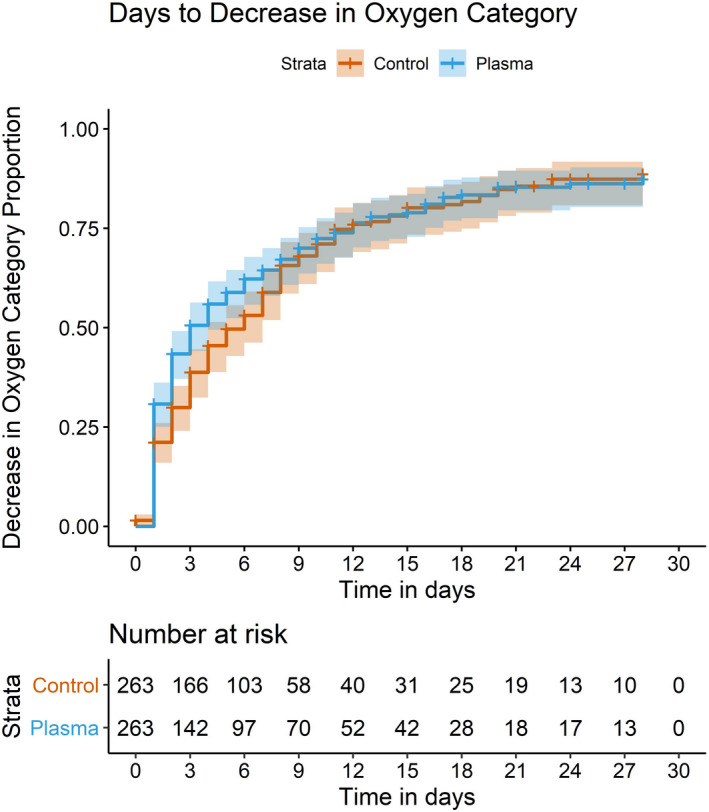
Days to improvement in oxygenation as defined by a decrease in oxygen delivery device category by one level.
Length of stay was longer for the CCP cases compared to the controls; 15·67 (SD 13·65) and 10·00 (SD 10·86) days, respectively (P < 0·001). A subanalysis of the length of stay of discharged patients only (omitting patients who died) also showed that the length of stay was longer for CCP cases compared to controls; 19·18 (SD 14·75) and 14·56 (SD 12·18) days, respectively, (P = 0·001).
The use of dexamethasone (31·52% vs. 9·7%, P < 0·001), methylprednisolone (32·73% vs. 12·12%, P < 0·001), remdesivir (50·50% vs. 3·64, P < 0·001), and tocilizumab (29·70% vs. 16·36%, P = 0·004) was more common among CCP cases. Hydroxychloroquine use was more common among the control group (41·82% vs. 1·82, P < 0·001). These imbalances continued to exist when limiting the analysis to more recent phases of the pandemic. Reducing the time to match CCP cases and control on date of arrival to the hospital to 14 days limited the number of CCP cases included in the study below the power required to attain significant results.
Discussion
Passive antibody therapy through convalescent‐plasma transfusion has shown benefit anecdotally in COVID‐19, primarily by case series and smaller studies utilizing matched controls showing 28‐day mortality benefit in patients transfused within 72 h of admission. 26 Previous results from the Mayo EAP show that the use of CCP transfusion is safe with minimal serious adverse events and a signal of mortality benefit; however, only small studies compared CCP treated patients to control patients who did not receive plasma. 26 This study showed that convalescent‐plasma therapy among hospitalized patients with COVID‐19 has an immediate mortality benefit when measured at seven days (P < 0·001) and 14 days (P = 0·01) after CCP transfusion compared to the standard treatment alone. While the difference in mortality between CCP cases and controls was not seen at 28 days (P = 0·06), there was a trend towards a benefit for CCP.
Convalescent plasma may provide clinical benefit when it contains high IgG antibody titres and when given early in the course of disease. 26 , 32 , 34 However, the optimal antibody titre for convalescent plasma required for viral neutralization is unknown. 30 , 35 It is also not known if additional benefit would be obtained by re‐treatment. A major constraint of this study was that antibody titres of transfused products were unknown. Transfused CCP was likely heterogeneous in antibody titre, and this could skew results. Additionally, patients’ pre‐existing comorbidities were not evaluated, and viral load through the clinical course was not known; both may potentially impact results. Pre‐existing comorbidities will need to be evaluated in further analyses of these data.
Given that the 7‐ and 14‐day mortality was better for CCP cases than controls, it was surprising that the length of stay was longer for the case cohort. Additionally, the impact of CCP seemed most significant for the first 14 days but did not impact mortality at 28‐days. It is possible that CCP transfusion does not impact evolving comorbid conditions that may complicate hospital stay such as kidney disease, cardiovascular complications, or thromboembolism, and a planned secondary analysis will look further into the hospital course of these patients. Additionally, for patients receiving low‐titre plasma, the passive immunity transferred could have been enough to mitigate severe disease but not enough to stop viral replication, inflammatory response, and downstream effects. 36
The SARS‐CoV‐2 virus is known to target the lower respiratory tract, and significant pulmonary compromise that leads to acute respiratory distress syndrome (ARDS) is a major complication in some patients. 38 , 39 Therefore, treatment modalities capable of improving respiratory compromise are valuable. In this study, the median days to improvement in the oxygen device delivery category was three days in the CCP cases compared to six days in the controls. While this was not statistically significant, it is remarkable that this trend was seen in all categories of respiratory support (Fig 3). Future studies using larger cohorts and/or high titre plasma will be better equipped to answer this question.
The evolution of treatment for COVID‐19 is rapidly changing; the timing and duration of medications administration during disease evolution and the target patient profile for treatment are still evolving. 22 , 23 , 39 Initial trial data suggest a clinical benefit with remdesivir and preliminarily, a mortality benefit with dexamethasone, but no other therapies have proven effective. 23 , 40 Remdesivir resulted in a faster time to recovery and a trend towards mortality benefit when given to patients with COVID‐19 and pulmonary involvement. 23 Dexamethasone reduced 28‐day mortality among patients hospitalized with COVID‐19 compared with standard care alone. 22 In this study, the use of dexamethasone, and remdesivir was more common among patients receiving CCP, and this could favourably impact the results observed in this study.
Limitations
The rapidity of onset of this pandemic and the severity of illness in many patients left the medical community struggling to find treatments for acutely ill hospitalized patients. The urgent need for effective treatments required that existing therapeutic modalities be re‐evaluated for clinical use. The Mayo EAP provided for use of convalescent plasma, a treatment used in other infectious outbreaks, early in the pandemic. However, due to time constraints, valuable metrics needed to assess the benefit of this treatment could not be utilized including randomized trials, rapid testing for SARS‐CoV‐2 viral loads and titres of plasma products.
Convalescent plasma was scarce early in the pandemic and required a national campaign to mobilize donors. The control cohort in this study was treated an average of 29 days prior to our patients, reflecting the lack of availability of CCP early in the pandemic but allowing these patients to serve as controls. Additionally, treatment strategies evolved during the pandemic, and patients treated earlier in the pandemic may have had a different ‘standard of care’ than patients treated later. Our case cohort on average was treated later than our control cohort and may have been subjected to different standards of care, including medication treatment protocols, which could add to imbalance in outcomes.
At the time of this study there was no single marker for disease severity that would aid in the matching of patients. The use of the oxygen delivery device category as a surrogate marker of disease severity to match the controls could underrepresent a patient's clinical picture. Additionally, days from hospital arrival for their acute illness was used as a surrogate for start of disease due to an inability to systematically obtain the true date of onset of symptoms.
The retrospective nature of this study does not allow for capture of all the clinical factors such as comorbidities, or inflammatory markers that may affect clinical outcomes and confound the analysis of data. Additionally, we did not have viral load or plasma IgG titre information that would also influence outcomes. Randomized clinical trials are planned that will avoid imbalance in patient characteristics, comorbidities, and treatment options.
Conclusion
In this retrospective, health system‐based, matched control study, we found an early mortality benefit at seven and 14 days of CCP transfusion but not at 28 days compared to controls. There was also a trend toward a quicker improvement in the oxygen device category in patients transfused with CCP, reflecting a more rapid respiratory recovery. This is the largest study to date, demonstrating a mortality benefit from COVID‐19 convalescent plasma compared to controls. Further studies controlling for patient characteristics and using high IgG titre plasma are needed.
Acknowledgements
The authors of this study would like to thank Michael Joyner, MD, the principal investigator of the Mayo Expanded Access Program, for coordinating the national program to make COVID‐19 convalescent plasma available for the treatment of our patients. We would also like to thank the members of the COVID‐19 Healthcare Coalition Convalescent Plasma Research Work Group, specifically Francis X. Campion, Rute M. Baptista, and Nigel Paneth, for their collaboration, and Dr. Cathy A. Conry‐Cantilena, director of the Blood Banking & Transfusion Medicine Department, for leading the collection and distribution of COVID‐19 convalescent plasma within our healthcare system.
This study was supported in part by a US Department of Health and Human Services (HHS), Biomedical Advanced Research and Development Authority (BARDA) grant, contract 75A50120C00096, National Center for Advancing Translational Sciences (NCATS) grant UL1TR002377, Schwab Charitable Fund (Eric E Schmidt, Wendy Schmidt, donors), United Health Group, National Basketball Association (NBA), Millennium Pharmaceuticals, Octopharma Octapharma USA, Inc, and the Mayo Clinic.
Contributorship statements and transparency declaration
AH and AS conceived of the presented idea. VB wrote the initial draft of the manuscript. JB collected the data, and SF developed performed the computations. AH and AS verified the analytical methods. All authors discussed the results and contributed to the final manuscript. AS is a guarantor of this study and accepts full responsibility for the work and the study's conduct. All authors had access to the data and controlled the decision to publish. VB affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted. Any discrepancies from the study as planned have been explained.
Supporting information
Table SI. Oxygen delivery device categories.
Table SII. Oxygen delivery device improvement in patients transfused within three days.
References
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID‐19 in an Integrated Health Care System in California. JAMA. 2020;323(21):2195. 10.1001/jama.2020.7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID‐19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–70. 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369(8249):1–15. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID‐19). JAMA. 2020;324(8):782. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 7. Wang D, Hu Bo, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen T, Wu Di, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368(1091):1–12. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically Ill patients with COVID‐19 in Washington State. JAMA. 2020;323(16):1612. 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao J, Tu W‐J, Cheng W, Yu L, Liu Y‐K, Hu X, et al. Clinical features and short‐term outcomes of 102 patients with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):748–55. 10.1093/cid/ciaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID‐19 pneumonia: a random association? Eur Heart J. 2020;41(19):1858. 10.1093/eurheartj/ehaa254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382(17):e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mao L, Jin H, Wang M, Hu Yu, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Yi, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Jiang W, He Qi, Wang C, Wang B, Zhou P, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID‐19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57–59. 10.1038/s41392-020-0158-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou W, Liu Y, Tian D, Wang C, Wang Sa, Cheng J, et al. Potential benefits of precise corticosteroids therapy for severe 2019‐nCoV pneumonia. Signal Transduct Target Ther. 2020;5(1):18–20. 10.1038/s41392-020-0127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID‐19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. 10.1016/S2665-9913(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970–5. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fragkou PC, Belhadi D, Peiffer‐Smadja N, Moschopoulos CD, Lescure F‐X, Janocha H, et al. Review of trials currently testing treatment and prevention of COVID‐19. Clin Microbiol Infect. 2020;26(8):988–98. 10.1016/j.cmi.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jean S‐S, Lee P‐I, Hsueh P‐R. Treatment options for COVID‐19: The reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–43. 10.1016/j.jmii.2020.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. RECOVERY Collaborative Group , Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. 2020. Online ahead of print. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 23. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid‐19 ‐ preliminary report. N Engl J Med. 2020:992–994. 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 24. McGuire LW, Redden WR. The use of convalescent human serum in influenza pneumonia‐a preliminary report. Am J Public Health (NY). 1918;8(10):741–4. 10.2105/ajph.8.10.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng Y, Wong R, Soo YOY, Wong WS, Lee CK, Ng MHL, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–6. 10.1007/s10096-004-1271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. Treatment of COVID‐19 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190(11):2290–2303. 10.1016/j.ajpath.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, Zhang W, Hu Yu, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19. JAMA. 2020;324(5):1–11. 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casadevall A, Pirofski L‐A. Antibody‐mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24(9):474–8. 10.1016/s1471-4906(03)00228-x [DOI] [PubMed] [Google Scholar]
- 29. Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, Lesser ER, Kunze KL. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID‐19: Initial Three‐Month Experience. Cold Spring Harbor Laboratory; 2020. 10.1101/2020.08.12.20169359 [DOI]
- 30. Zhou G, Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS‐CoV‐2. Int J Biol Sci. 2020;16(10):1718–23. 10.7150/ijbs.45123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Casadevall A, Pirofski LA. The convalescent sera option for containing COVID‐19. J Clin Invest. 2020;130(4):1545–8. 10.1172/JCI138003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joyner MJ, Wright RS, Fairweather DeLisa, Senefeld JW, Bruno KA, Klassen SA, et al. Early safety indicators of COVID‐19 convalescent plasma in 5000 patients. J Clin Investig. 2020;130(9):4791–7. 10.1172/JCI140200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, et al. Safety update. Mayo Clin Proc. 2020;95(9):1888–97. 10.1016/j.mayocp.2020.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murphy M, Estcourt L, Grant‐Casey J, Dzik S. International survey of trials of convalescent plasma to treat COVID‐19 infection. Transfus Med Rev. 2020;34(3):151–7. 10.1016/j.tmrv.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brouwer PJM, Caniels TG, van der Straten K, Snitselaar JL, Aldon Y, Bangaru S, et al. Potent neutralizing antibodies from COVID‐19 patients define multiple targets of vulnerability. Science. 2020;369(6504):643–50. 10.1126/science.abc5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically Ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582–9. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang D, Lian X, Song F, Ma H, Lian Z, Liang Y, et al. Clinical features of severe patients infected with 2019 novel coronavirus: a systematic review and meta‐analysis. Ann Transl Med. 2020;8(9):576. 10.21037/atm-20-2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID‐19. JAMA. 2020;324(11):1048. 10.1001/jama.2020.16349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid‐19. N Engl J Med. 2020;382(24):2327–36. 10.1056/NEJMoa2007016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. Oxygen delivery device categories.
Table SII. Oxygen delivery device improvement in patients transfused within three days.


