Dear Editor,
1.
With the initial cases of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection first reported in December 2019 in the Wuhan city of China, 1 the coronavirus disease 2019 (COVID‐19) pandemic has completed it's first‐anniversary and has had an unprecedented adverse impact on almost all domains of basic and clinical research worldwide. Several authors 2 , 3 , 4 , 5 have elaborated the short‐term and long‐term adverse consequences of the COVID‐19 pandemic on various facets of scientific research and its dissemination, and training. However, every adversity is an opportunity and brings positive changes as well, and so is the COVID‐19 pandemic. Several constructive changes that have been brought forward by the COVID‐19 pandemic will continue to shape the scientific research and training in the years to come (Figure 1).
Figure 1.
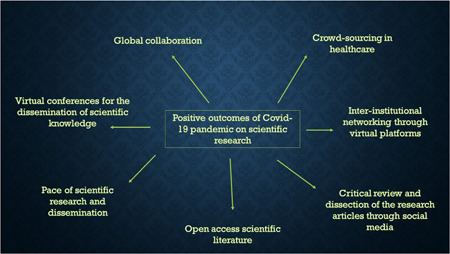
Displays the positive impact of COVID‐19 pandemic on scientific research and publications [Color figure can be viewed at wileyonlinelibrary.com]
The sheer pace with which the scientific community responded to the difficulties posed by the COVID‐19 pandemic is commendable considering that scientific research and dissemination is traditionally considered a slow process. A Pubmed search with the keyword “COVID” yielded 92,869 articles on January 21st, 2021 confirming a “Publication‐tsunami” of COVID‐related literature. However, the COVID‐19 pandemic has also provided an excellent opportunity for merging the latent and emerging issues with scientific review and research dissemination. The rapid output of scientific research in response to the COVID‐19 pandemic has reinforced that internal validity, credibility, and reproducibility are the critical components of scientific communication. Any loopholes in these critical components have tremendous potential for real‐world harm—akin to the Surgisphere debacle. 6 There is a need to share data generated through a large number of trials that are being conducted worldwide as it would bring in transparency and promote reusability of the data for secondary research. 7 At the same time, the medical diaspora and the scientific community must be wary of p‐hacking, file‐drawer effect, salami‐slicing, or any other identifiable biases that plague the clinical research and culminates into compromised patient care/treatment outcomes.
Another positive impact of the current COVID‐19 pandemic has been a growing impetus to crowdsourcing in the health sector and clinical research. Crowdsourcing classically means involving a large number of people who work collectively to solve a problem or to complete a task with some objectives adhering to an adage—No one knows everything, but everyone knows something—collective intelligence. We believe that focussed crowdsourcing in healthcare should also include group participation (interinstitutional within a country or across the countries) and sharing solutions for the larger public benefit. The current pandemic has brought together researchers and clinicians from all over the world to crowdsource their innovative ideas, disease‐related data, and resources to quickly develop the treatment guidelines and research strategies. 8 The oncology community has also acclimatized to the existing trends and is promoting a tailored treatment approved by a multidisciplinary team consensus which is feasible and in the best interest of the patient. The zeal with which the scientists are collaborating worldwide with unwavering unity to confront the virus is unprecedented and unparallel. It has provided a new impetus to the progress of science. This crowdsourcing and global collaboration are especially crucial for developing countries lacking health resources and comprehensive research infrastructure. A review of early publications related to cancer and COVID‐19 infection highlighted that almost one‐fifth of the articles (38 of 212) was published as a part of international collaborations. 9 In an excellent example of crowdsourcing, COVIDsurg collaborative 10 analyzed the mortality and pulmonary complications in 1128 patients with perioperative SARS‐CoV‐2 infection; the data was collected from 235 hospitals in 24 countries.
In conclusion, the COVID‐19 pandemic has certainly opened up new avenues for crowdsourcing and global collaboration through virtual meetings and networking for research in medicine and healthcare, as well as to harness the human intelligence and creativity of a large number of people residing in developing countries with scarce resources. There is also an urgent need to emphasize and ensure that the growing global public health research and evidence‐based medicine must translate into better patient care.
REFERENCES
- 1. Wang Y, Zhou Y, Yang Z, Xia D, Hu Y, Geng S. Clinical characteristics of patients with severe pneumonia caused by the SARS‐CoV‐2 in Wuhan, China. Respiration. 2020;99(8):649‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang L, Zhou W, Zhou W, You R. The impact of COVID‐19 pandemic on medical research. Ann Acad Med Singap. 2020;49(10):829‐830. [PubMed] [Google Scholar]
- 3. Shyr Y, Berry LD, Hsu C‐Y. Scientific rigor in the age of COVID‐19. JAMA Oncol. 2020. 10.1001/jamaoncol.2020.6639 [DOI] [PubMed] [Google Scholar]
- 4. Sohrabi C, Mathew G, Franchi T, et al. Impact of the coronavirus (COVID‐19) pandemic on scientific research and implications for clinical academic training—a review. Int J Surg. 2021;86:57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vissio E, Falco EC, Collemi G, et al. Impact of COVID‐19 lockdown measures on oncological surgical activity: analysis of the surgical pathology caseload of a tertiary referral hospital in Northwestern Italy. J Surg Oncol. 2021;123(1):24‐31. [DOI] [PubMed] [Google Scholar]
- 6. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in COVID‐19. N Engl J Med. 2020;382(25):e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7. Zariffa N, Haggstrom J, Rockhold F. Open science to address COVID‐19: sharing data to make our research investment go further. Ther Innov Regul Sci. 2020. 10.1007%2Fs43441‐020‐00250‐z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garg PK, Kaul P, Choudhary D, Singh MP, Tiwari AR. Cancer surgery in the era of COVID‐19 pandemic: changing dynamics. J Surg Oncol. 2020;122:1262‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garg PK, Kaul P, Choudhary D, et al. Discordance of COVID‐19 guidelines for patients with cancer: a systematic review. J Surg Oncol. 2020;122:579‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collaborative CO. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet. 2020;396(10243):27‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]


