Abstract
Background and Objectives
The coronavirus disease 2019 (COVID‐19) affected millions of people worldwide and caused disruptions at the global level including in healthcare provision. Countries of the WHO African region have put in place measures for the COVID‐19 pandemic containment that may adversely affect blood system activities and subsequently reduce the supply and demand of blood and blood components. This study aims to assess the impact of the COVID‐19 pandemic on blood supply and demand in the WHO African Region and propose measures to address the challenges faced by countries.
Materials and Methods
A survey questionnaire was sent to all 47 countries in the WHO African Region to collect information on blood supply and demand for the first 5 months of 2019 and 2020, respectively, and on COVID‐19 Convalescent Plasma therapy in September 2020.
Results
Thirty‐seven countries provided responses. The total number of blood donations dropped in 32 countries while it increased in five countries. The proportion of blood drives also decreased in 21 countries and increased in nine countries. The blood requested and issued for transfusion decreased for blood demand and for blood issued for transfusion in 30 countries. Ten countries reported some activities of convalescent plasma. However, very few units of this product collected have been transfused to COVID‐19 patients.
Conclusion
The COVID‐19 pandemic has led to a reduction of blood related activities in the region, including the supply and demand. Countries preparedness plans for health emergencies need more emphasis to maintaining blood stock.
Keywords: blood demand, blood donation, blood supply, convalescent Plasma, COVID‐19 pandemic, WHO African Region
Introduction
The coronavirus disease (COVID‐19) pandemic is caused by the coronavirus 2 (SARS‐CoV‐2) primarily transmitted by the respiratory route. The characteristics of SARS‐CoV‐2 infection have been described by multiple reports [1, 2]. The pandemic has caused significant socio‐economic disruptions at the global level with an impact on healthcare provision. The COVID‐19 outbreak continues to evolve in the WHO African Region (AFRO) since it was first detected in Algeria on 25 February 2020 with 8 confirmed cases. As of 23 June 2020, the 47 countries in AFRO are affected and have reported 236 909 cumulative confirmed cases of COVID‐19 with 5257 deaths [3]. For blood transfusion services, experience with outbreaks of other coronaviruses suggested that there will be significant impact on blood supply due to reduced blood donation [4, 5, 6]. The COVID‐19 pandemic has the potential to reduce the supply of blood and blood components and adversely affect blood system activities.
The World Health Organization (WHO) has developed and disseminated an interim guidance on maintaining a safe and adequate blood supply during the COVID‐19 pandemic [7]. This guidance recommends (1) mitigating potential risk of transmission through blood transfusion, staff risk and donor exposure to COVID‐19, as well as risk of reduced availability of blood donors; (2) managing blood demand; (3) ensuring undisrupted supply of critical materials and equipment; (4) communicating to ensure that donors, recipients, all staff, relevant stakeholders and the population are properly informed; and (5) collection of convalescent plasma from patients who have recovered from COVID‐19.
WHO estimated that the COVID‐19 pandemic caused 20% to 30% reduction of blood supply in all its six regions, and it was noted that the donor attendance rate has fallen by 10–30% in the state of Washington in the United States of America (USA) and by 30% at Canadian Blood Services. However, in the early stages of the pandemic, this trend was compensated by a reduction in demand for blood because of a decrease in elective surgery and medical treatment [8, 9]. The USA blood centres reported their lowest blood supply levels since the beginning of the pandemic, and blood drives continued to be cancelled since many businesses, schools and other organizations remained closed [10].
After imported cases of COVID‐19 were reported in Saudi Arabia, donor attendance and blood supply at blood bank‐based collection centres showed a drop of 39·5% and, on the other hand, blood demand during the same period was reduced by 21·7% [11]. In Malaysia, despite various promotional activities, the status of blood collection had not been satisfactory reaching only 57% of the target, and during the movement control order (MCO) enforcement, blood supply at the national blood centre and other blood centres throughout the country had decreased by 40% compared with previous years [12]. Due to the COVID‐19 pandemic, the number of whole blood donors also dropped by 67% and the success rate of recruitment for donations dropped by 60% in Zhejiang province in China [13].
Regarding the COVID‐19 convalescent plasma (CCP), clinical trials have been conducted in USA and other clinical trials are underway around the world to evaluate the effectiveness of using plasma derived from the blood of recovered COVID‐19 patients to reduce the severity of illness among people infected with COVID‐19 [14]. Furthermore, medical researchers in the Netherlands had recruited up 1500 people recovered from the new coronavirus to donate blood as part of an international push to develop a treatment for the virus from their plasma [15]. However, in Malaysia the Ministry of Health had 22 blood plasma packs donated by former COVID‐19 patients for treatment and further research [16]. In the African Region, this approach was used by Mauritius in the current epidemic, in line with the national decision to use serum plasma therapy for critically ill COVID‐19 patients [17].
Before the current COVID‐19 pandemic, results of surveys on availability and access to safe blood and blood products showed that some improved functionality metrics for the national blood transfusion systems in most countries in the African Region. However, significant challenges still remain, the biggest one being the insufficient and unsustainable resources at the disposal of the national blood transfusion services (NBTS). This seriously compromises timely availability and access to safe blood for all patients who need blood in the region [18, 19, 20]. During the current COVID‐19 pandemic, it was therefore necessary to assess the level of preparedness of countries in the region to maintain blood supply and demand and to respond appropriately to the emerging challenges.
The purpose of this paper is to outline key findings of the survey, discuss the impact of the COVID‐19 pandemic on blood supply and demand in the African Region and propose measures to address challenges countries encountered as a result of the pandemic.
Materials and methods
We conducted a quick survey on the impact of COVID‐19 on blood supply and demand in the WHO African Region. All 47 countries in the Region were invited to complete a structured questionnaire and send in their response from 21 May to 14 June 2020. For comparison purpose, countries were requested to provide data for the period from 1 January to 31 May 2019 and 1 January to 31 May 2020. A second round of the survey was conducted during the first two weeks of September 2020 especially focused on the COVID‐19 convalescent plasma (CCP).
The survey questionnaire covered selected blood supply and demand indicators across key transfusion system metrics, namely blood donors and blood collection, blood demand and blood issued for transfusion and some key managerial aspects relevant for blood services including funding. With regard to CCP, the questionnaire covered the mapping of available study protocols on its use and the institutions that are performing the CCP collection; the study setting such as randomized controlled trial (RCT), observational study (OS), compassionate use (CU) or other; as well as the appreciation whether the protocols had regulatory authorization or not. The CCP donors who had consented to donate were recruited according to the guidelines from the organizing committee of the ISBT Working Party on Global Blood Safety [21].
All data provided were verified with countries during a virtual meeting for completeness and accuracy. Variables reported were expressed in number and or percentage of increase or decrease for blood donations, blood drives, blood demand, blood use and CCP. The difference between the high and low figures and averages with ranges of key indicators were calculated for each set of data, where applicable. Data entry and analysis were performed using the Microsoft Office 365 Pro Plus Excel and presented in the form of tables. The figures with minus signs mean decrease and those without an increase.
The limitation of the study is that although countries put in place appropriate measures around end of June 2020 to encourage blood donation, we were not able to continue with the survey because the blood supply started to stabilize.
Results
Out of the 47 countries in the WHO African Region, 37 (78·7%) provided responses to the questionnaire as of 14 June 2020 of which two provided partial data limited to a single centre. The ten countries that did not provide any data were left out of the analysis. Figure 1 shows country responses to the survey on COVID‐19 and blood supply and demand in the WHO African Region, over the period.
Fig. 1.
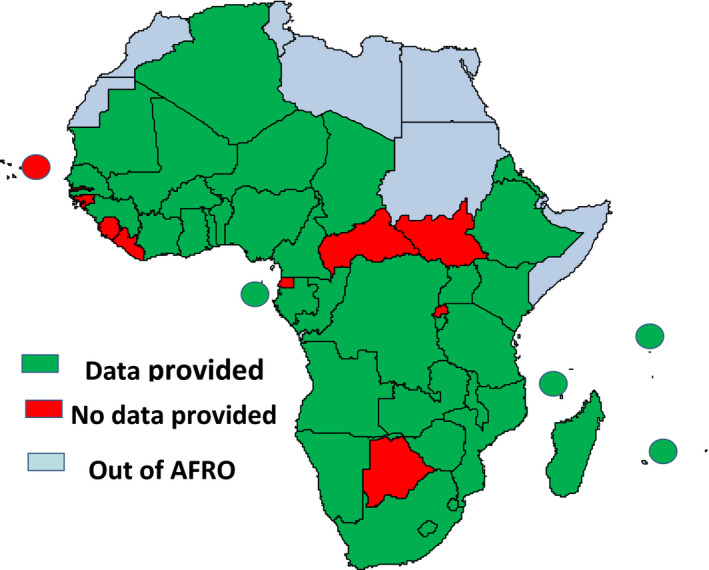
Country responses to the survey on COVID‐19 and blood supply and demand in the WHO African Region.
Overall, while blood donation rate reduction varied in 32 countries from 0·07% to 44·2%, five countries reported an increase ranging from 0·4% to 14% during the reported period. Out of 19 countries that had reached the regional target of 80–100% VNRBD in 2019, two countries failed to meet this target in the first half of 2020. One country reported 8343 paid blood donations in 2020 representing 5·7% of the total number of blood donations collected in that country.
In 21 countries, the number of blood drives also decreased from 24 467 in 2019 to 18 509 in 2020. This represents a proportion decrease ranging from 12·1% to 100%. Regular blood donation drives were drastically reduced as secondary schools, universities and most of the non‐essential sectors were closed; thus, it was not possible to recruit blood donors in these institutions. However, nine countries reported an increase in the proportion of mobile drive ranging from 0·8% to 388·9%. In these countries, the number of blood donations remained on the pre‐COVID marks or even increased. Satisfactory volunteer recruitment rates were reported to have been associated with the following considerations: late entry into countrywide lockdown, collaboration with national blood donor associations, civil society organizations, armed and security forces and good coordination with the national COVID‐19 task force. In one country, there was no change in the number of blood drives, while 6 countries did not report any data on such collections.
In 26 countries, the number of deferred donors dropped from 151 459 in 2019 to 105 755 in 2020 representing a percentage of decrease range from 1·6% to 100%. Two countries reported an increased deferred donor of 73·7% and 88·4%, respectively, while nine countries did not provide any data on donor deferred over the reported period.
The proportional drops ranged from 0·1% to 44% for blood demand and from 0·5% to 46·6% for blood issued for transfusion in 30 countries. However, seven countries reported an increase of blood demand ranging from 0·7% to 13·7%, while nine countries did so for the blood issued for transfusion ranging from 1·6% to 39%. The detailed results for each country for 2019 and 2020 related to the number of blood donations, blood drives, blood demand and blood issued are reported in Table 1 (Gap between blood donations in 2019 and 2020 in the African Region), in Table 2 (Gap between blood drives in 2019 and 2020 in the African Region) and in Table 3 (Gap between blood demand and blood issued for transfusion in 2019 and 2020 in the African Region). For most countries, blood issued was lower than blood demand and this could be explained by non‐availability of sufficient blood to meet the overall requests/demands. In DRC, Senegal and South Africa the demand numbers and issue numbers were exactly the same because no complete data was given for blood demand or blood issued in these three countries.
Table 1.
Gap between blood donations in 2019 and 2020 in the African Region
| Countries | Number of blood donations | |||
|---|---|---|---|---|
| 2019 | 2020 | Difference | Proportion of decrease or increase (%) | |
| Algeria a | 6025 | 4470 | −1555 | −25·8 |
| Angola | 68 965 | 49 353 | −19 612 | −28·4 |
| Benin | 29 636 | 25 352 | −4284 | −14·5 |
| Burkina Faso | 45 229 | 29 391 | −15 838 | −35·0 |
| Burundi | 34 237 | 39 041 | 4804 | 14·0 |
| Cameroon | 47 275 | 32 328 | −14 947 | −31·6 |
| Chad | 9860 | 9903 | 43 | 0·4 |
| Comoros | 1 106 | 848 | −258 | −23·3 |
| Congo | 25 998 | 26 098 | 100 | 0·4 |
| Côte d'Ivoire | 60 201 | 51 374 | −8 827 | −14·7 |
| Democratic Republic of Congo | 255 460 | 146 693 | −108 767 | −42·6 |
| Eritrea | 4536 | 4593 | 57 | 1·3 |
| Eswatini | 7185 | 5174 | −2011 | −28·0 |
| Ethiopia | 98 340 | 106 582 | 8242 | 8·4 |
| Gabon | 9175 | 6999 | −2176 | −23·7 |
| Ghana | 73 063 | 57 269 | −15 794 | −21·6 |
| Guinea | 8543 | 6806 | −1737 | −20·3 |
| Kenya | 59 858 | 33 419 | −26 439 | −44·2 |
| Lesotho | 2765 | 2550 | −215 | −7·8 |
| Madagascar | 21 868 | 16 733 | −5135 | −23·5 |
| Malawi | 22 560 | 21 210 | −1 350 | −6·0 |
| Mali | 22 747 | 16 765 | −5 982 | −26·3 |
| Mauritania | 8017 | 7968 | −49 | −0·6 |
| Mauritius | 19 685 | 13 727 | −5958 | −30·3 |
| Mozambique | 54 811 | 49 207 | −5604 | −10·2 |
| Namibia | 15 204 | 14 900 | −304 | −2·0 |
| Niger | 9619 | 8560 | −1059 | −11·0 |
| Nigeria a | 9450 | 5879 | −3571 | −37·8 |
| Sao Tome and Principe | 499 | 364 | −135 | −27·1 |
| Senegal | 16 442 | 13 345 | −3097 | −18·8 |
| Seychelles | 675 | 594 | −81 | −12·0 |
| South Africa | 385 983 | 384 263 | −1720 | −0·4 |
| Togo | 16 054 | 16 043 | −11 | −0·1 |
| Uganda | 122 598 | 116 856 | −5742 | −4·7 |
| United Republic of Tanzania | 130 038 | 100 764 | −29 274 | −22·5 |
| Zambia | 55 085 | 46 453 | −8632 | −15·7 |
| Zimbabwe | 41 444 | 26 899 | −14 545 | −35·1 |
| Total | 1 800 236 | 1 498 773 | −301 463 | −16·7 |
Data from BTS of Blida for Algeria and NBTS of Lagos State for Nigeria.
Table 2.
Gap between blood drives in 2019 and 2020 in the African Region
| Countries | Number of blood drives | |||
|---|---|---|---|---|
| 2019 | 2020 | Difference | Proportion of decrease or increase (%) | |
| Algeria a | 17 | 19 | 2 | 11·8 |
| Angola | ‐ | ‐ | ‐ | ‐ |
| Benin | 553 | 386 | −167 | −30·2 |
| Burkina Faso | 994 | 450 | −544 | −54·7 |
| Burundi | 638 | 643 | 5 | 0·8 |
| Cameroon | ‐ | ‐ | ‐ | ‐ |
| Chad | 9 | 44 | 35 | 388·9 |
| Comoros | ‐ | ‐ | ‐ | ‐ |
| Congo | 70 | 71 | 1 | 1·4 |
| Côte d'Ivoire | 155 | 87 | −68 | −43·9 |
| Democratic Republic of Congo | 6387 | 3667 | −2720 | −42·6 |
| Eritrea | 53 | 46 | −7 | −13·2 |
| Eswatini | ‐ | ‐ | ‐ | ‐ |
| Ethiopia | 889 | 1094 | 205 | 23·1 |
| Gabon | 18 | 19 | 1 | 5·6 |
| Ghana | 506 | 240 | −266 | −52·6 |
| Guinea | 25 | 11 | −14 | −56·0 |
| Kenya | 1613 | 378 | −1235 | −76·6 |
| Lesotho | 56 | 9 | −47 | −83·9 |
| Madagascar | 46 | 8 | −38 | −82·6 |
| Malawi | 893 | 629 | −264 | −29·6 |
| Mali | 57 | 43 | −14 | −24·6 |
| Mauritania | 67 | 56 | −11 | −16·4 |
| Mauritius | 925 | 759 | −166 | −17·9 |
| Mozambique | ‐ | ‐ | ‐ | ‐ |
| Namibia | 1410 | 1159 | −251 | −17·8 |
| Niger | ‐ | ‐ | ‐ | ‐ |
| Nigeria a | 96 | 99 | 3 | 3·1 |
| Sao Tome and Principe | 3 | 3 | 0 | 0·0 |
| Senegal | 129 | 72 | −57 | −44·2 |
| Seychelles | 4 | 1 | −3 | −75·0 |
| South Africa | 4867 | 4279 | −588 | −12·1 |
| Togo | 44 | 24 | −20 | −45·5 |
| Uganda | 2667 | 2201 | −466 | −17·5 |
| United Republic of Tanzania | 1 | 0 | −1 | −100·0 |
| Zambia | 918 | 1548 | 630 | 68·6 |
| Zimbabwe | 657 | 464 | −193 | −29·4 |
| Total | 24 467 | 18 509 | −6258 | −25·3 |
Data from BTS of Blida for Algeria and NBTS of Lagos State for Nigeria.
Table 3.
Gap between blood demand and issued for transfusion in 2019 and 2020 in the African Region
| Countries | Number of blood units demand | Number of blood units issued/transfused | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | Difference | % | 2019 | 2020 | Difference | % | |||||
| Algeria* | 18 434 | 15 647 | −2787 | −15·1 | 10 337 | 8 186 | −2151 | −20·8 | ||||
| Angola | 129 796 | 99 616 | −30 180 | −23·3 | ‐ | ‐ | ‐ | ‐ | ||||
| Benin | 42 418 | 43 520 | 1102 | 2·6 | 31 769 | 36 926 | 5157 | 16·2 | ||||
| Burkina Faso | 47 571 | 30 473 | −17 098 | −35·9 | 38 019 | 37 501 | −518 | −1·4 | ||||
| Burundi | ‐ | ‐ | ‐ | ‐ | 30 836 | 33 123 | 2287 | 7·4 | ||||
| Cameroon | ‐ | ‐ | ‐ | ‐ | 43 128 | 27 692 | −15436 | −35·8 | ||||
| Chad | 5350 | 5755 | 405 | 7·6 | 6832 | 9500 | 2668 | 39·1 | ||||
| Comoros | ‐ | ‐ | ‐ | ‐ | 1010 | 805 | −205 | −20·3 | ||||
| Congo | ‐ | ‐ | ‐ | ‐ | 20 330 | 26 264 | 5934 | 29·2 | ||||
| Côte d'Ivoire | ‐ | ‐ | ‐ | ‐ | 73 262 | 58 123 | −15139 | −20·7 | ||||
| Democratic Republic of Congo | 237 218 | 141 677 | −95 541 | −40·3 | 237 218 | 141 677 | −95541 | −40·3 | ||||
| Eritrea | 5740 | 6526 | 786 | 13·7 | 4511 | 4490 | −21 | −0·5 | ||||
| Eswatini | 6343 | 3543 | −2800 | −44·1 | 5887 | 3543 | −2344 | −39·8 | ||||
| Ethiopia | ‐ | ‐ | ‐ | ‐ | 123 581 | 109 773 | −13808 | −11·2 | ||||
| Gabon | 9483 | 10 483 | 1000 | 10·5 | 9194 | 7795 | −1399 | −15·2 | ||||
| Ghana | 28 698 | 27 014 | −1684 | −5·9 | 30 480 | 23 276 | −7204 | −23·6 | ||||
| Guinea | 5 145 | 4 617 | −528 | −10·3 | 5120 | 5379 | 259 | 5·1 | ||||
| Kenya | 127 182 | 93 637 | −33 545 | −26·4 | 57 936 | 30 943 | −26993 | −46·6 | ||||
| Lesotho | 3924 | 3457 | −467 | −11·9 | 2784 | 2244 | −540 | −19·4 | ||||
| Madagascar | ‐ | ‐ | ‐ | ‐ | 24 395 | 17 988 | −6407 | −26·3 | ||||
| Malawi | 42 207 | 40 762 | −1 445 | −3·4 | 28 079 | 23 276 | −4803 | −17·1 | ||||
| Mali | ‐ | ‐ | ‐ | ‐ | 13 011 | 12 177 | −834 | −6·4 | ||||
| Mauritania | 13 552 | 14 108 | 556 | 4·1 | 9 130 | 9 560 | 430 | 4·7 | ||||
| Mauritius | 50 943 | 46 052 | −4891 | −9·6 | 38 384 | 33 856 | −4528 | −11·8 | ||||
| Mozambique | 63 301 | 63 768 | 467 | 0·7 | 60 309 | 61 330 | 1021 | 1·7 | ||||
| Namibia | − | − | − | − | 15 657 | 15 354 | −303 | −1·9 | ||||
| Niger | 16 578 | 15 110 | −1468 | −8·9 | 7266 | 6948 | −318 | −4·4 | ||||
| Nigeria a | 6230 | 3610 | −2620 | −42·1 | 6230 | 3610 | −2620 | −42·1 | ||||
| Sao Tome and Principe | 517 | 440 | −77 | −14·9 | 514 | 439 | −75 | −14·6 | ||||
| Senegal | 17 659 | 13 606 | −4053 | −23·0 | 17 659 | 13 606 | −4053 | −23·0 | ||||
| Seychelles | 2770 | 894 | −1876 | −67·7 | − | − | − | − | ||||
| South Africa | 448 529 | 438 985 | −9544 | −2·1 | 448 529 | 438 985 | −9544 | −2·1 | ||||
| Togo | 26 070 | 24 331 | −1739 | −6·7 | 20 046 | 20 360 | 314 | 1·6 | ||||
| Uganda | 164 365 | 164 230 | −135 | −0·1 | 106 809 | 105 841 | −968 | −0·9 | ||||
| United Republic of Tanzania | − | − | − | − | 105 953 | 78 012 | −27941 | −26·4 | ||||
| Zambia | 116 452 | 120 270 | 3818 | 3·3 | 47 631 | 53 499 | 5868 | 12·3 | ||||
| Zimbabwe | 46 870 | 38 957 | −7913 | −16·9 | 36 758 | 27 630 | −9128 | −24·8 | ||||
| Total | 1 683 345 | 1 471 088 | −212 257 | −12·6 | 1 718 594 | 1 489 711 | −228883 | −13·3 | ||||
Data from BTS of Blida for Algeria and NBTS of Lagos State for Nigeria.
Apart from platelet concentrates (PLT), for which the demand was slightly up, by 0·6%, demands for other blood components dropped by 21·1% for whole blood (WB), 10·8% for red blood cell (RBC), 4·0% for fresh frozen plasma (FPP) and 9·0% for cryoprecipitates. As for blood demand, blood issued for transfusion also dropped by 22% for WB, 8·3% for RBC, 14·9% for PLT, 3·5% for FFP and 23·4% for cryoprecipitate. Blood demanded and blood issued for transfusion decreased in healthcare facilities in most countries were generally due to reasons such as (1) decrease in requests for blood from the prescribers that has contributed to making the shortage of blood less pronounced; (2) outpatient department shut down; (3) routine surgery suspension; (4) difficulty in transporting blood and blood components from a blood bank to another; (5) management of the blood request by a good communication between health professionals working in health facilities to give priority to emergency situations; (6) most of NBTS do not have records to capture all blood requested and only the blood units issued get recorded or health facilities report only the quantity of units transfused without giving a break‐down by components; and (g) non‐availability of data on blood demanded and blood issued in some countries.
Even though countries that reported an increase in blood demanded and blood issued did not provide a specific reason, this might be linked to the maintaining normal clinical routine activities. Whatever, the situation might be with routine clinical practices, and in preparation for the resumption of clinical activities after the COVID‐19 pandemic, the NBTS should reach out to healthcare professionals responsible for transfusion activities. The NBTS should advise on the use of blood and components only when clinically appropriate.
With regard to the CCP survey, out of 47 countries in the region, 29 provided information of which ten showed some CCP activities. The mapping of the CCP therapy is indicated in Table 4 on COVID‐19 convalescent plasma therapy in the WHO African Region.
Table 4.
COVID‐19 Convalescent plasma therapy in the WHO African Region
| Country | National protocol for collection and use of CCP and regulatory authorization | Institutions that collect CCP | Use of CCP | CCP collected and patients treated | Comments and any additional information |
|---|---|---|---|---|---|
| Algeria | No | A protocol has been developed by the University Hospital of Blida, but still waiting for approval | |||
| Ethiopia | No: The NBTS however has a protocol for the selection of donor and collection of plasma from recovered patients. There were efforts by the national public health institute to get approval of CCP trial by the regulatory authority | NBTS | Compassionate Use | 12 units (using routine whole blood collection and processing techniques) | The activity was halted as the Apheresis techniques was deemed more convenient and the process of procuring consumables for Apheresis collection was initiated. |
| Ghana | Yes: the protocol has received regulatory approval from the Ghana Foods and Drugs Authority | NBTS (Southern and Central Zonal Blood Centres) | Observational Study planned, but not commenced. | Collection has not started due to challenges with implementation | |
| Guinea | Yes: not yet approved | NBTS | Compassionate Use | 15 units were collected, and 8 ICU COVID‐19 patients treated. | |
| Mauritius | Yes | NBTS | Compassionate Use | 3 ICU ventilated patients received CCP, 2 recovered well and the third patient passed away due to the development of sepsis and renal failure |
150 recovered donors have consented to donate CCP Country does not have any community transmission of COVID 19 presently |
| Namibia | No | The country has not started collecting CCP, however, such an activity could be integrated in the plasmapheresis programme introduced in February 2020 | |||
| Nigeria | Yes: There is a protocol for collection and use. This yet to be captured on the NBTS data. | Nigeria Institute for Medical Research (NIMR) and for now, Lagos State Blood Transfusion Services | Regulatory authority was sought and received by the NIMR. Country is working on harmonizing details through the Federal Ministry of Health | ||
| South Africa | Yes: received regulatory authorization | SANBS and WCBS | Randomized Controlled Trial | More than 50% of the required CCP for the RCT have been collected | SANBS and WCBS have collaborated on two studies with regard to CCP under the title of PROTECT trials. No data have been published |
| Uganda | Yes | Uganda blood transfusion service | Randomized Controlled Trial | The trial regulatory authorization certificate indicates a sample size of 136 patients. The projected need for the study period is 300 units | The trial is registered under clinicaltrials.gov. |
| Zimbabwe | Yes: the protocol has neither been shared publicly nor sent for regulatory approval | NBSZ | Compassionate Use | There has not been any collection done and no requests received |
The developed protocols varied from the institutional level to the national level. The use of CCP was also varied from RCT, OS or CU.
The risk of stock‐out of reagents and consumables used along the blood transfusion chain, from the blood collection to transfusion to patients, increased from eleven (29·7%) countries in 2019 to 22 (59·5%) in 2020. Despite the COVID‐19 pandemic, no country in the region has reported any specific budget allocation from the government related to the pandemic to strengthen national blood supply system. However, in 18 countries efforts have been undertaken by the NBTS to mobilize resources from other sources, mainly through (1) awareness raising; (2) advocacy with health authorities and partners; (3) risk mitigation to ensure business continuity; (4) development and submission of proposals to potential donors for funding; (5) creating partnerships; and (6) cost recovery initiatives of blood issued in government and private hospitals. In four countries, 15 staff were reported to have been infected with COVID‐19.
Discussions
Like all regions in the world, the African Region is facing socio‐economic disruption due to the COVID‐19 pandemic. It makes a significant impact on health service delivery. Overall, it was revealed through the survey that the safe blood supply and demand were at risk, since there was a decrease of these activities in most of respondent countries in the region, especially in the beginning of the COVID‐19 pandemic. Most NBTS conducted a risk assessment, which focused on blood collection, laboratory testing and workplace transmission to adapt their response to the pandemic. The authors are cognizant of the fact that many African countries only started to experience the early infections of the pandemic in mid to late March 2020, with lockdown being implemented. However, this was a quick survey carried out over the considered period in order to monitor the impact of the COVID‐19 pandemic on blood supply and demand.
The proportion of the reduction in the number of blood donations both at the regional and country levels were also reported by other countries such as Saudi Arabia, Canada, USA, Malaysia and in Zhejiang province in China [8, 9, 10, 11, 12, 13]. In the countries with reduced blood donations, lockdown orders, donor anxiety and fear of COVID‐19 infection during blood donation, which often stems from popular misconceptions and misinformation, have hindered blood donors from accessing blood transfusion services. Unfortunately, it was not possible to correlate the blood collection and usage to the time period where the countries enforced the social distancing measures and when infections started rising due to the declining of the COVID‐19 cases in the countries of the region.
To improve blood donations and mitigate potential risks for blood donors, countries implemented measures such as (1) public awareness campaigns through local radio and TV, newspapers, social media platforms, bulk text messaging and direct call donors; (2) transporting donors to and from their homes with authorization from relevant national authorities; (3) providing facemasks, hand washing equipment and sanitizers; (4) modifying donor screening questionnaire for exposure to COVID‐19; and (5) temperature measurement. Comparable measures have also been implemented by blood banks in China during the COVID‐19 pandemic to reduce panic among potential blood donors [22].
However, after the strategies put in place during the lockdown, countries in the region need to think forward and identify appropriate ways and methods of encouraging and increasing blood donations after the lifting of lockdown because blood donors may always be reluctant to come and donate, whereas needs of blood will expectedly increase again, especially in countries where routine clinical activities have been reduced, postponed or even suspended. Special attention needs to be given to the most affected countries in the region.
The CCP collected and transfused in the region remain very weak. The main issue for the use of this therapy in most countries in the region was the insufficient capacity of NBTS to safely collect, process and store it in a quality‐assured manner. The challenges raised by some countries included the approval still awaited from their National Regulatory Authorities to conduct clinical trials and the lack of test kits for determination of anti‐COVID‐19 titres in the CCP due to lack of resources. However, some countries planned to establish partnerships with institutions taking part in ongoing RCT or OS or CU to collect CCP from recovered patients for the management of critically ill COVID‐19 patients. Considering the trends of the COVID‐19 pandemic in the African Region, whose cases are decreasing in most countries, it was not possible to collect statistically enough numbers of CCP.
The main reasons observed in 2019 regarding the stock‐outs were related to delays in release and allocation of budget, whereas in 2020, the additional reasons were (1) border control measures; (2) transport and movement restrictions that led to the shortages of critical supplies and equipment needed for blood donation, processing, testing and transfusion to patients. Furthermore, the capacities of the central medical stores in most countries were exceeded because of the urgent need for procurement and supply of other commodities used for the COVID‐19 pandemic response. As part of its working procedures, South Africa reported that it had put in place a risk mitigation measure through the procurement of additional stock items prior to the pandemic taking off to ensure business continuity. The supply chain systems with the national procurement agencies were facing some challenges due to COVID‐19 pandemic such as borders closure, transport restrictions in Algeria, Benin, Burundi, Cameroon, Cote d’Ivoire, Ethiopia, Eritrea, Gabon, Mauritania, Mozambique, Niger, Tanzania and Zambia; inadequate funding from national governments to procure sufficient blood reagents and consumables in Burkina Faso, Eswatini and Lesotho as well as donor funding withdrawal in Kenya.
The biggest challenge with blood supply in many African countries is the insufficient resources allocated to NBTS by governments and drastic cut in funding from partners to make available safe and quality‐assured blood and blood components for all patients who need blood. Countries also reported that there was no specific blood transfusion budget related to COVID‐19 pandemic. Regarding staff, the challenges were the shortage of human resources due to travel restrictions within the same country, which prevented many employees from reaching places of work because of lockdown; staff safety especially the provision of personal protection equipment (PPE) for every employee; training for COVID‐19 exclusion; social distancing during donor sessions and temperature monitoring.
To address the resource challenges faced by countries, most NBTS have undertaken initiatives such as (1) advocating for more allocation from the central government and other non‐traditional organizations to support the service; (2) drawing up costed work plans to share with the MOH and the partners; (3) using available funds to implement workplace related measures to prevent COVID‐19 as alternative means of mobilizing blood donors will in the long run affect ability to sustain operations if no replacement funding is available.
Conclusion
The COVID‐19 pandemic has led to an overall reduction of blood transfusion activities in most countries in the region, in particular blood donations, blood demands and use in health facilities. However, the experience and measures implemented by countries to overcome the reduced activities and gain confidence of donors in safe blood donation yielded results. These experiences and measures should find an appropriate place in preparedness plans for future similar shocks to the blood transfusion system.
There is need to continue supporting countries through strengthening capacity of national blood transfusion systems, enhancing risk mitigation to ensure business continuity, developing a preparedness plan for future similar shocks to the blood transfusion system, raising awareness through advocacy with national health authorities and partners for sustainable funding of blood supply and demand in the region.
Conflicts of interests
The authors declared no conflict of interest.
Authorship
André Loua: participated in the development of the assessment tool, the study design and supervision, data analysis and interpretation, and wrote the draft of the manuscript. Other co‐authors: contributed to the revision of the draft of the manuscript and approved the final version submitted for publication.
Acknowledgements
The following Officers from National Blood Transfusion Services provided response to the survey questionnaire: Chaib Mohamed (Algeria), Deodete Machado (Angola), Orou Bagou Yorou Chabi (Benin), Alice Kiba Koumare (Burkina Faso), Félicien Nzotungwanayo (Burundi), Noah Owona Appolonie (Cameroon), Djimadoum Mbanga (Chad), Djamal Mohamed Chanfi (Comoros), Serge Oscar Mokono (Congo), Konate Seidou (Cote d’Ivoire), Pacifique Misingui (Democratic Republic of Congo), Yohannes Tekeste (Eritrea), Gugu Maphalala (Eswatini), Yaregal Bante Demem (Ethiopia), Olivier Rebienot Pellegrin (Gabon), Justina Kordai Ansah (Ghana), Nyankoye Haba (Guinea), Charles Rombo (Kenya), Maleqhoa Nyopa (Lesotho), Randriamanantany Zely Arivelo (Madagascar), Caroline T. Masangalawe (Malawi), Amadou B. Diarra (Mali), Khadijetou Ba (Mauritania), Janaki Sonoo (Mauritius), Dina Safina Ambasse Ibraimo (Mozambique), Israel Chipare (Namibia), Zoubeida Mayaki (Niger), Oluwatoyin A. Smith (Nigeria), Cristiano Santos (Sao Tome and Principe), Saliou Diop (Senegal), Joanne Pragassen (Seychelles), Jackie Thomson (South Africa), Magdalena A. Lyimo (Tanzania), Lochina Feteke (Togo), Dorothy Kyeyune (Uganda), Joseph Mulenga (Zambia) and Lucy Marowa (Zimbabwe).
References
- 1. Guan WJ, Ni ZY, Hu Y, et al.: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan M, et al.: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. COVID‐19. WHO African Region ‐ External situation Report 17. Available at: https://apps.who.int/iris/bitstream/handle/10665/332705/SITREP_COVID‐19_WHOAFRO_20200624‐eng.pdf. Accessed 24 June 2020
- 4. Shan H, Zhang P: Viral attacks on the blood supply: the impact of severe acute respiratory syndrome in Beijing. Transfusion 2004; 44:467–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teo D: Blood supply management during an influenza pandemic. ISBT Sci Ser. 2009; 4(n2):293–298 [Google Scholar]
- 6. Kwon SY, Lee EH, Kim HS, et al.: Middle East Respiratory Syndrome Coronavirus (MERS‐COV) outbreak in South Korea: risk management at the Korean Red Cross Seoul Nambu Blood Center (abstract). Vox Sang 2015; 109(Suppl. 2):18 25827316 [Google Scholar]
- 7. World Health Organization : Interim guidance on maintaining a safe and adequate blood supply during the pandemic outbreak of coronavirus disease (COVID‐19), 20 March 2020. Available at: https://www.who.int/publications‐detail/maintaining‐a‐safe‐and‐adequate‐blood‐supply‐during‐the‐pandemic‐outbreak‐of‐coronavirus‐disease‐(covid‐19) ‐ Accessed 28 May 2020
- 8. NUSADAILY.COM, WHO :Covid‐19 Effect, Blood Supply is Reduced up to 30 Percent. Available at: https://nusadaily.com/en/news/covid‐19‐effect‐who‐blood‐supply‐is‐reduced‐up‐to‐30‐percent.html. Accessed 21 June 2020
- 9. Stanworth Simon J, New Helen V, Apelseth Torunn O, et al.: Effects of the COVID‐19 pandemic on supply and use of blood for transfusion. Lancet Haematol 2020; 7:e756–e764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. AABB : AABB COVID‐19 Weekly Hospital Transfusion Services Survey: COVID‐19 pandemic impact on transfusion services: elective surgeries resume, convalescent plasma use increases, and blood supply drops to critical levels. Available at : https://transfusionnews.com/2020/06/02/covid‐19‐pandemic‐impact‐on‐transfusion‐services‐elective‐surgeries‐resume‐convalescent‐plasma‐use‐increases‐and‐blood‐supply‐drops‐to‐critical‐levels/ ‐ Accessed 20 June 2020
- 11. Yahia AIO: Management of blood supply and demand during the COVID‐19 pandemic in King Abdullah Hospital, Bisha, Saudi Arabia. Transfus Apher Sci 2020; 59:102836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. TheStar : National Blood Centre sends out SOS. https://www.thestar.com.my/news/nation/2020/05/21/national‐blood‐centre‐sends‐out‐sos#cxrecs_s ‐ (Accessed 21 June 2020)
- 13. Wang Yongjun, Han Wenjuan, Pan Lingling, et al.: Impact of COVID‐19 on blood centres in Zhejiang province China. Vox Sang 2020; 115:502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joyner MJ, Wright RS, Fairweather D, et al.: Early safety indicators of COVID‐19 convalescent plasma in 5,000 patients [published online ahead of print, 2020 Jun 11]. J Clin Invest. 2020; 130(9):4791–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. TheStar : Dutch donors join global effort on plasma treatment for coronavirus. Available at https://www.thestar.com.my/news/world/2020/05/21/dutch‐donors‐join‐global‐effort‐on‐plasma‐treatment‐for‐coronavirus#cxrecs_s (Accessed 21 June 2020)
- 16. TheStar, Health DG : Govt has 22 blood plasma packs from recovered Covid‐19 patients. Available at https://www.thestar.com.my/news/nation/2020/05/17/health‐dg‐govt‐has‐22‐blood‐plasma‐packs‐from‐recovered‐covid‐19‐patients#cxrecs_s ‐ (Accessed 21 June 2020)
- 17. Republic of Mauritius . Recovery cases of Covid‐19 rise to 19 and plasma therapy start for critically ill patients. Available at http://www.govmu.org/English/News/Pages/Recovery‐cases‐of‐Covid‐19rise‐to‐19‐and‐plasma‐therapy‐start‐for‐critically‐ill‐patients.aspx (Accessed 31 May 2020)
- 18. Tapko JB, Toure B, Luis GS: Status of Blood Safety in the WHO African Region: Report of the 2010 Survey. Brazzaville, Republic of Congo, World Health Organization, 2014. [Google Scholar]
- 19. Loua A, Nikiema JB, Kasilo OMJ, et al.: Blood safety and availability in the WHO African region. Global Surg 2018; 4:1–7. https://www.oatext.com/blood‐safety‐and‐availability‐in‐the‐who‐african‐region.php [Google Scholar]
- 20. Loua A, Nikiema JB, Sougou A, et al.: Transfusion in the WHO African Region. Transfus Clin Biol 2019; 26:155–159 [DOI] [PubMed] [Google Scholar]
- 21. Martin Smid W, Burnouf T, Epstein J, Kamel H, et al: Points to consider in the preparation and transfusion of COVID‐19 convalescent plasma in low‐ and middle‐ income countries. Available at http://www.isbtweb.org/fileadmin/user_upload/FINAL_Points_to_consider_in_the_preparation_of_COVID_convalescent_plasma_in_LMIC.pdf (Accessed 4 November 2020) [DOI] [PMC free article] [PubMed]
- 22. Zhang C, Wang M: MRCA time and epidemic dynamics of the 2019 novel coronavirus. bioRxiv. 2020. 1–8. 10.1101/2020.01.25.919688 [DOI] [Google Scholar]


