Key Points
Question
Does the addition of revacept, a novel platelet glycoprotein VI antagonist, in addition to currently recommended antithrombotic therapy in the setting of percutaneous coronary intervention (PCI) in patients with stable ischemic heart disease (SIHD) have an effect on the myocardial injury rate?
Findings
In this phase 2 randomized clinical trial, revacept did not reduce myocardial injury in patients with SIHD undergoing PCI. There were few bleeding events and no significant differences between treatment arms, and the 160-mg dose of revacept had a small but statistically significant effect on collagen-induced but not adenosine 5′-diphosphate–induced platelet aggregation.
Meaning
In patients with SIHD undergoing PCI, addition of revacept to standard antithrombotic therapy does not reduce the incidence of myocardial injury.
Abstract
Importance
The assessment of new antithrombotic agents with a favorable safety profile is clinically relevant.
Objective
To test the efficacy and safety of revacept, a novel, lesion-directed antithrombotic drug, acting as a competitive antagonist to platelet glycoprotein VI.
Design, Setting, and Participants
A phase 2 randomized clinical trial; patients were enrolled from 9 centers in Germany from November 20, 2017, to February 27, 2020; follow-up ended on March 27, 2020. The study included patients with stable ischemic heart disease (SIHD) undergoing elective percutaneous coronary intervention (PCI).
Interventions
Single intravenous infusion of revacept, 160 mg, revacept, 80 mg, or placebo prior to the start of PCI on top of standard antithrombotic therapy.
Main Outcomes and Measures
The primary end point was the composite of death or myocardial injury, defined as an increase in high-sensitivity cardiac troponin to at least 5 times the upper limit of normal within 48 hours from randomization. The safety end point was bleeding type 2 to 5 according to the Bleeding Academic Research Consortium criteria at 30 days.
Results
Of 334 participants (median age, 67.4 years; interquartile range, 60-75.1 years; 253 men [75.7%]; and 330 White participants [98.8%]), 120 were allocated to receive the 160-mg dose of revacept, 121 were allocated to receive the 80-mg dose, and 93 received placebo. The primary end point showed no significant differences between the revacept and placebo groups: 24.4%, 25.0%, and 23.3% in the revacept, 160 mg, revacept, 80 mg, and placebo groups, respectively (P = .98). The high dose of revacept was associated with a small but significant reduction of high-concentration collagen-induced platelet aggregation, with a median 26.5 AU × min (interquartile range, 0.5-62.2 AU × min) in the revacept, 160 mg, group; 43.5 AU × min (interquartile range, 22.8-99.5 AU × min) in the revacept, 80 mg, group; and 41.0 AU × min (interquartile range, 31.2-101.0 AU × min) in the placebo group (P = .02), while adenosine 5′-diphosphate–induced aggregation was not affected. Revacept did not increase Bleeding Academic Research Consortium type 2 or higher bleeding at 30 days compared with placebo: 5.0%, 5.9%, and 8.6% in the revacept, 160 mg, revacept, 80 mg, and placebo groups, respectively (P = .36).
Conclusions and Relevance
Revacept did not reduce myocardial injury in patients with stable ischemic heart disease undergoing percutaneous coronary intervention. There were few bleeding events and no significant differences between treatment arms.
Trial Registration
ClinicalTrials.gov Identifier: NCT03312855
This randomized clinical trial evaluates the efficacy and safety of revacept, a novel, lesion-directed antithrombotic drug, acting as a competitive antagonist to platelet glycoprotein VI.
Introduction
Patients undergoing percutaneous coronary intervention (PCI) for stable ischemic heart disease (SIHD) or acute coronary syndromes (ACS) receive dual antiplatelet therapy (DAPT) consisting of aspirin in combination with a P2Y12 inhibitor to prevent periprocedural and postprocedural ischemic events.1,2,3,4 Despite routine use of DAPT, patients undergoing PCI continue to experience periprocedural ischemic events, which represent strong and independent predictors for unfavorable outcome including mortality.5,6 Clopidogrel, the P2Y12 inhibitor recommended in patients with SIHD undergoing elective PCI, is limited by a delayed onset and considerable interpatient variability of its antiplatelet action.3 Rapidly acting antiplatelet agents that can be applied intravenously or intra-arterially are another alternative. Yet antiplatelet drugs that exert rapid and reliable antithrombotic efficacy without increasing the risk of bleeding represent an important unmet clinical need.
An optimal antithrombotic agent inhibits platelet function selectively at the site of atherosclerotic plaque injury (spontaneous or PCI-induced) without affecting systemic hemostasis. This requires targeting of thrombotic pathways that differ between healthy and atherosclerotic vasculature. Collagen fibers constitute the most thrombogenic macromolecular components of the extracellular matrix of atherosclerotic plaques.7 When collagen is exposed during atherosclerotic plaque rupture, it binds platelet glycoprotein VI (GPVI), the major platelet collagen receptor. Glycoprotein VI in turn mediates local platelet recruitment, activation, and aggregation.8,9 Glycoprotein VI is an attractive antiplatelet target because GPVI-mediated platelet response plays a central role during myocardial infarction and stroke but is less relevant in physiological hemostasis.10,11
We described a novel competitive antagonist to collagen-GPVI signaling named revacept (advanceCOR GmbH).10,12,13,14,15,16 Revacept is a dimeric, soluble fusion protein composed of the extracellular domain of the GPVI receptor and the human Fc-fragment. It competes with endogenous platelet GPVI for binding to exposed collagen fibers and inhibits collagen-mediated platelet adhesion and aggregation selectively at the site of plaque rupture.17 In addition, revacept blocks binding of von Willebrand factor to collagen and inhibits von Willebrand factor–mediated platelet activation.16 As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the atherosclerotic lesion. As a consequence, revacept inhibits atherothrombosis but has little effect on systemic hemostasis or bleeding in animal models10,13,15,16,18 and in a phase 1 clinical trial.14 However, data on the clinical efficacy and safety of revacept in patients undergoing PCI are currently lacking.
The Intracoronary Stenting and Antithrombotic Regimen: Lesion Platelet Adhesion as Selective Target of Endovenous Revacept in Patients With Chronic Coronary Syndromes Undergoing Percutaneous Coronary Intervention (ISAR-PLASTER) trial is a randomized, double-blind phase 2 trial designed to test, for the first time to our knowledge, the efficacy and safety of 2 different doses of revacept in patients with SIHD undergoing elective PCI administered in addition to standard DAPT.
Methods
Study Design
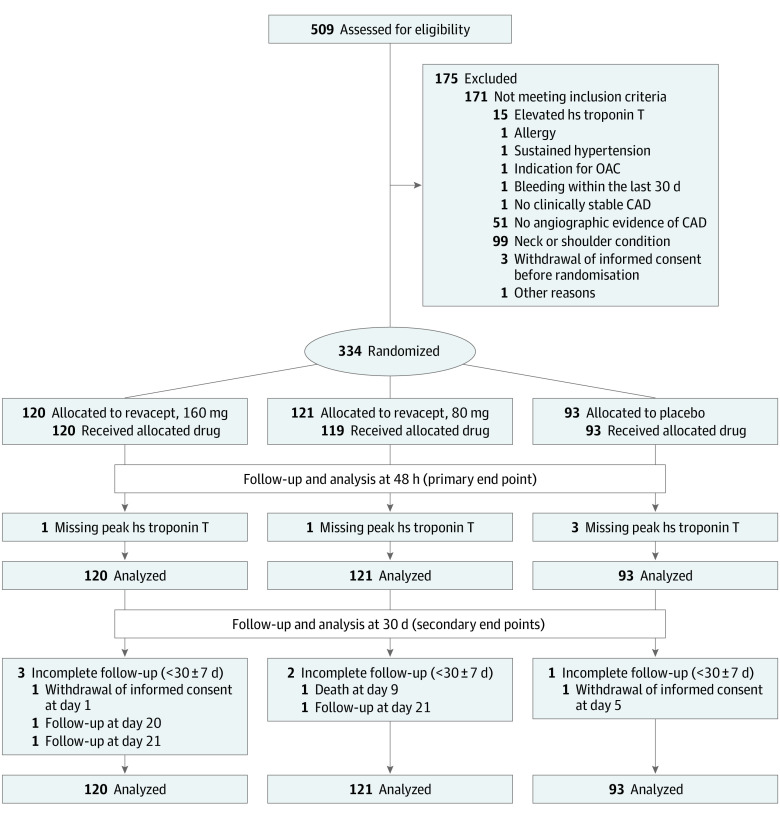
The ISAR-PLASTER was an investigator-initiated, multicenter, randomized, double-blind trial that enrolled patients with SIHD undergoing PCI at 9 participating study sites in Germany. The flow of patients in the trial is shown in Figure 1. The study had an academic sponsor (Deutsches Herzzentrum München) and was approved by the relevant ethics committee for each participating site. It was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was provided by all the patients before enrollment. An independent data safety and monitoring board oversaw the trial. An external service provider did study monitoring for all patients. The formal trial protocol can be found in Supplement 1. A detailed list of trial committee members, participating centers and investigators (eAppendix 1), the coordinating center (eAppendix 2), and statisticians (eAppendix 3) is provided in Supplement 2.
Figure 1. Screening, Randomization, Treatment, and Follow-up.
Patients were evaluated from randomization until death, withdrawal of consent, or the last contact date. CAD indicates coronary artery disease; hs, high-sensitivity; OAC, oral anticoagulation.
Patient Selection
Eligible patients were 18 years or older, presented with SIHD, had normal high-sensitivity cardiac troponin T (hsTnT) level, and angiographic evidence of coronary artery disease (CAD) with an indication for PCI. A comprehensive list of inclusion and exclusion criteria is provided in the eMethods in Supplement 2. Details of the trial rationale, design, and methods have been reported previously.19
Study Procedures
We randomly assigned eligible patients to receive either placebo, revacept, 80 mg, or revacept, 160 mg. We selected these doses based on the phase 1 study testing different revacept doses ranging from 10 to 160 mg in healthy volunteers.14 In that study, inhibition of collagen-induced platelet aggregation at 2 hours after administration was greatest in the 80- and 160-mg dose groups. The mean (SD) reported plasma half-life of revacept after 80- and 160-mg doses was 137.6 (27.2) hours and 136.6 (36.7) hours, respectively.14 No drug-related adverse events were observed with these doses.
Randomization was performed in a double-blinded manner stratified by study center with the use of a centralized computer system, embedded in the electronic case report form. Patients received the study drug (revacept, 80 mg, revacept, 160 mg, or placebo) in the form of a single intravenous infusion as soon as possible after the decision to perform PCI had been made and prior to the start of the PCI procedure (defined as guidewire passage over the stenosis). In addition to the study drug, all patients were treated with standard periprocedural antithrombotic therapy composed of clopidogrel, aspirin, and heparin (or bivalirudin) based on local practice and current guideline recommendations on myocardial revascularization.20,21
We performed 3 visits during the trial. Visit 1 included screening and administration of study medication. Visit 2 was performed at a mean (SD) of 48 (12) hours after randomization to assess the primary end point. Visit 3 was an outpatient visit done at a mean (SD) of 30 (7) days after randomization for the assessment of secondary and safety end points.
Outcomes and Definitions
The primary end point was a composite of death or myocardial injury, defined as the increase in hsTnT value to at least 5 times the upper limit of normal within 48 hours from randomization (eMethods in Supplement 2).22
The secondary end points included peak hsTnT within 48 hours from randomization as well as all-cause mortality, myocardial infarction (defined according to the third universal definition of myocardial infarction22), PCI-related (type 4a) myocardial infarction, stroke, definite stent thrombosis defined according to Academic Research Consortium (ARC) criteria,23 and urgent coronary revascularization within the first 30 days after randomization. Bleeding type 2 or higher according to the Bleeding Academic Research Consortium (BARC) criteria at 30 days was defined as the safety end point.24
Detailed definitions of the clinical outcomes are previously published.19 An independent event adjudication committee (EAC) blinded to the randomly assigned treatment adjudicated all suspected clinical events.
Assessment of Platelet Function
Venous whole blood was obtained from patients using 1.6-mL hirudin tubes (Sarstedt). The blood samples were planned to be collected from patients enrolled in Deutsches Herzzentrum München (n = 168) for the assessment of both collagen-induced and adenosine 5′-diphosphate (ADP)–induced platelet aggregation and in Klinikum rechts der Isar, Munich (n = 37), for the assessment of ADP-induced platelet aggregation.
Platelet aggregation was assessed with the Mulitplate Analyzer (Roche Diagnostics) after stimulation with adenosine diphosphate (ADP-test) or with 3 different concentrations of collagen, 31 μg/mL, 93 μg/mL, and 253 μg/mL.14 The results of the tests were quantified as area under the curve (AUC) of aggregation units (AU): AUC = AU × min.
Statistical Analysis
Details of the sample size calculation are provided in a previous publication.19 The statistical analysis plan can be found in Supplement 3.
Confirmatory hypothesis testing of the primary efficacy end point was performed in a sequential order. First, significance of the treatment effect across the 3 groups was assessed by a test for trend on a 2-sided 5% significance level using a binary logistic regression model and by using the values 0, 1, and 2 to code the placebo group, the lower-dose revacept group, and the higher-dose revacept group as a continuous variable. The logistic regression model accounted for stratification by inclusion of centers as a factor variable. In case of a significant difference between the 3 groups, the 2 revacept groups were to be compared by using a χ2 test on a 2-sided 20% significance level.25 Five missing values in the primary end point were conservatively imputed (ie, as an event in the revacept arms and as no event in the control arm) in an additional exploratory sensitivity analysis. Further exploratory analyses of secondary and safety end points were performed similar to the analysis of the primary end point using binary logistic regression models and linear regression models. Time-dependent risks until 30 days are given by Kaplan-Meier estimates as there were no competing risks. Exploratory hypothesis testing was performed at 2-sided 5% significance levels.
Three populations were used for the statistical analysis. The full-analysis set included all patients who have been randomized in concordance with the intention-to-treat principle. The modified intention-to-treat population included all patients belonging to the full analysis set who received the study medication. The per-protocol population included all patients of the full analysis set who did not show major protocol deviations. Baseline characteristics were analyzed in the full-analysis set. Confirmatory analysis of the primary end point was performed using the full analysis set (intention-to-treat population). Safety end points as well as laboratory and electrocardiogram (ECG) parameters at 48 hours after randomization were analyzed using the modified intention-to-treat population. The per-protocol population was used for additional exploratory analyses.
Descriptive statistics for quantitative data are median and interquartile range (IQR). Qualitative data are presented by absolute and relative frequencies. Exploratory hypothesis testing of group differences in baseline characteristics, as well as in laboratory and ECG parameters at 48 hours after randomization, was performed by Kruskal-Wallis rank sum tests and χ2 tests.
Sample Size Calculation
We planned to test for 2 ordered hypotheses. First, we planned to test for a significant difference in the primary end point among the 3 study groups in favor of revacept treatment. Sample size calculation was based on the following assumptions: incidence of the primary end point of 25% in the placebo group (as shown by an analysis of 2000 patients meeting study criteria from the database of the Deutsches Herzzentrum München) and 8% and 17% in the higher 160-mg and lower 80-mg revacept dose groups, respectively. Our assumptions were based on large reductions in the primary end point by revacept because myocardial injury as defined in this trial is a very soft end point and only large reductions in its incidence might be expected to have a measurable effect on hard events in a future trial sufficiently powered for clinical end points. Hence, this trial was only powered to see a very large treatment effect because such a large reduction in myocardial injury would likely translate into a clinically meaningful benefit. Use of a 2-sided α level of .05 and a power of 80% leads to a total sample size of 270 patients (90 patients in each of the 3 study groups). Second, in the case of a statistically significant difference between the 3 study groups, we planned to test for a difference in the 2 revacept dose groups. Instead of considering superior the dose with the larger response rate, we preferred to increase the type I error rate at α = 20%.19 References in support of this method are given in the previous publication of trial design. Detection of a difference between 8% and 17% with a power of 80% using a type I error rate of 20% required 121 patients in each of the 2 revacept treatment arms for a total of 242 patients. The sample size of 90 patients in the placebo group was added to the 242 patients in the 2 revacept groups to form the total sample size of 332 patients for this trial.
Results
Study Population
Between November 20, 2017, and February 27, 2020, we screened 509 patients who had signed the informed consent as possible candidates for participation in the study. Figure 1 reports the reasons why 175 patients were not randomized. Thus, 334 patients were successfully randomized and constitute the intention-to-treat population (Figure 1). Baseline clinical, angiographic, and interventional characteristics are shown in Table 1 as well as in eTables 1 and 2 in Supplement 2.
Table 1. Baseline Patient Characteristicsa.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Revacept | Placebo (n = 93) | ||
| 160 mg (n = 120) | 80 mg (n = 121) | ||
| Age, median (IQR), y | 67.3 (61.5-75.5) | 67.4 (60.4-75.1) | 67.8 (60.8-74.8) |
| Female | 38 (31.7) | 24 (19.8) | 19 (20.4) |
| Weight, median (IQR), kg | 82.0 (70.8-92.0) | 82.0 (74.0-94.0) | 82.0 (73.0-91.0) |
| Race/ethnicity | |||
| White | 116 (96.7) | 121 (100) | 93 (100) |
| Black | 1 (0.8) | 0 | 0 |
| Asian | 3 (2.5) | 0 | 0 |
| Cardiovascular risk factors | |||
| Diabetes | 32 (26.7) | 35 (28.9) | 22 (23.7) |
| Insulin therapy, No./total No. | 12/32 (37.5) | 11/35 (31.4) | 8/22 (36.4) |
| Current smoker | 24 (20.0) | 20 (16.5) | 23 (24.7) |
| Arterial hypertension | 160 (88.3) | 103 (85.1) | 87 (93.5) |
| Hypercholesterolemia | 110 (91.7) | 108 (89.3) | 80 (86.0) |
| Medical history | |||
| Myocardial infarction | 21 (17.5) | 27 (22.3) | 26 (28.0) |
| PCI | 60 (50.0) | 71 (58.7) | 57 (61.3) |
| CABG | 10 (8.3) | 11 (9.1) | 6 (6.5) |
| Stroke | 2 (1.7) | 3 (2.5) | 3 (3.2) |
| Peripheral arterial occlusive disease | 11 (9.2) | 8 (6.6) | 7 (7.5) |
| COPD | 8 (6.7) | 4 (3.3) | 5 (5.4) |
| Kidney insufficiency | 14 (11.7) | 12 (9.9) | 5 (5.4) |
| Family history of premature CAD, No./total No. (%)b | 53 (44.2) | 52/119 (43.7) | 36/92 (39.1) |
| High-sensitivity cardiac troponin T, median (IQR), ng/L | 11.0 (8.8-13.0) | 11.0 (8.0-13.0) | 10.0 (7.0-13.0) |
| Creatinine, median (IQR), mg/dL | 1.0 (0.9-1.1) | 1.0 (0.8-1.1) | 0.9 (0.8-1.1) |
| Body temperature, median (IQR), °Cc | 36.5 (36.4-36.7) | 36.5 (36.4-36.8) | 36.6 (36.5-36.8) |
| Heart rate, median (IQR), bpm | 64.5 (58.8-72.2) | 66.0 (58.0-72.0) | 66.0 (57.0-71.0) |
| Blood pressure, median (IQR), mm Hg | |||
| Systolic | 145.0 (128.8-154.0) | 137.0 (124.0-150.0) | 141.0 (130.0-154.0) |
| Diastolic | 80.0 (72.0-90.0) | 78.0 (71.0-83.0) | 80.0 (72.0-87.0) |
| Access site | |||
| Femoral | 57 (47.5) | 67 (55.4) | 40 (43.0) |
| Radial | 62 (51.7) | 54 (44.6) | 52 (55.9) |
| Brachial | 1 (0.8) | 0 | 1 (1.1) |
| No. of diseased coronary vessels | |||
| 1 | 19 (15.8) | 23 (19.0) | 15 (16.1) |
| 2 | 41 (34.2) | 36 (29.8) | 28 (30.1) |
| 3 | 60 (50.0) | 62 (51.2) | 50 (53.8) |
| Closure device | 39 (32.5) | 48 (39.7) | 26 (28.0) |
Abbreviations: CABG, coronary-artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention.
There were no significant between-group differences in clinical and angiographic characteristics at baseline.
Information about family history of premature CAD not available in 3 patients (2 in the revacept, 80 mg, group and 1 in the placebo group).
Body temperature not available in 3 patients (1 in the revacept, 160 mg, group; 1 in the revacept, 80 mg, group; and 1 in the placebo group).
Efficacy
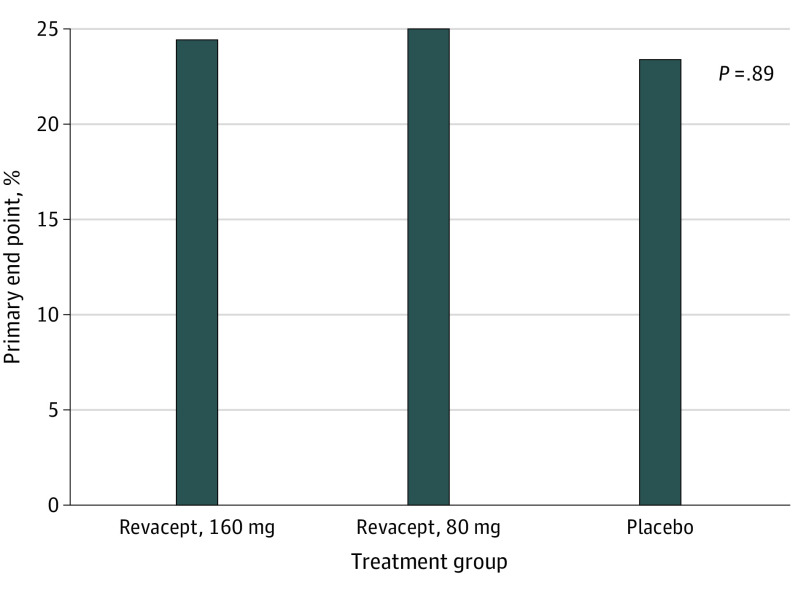
At 48 hours, the combined primary end point occurred in 80 of 329 patients with peak hsTnT measurements (24.3%) without significant differences between the revacept and placebo groups (29 of 119 patients allocated to revacept, 160 mg [24.4%]; 30 of 120 patients allocated to revacept, 80 mg [25.0%]; and 21 of 90 patients allocated to placebo [23.3%]; P = .98) (Figure 2). Because there was only 1 patient who died, this result practically consists of the myocardial injury component. There was no statistically significant difference in the incidence of the primary end point between patients with and those without clopidogrel loading prior to PCI (63 of 270 [23.3%] and 17 of 59 [28.8%], respectively; P = .37). Within 48 hours, the median peak hsTnT was 31.5 ng/L (IQR, 18.0-66.8 ng/L) and 28.0 ng/L (IQR, 16.0-65.0 ng/L) in the revacept, 80 mg and 160-mg groups, respectively, vs 32.0 ng/L (IQR, 20.8-62.3 ng/L) in the placebo group (P = .44). Only 4 patients (2 patients in the revacept, 160 mg, group and 1 in the revacept, 80 mg, and placebo groups, respectively) showed an increase in hsTnT of at least 70 times the upper limit of normal within 48 hours from randomization. Risk estimates of the primary end point with imputation of the missing values in the modified intention-to-treat and in the per protocol population did not differ by more than 0.6% from the values obtained in the intention-to-treat population, confirming the robustness of the finding. These analyses also showed no significant difference between the groups (data not shown).
Figure 2. Primary End Point in the 3 Treatment Groups.
The primary end point was a composite of death or myocardial injury, defined as increase in high-sensitivity cardiac troponin T to at least 5 times the upper limit of normal within 48 hours from randomization. There was only 1 death in the trial; thus, the end point largely reflects myocardial injury.
The composite of all-cause death, myocardial infarction, stroke, or urgent revascularization within 30 days from randomization was observed in 9 of 334 patients (2.7%) of the entire cohort without significant difference between the groups (3 of 120 patients [2.5%] allocated to revacept, 160 mg, 4 of 121 patients (3.3%) allocated to revacept, 80 mg, and 2 of 93 patients (2.2%) in the placebo group; P = .91) (eTable 3 and the eFigure in Supplement 2). Distribution of the individual adverse events across the 3 study treatment groups is also shown in Table 2. In the revacept, 80 mg, group, 1 patient in whom both right coronary artery and left circumflex coronary were treated during the index procedure died on day 9 of acute inferior myocardial infarction without having a diagnostic coronary angiography.
Table 2. Safety End Point at 30 Days.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| Revacept | Placebo (n = 93) | |||
| 160 mg (n = 120) | 80 mg (n = 119) | |||
| BARC type 2-5 | 6 (5.0) | 7 (5.9) | 8 (8.6) | .36 |
| Type 2 | 5 | 5 | 7 | NA |
| Type 3a | 1 | 1 | 1 | NA |
| Type 3b | 0 | 1 | 0 | NA |
| Type 3c | 0 | 0 | 0 | NA |
| Type 4 | 0 | 0 | 0 | NA |
| Type 5 | 0 | 0 | 0 | NA |
Abbreviations: BARC, Bleeding Academic Research Consortium; NA, not applicable.
Safety
The incidence of the key secondary safety end point of BARC type 2 to 5 bleeding differed marginally between the groups in the modified intention-to-treat population: it was 5.0% (6 patients) in the revacept, 160 mg, group; 5.9% (7 patients) in the revacept, 80 mg, group; and 8.6% (8 patients) in the placebo group; P = .36 (eFigure in Supplement 2), with all of the events recorded during the first 2 days after randomization. Table 2 summarizes all BARC type 2 to 5 bleedings across groups and shows that most of these events were classified as less severe BARC type 2 bleeding. The BARC type 1 bleeding was observed in 15 of 120 patients (12.5%) in the revacept, 160 mg, groups; 13 of 119 patients (10.9%) in the revacept, 80 mg, group; and 5 of 93 patients (5.4%) in the placebo group (P = .09).
In the modified intention-to-treat population, laboratory parameters at 48 hours were not significantly different between the groups (eTable 4 in Supplement 2). In addition, there were no significant between-group differences in ECG parameters at 48 hours, except for a slight but significant prolongation of the QTc interval in the revacept groups (median, 423 milliseconds, IQR, 404-447 milliseconds in the revacept, 160 mg, group; 421 milliseconds, IQR, 400-436 milliseconds in the revacept, 80 mg, group; and 414 milliseconds, IQR, 399-435 milliseconds in the placebo group (P = .03; eTable 5 in Supplement 2).
Platelet Function
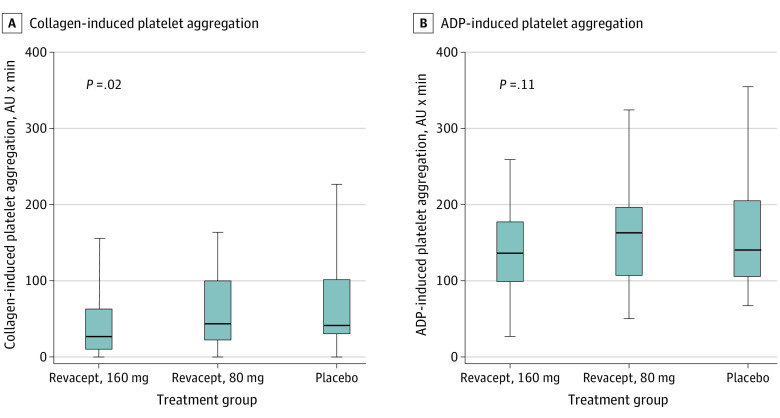
The characteristics of patients with and without planned platelet function evaluation are shown in eTables 6 and 7 in Supplement 2. Revacept significantly reduced platelet aggregation in response to collagen in the 93 μg/mL (P = .03) and 253 μg/mL concentration (Figure 3A; P = .02) as compared with placebo. Enhanced platelet inhibition by revacept was specific to the collagen-GPVI axis because we did not observe significant intergroup differences in the platelet response to ADP (median, 136.0 AU, IQR, 99.0-177.0 AU in the revacept, 160 mg, group; 167.0 AU, IQR, 109.5-204.5 AU in the revacept, 80 mg, group; and 143.0 AU, IQR, 108.2-209.2 AU in the placebo group; P = .11; Figure 3B). A detailed presentation of the platelet function data is included in eTable 8 in Supplement 2.
Figure 3. Inhibition of Collagen-Induced Platelet Aggregation (A) and ADP-Induced Platelet Aggregation (B).
Horizontal bars indicate the median values, boxes indicate the 25th and 75th percentiles, and vertical lines indicate the 5th and 95th percentiles. The results are shown for collagen concentration of 253 μg/mL. ADP indicates adenosine 5′-diphosphate; AU, aggregation unit.
Discussion
Drugs that allow a rapid and profound platelet inhibition without increasing the risk of bleeding are conceptually appealing for periprocedural antithrombotic management in patients undergoing PCI. The ISAR-PLASTER is a phase 2 study that, for the first time to our knowledge, tests the efficacy and safety of revacept in patients with SIHD undergoing elective PCI. Revacept is a novel lesion-directed competitive inhibitor to the platelet collagen receptor GPVI that efficiently prevents arterial thrombosis, but has little effect on physiologic hemostasis in animal models and did not cause bleeding in healthy individuals in a phase I clinical trial.10,14 The trial shows that in patients with low-risk SIHD, revacept administered on top of standard DAPT did not affect the primary clinical efficacy end point, a myocardial injury surrogate. However, a high dose of revacept leads to a significant additional increase in platelet inhibition in patients treated with standard antithrombotic therapy. Despite providing a more robust platelet inhibition, the high dose of revacept on top of standard DAPT was not associated with an increase in bleeding.
The functional target of revacept is GPVI, a type I transmembrane protein expressed by megakaryocytes and platelets that belongs to the immunoglobulin superfamily. Glycoprotein VI was first described in 1982 as a protein that was missing in a patient with a severe collagen-activation defect in platelets.26 Since then, GPVI has been identified as the central collagen receptor expressed on platelets.7,8 When thrombogenic collagen fibers are exposed during atherosclerotic plaque rupture, binding of platelet GPVI to exposed collagen triggers local adhesion. This step in the platelet adhesion cascade is essential for subsequent activation and aggregation of platelets. In addition, GPVI binds to fibrin and has major roles in thrombus growth and stability.27,28 Inhibition or ablation of GPVI therefore yields strong protection against arterial thrombosis in animal models.7,8,9 However, despite major roles of GPVI in several critical aspects of atherothrombosis, GPVI deficiency is generally associated with minimal effects on hemostasis in animal models.29,30 This suggests that physiologic hemostasis does not require GPVI and indicates that pharmacologic GPVI modulation may provide a novel concept of antithrombotic therapies that do not increase bleeding.11
Research on new antiplatelet drugs is increasingly focusing on preserving hemostasis.31 The ISAR-PLASTER is, to our knowledge, the first phase 2 clinical trial testing the efficacy and safety of a pharmacologic approach targeting GPVI in patients with CAD. Different approaches to block platelet GPVI have been developed and are currently tested in preclinical models, predominantly including inhibitory monoclonal antibodies that bind to GPVI on circulating blood platelets.17,32 The results of phase 2 clinical studies with inhibitors of platelet GPVI-mediated adhesion pathways in patients with cerebrovascular disease, such as Revacept in Symptomatic Carotid Stenosis (Revacept/CS/02) and Acute Ischemic Stroke Interventional Study (ACTIMIS), are being awaited.
Two aspects are likely to explain why enhanced platelet inhibition provided by revacept did not translate into increased clinical efficacy in the ISAR-PLASTER trial. First, the primary end point of our trial included the 5-time increase in hsTnT, a surrogate end point of myocardial injury that has little prognostic impact.33 In fact, only much higher increases in hsTnT (70 times) have been found with a significant prognostic value.34,35 The incidence of major adverse cardiovascular events at 30 days was 2.5%, indicating that low-risk patients were enrolled in the ISAR-PLASTER trial. Future studies are being planned to address whether patients at higher risk of ischemic events triggered by collagen-induced platelet activation, in particular patients with ACS, derive benefit from a 160-mg dose of revacept. Second, the surrogate of myocardial injury used in our trial is subject to triggers that may not be modifiable by revacept. As a lesion-directed drug, revacept does not interfere with the function of circulating platelets beyond the coronary lesion. Hence, key triggers of type 4a myocardial infarction, including side branch occlusion owing to plaque shifting or distal plaque material embolization, are unlikely to respond to inhibition of GPVI-collagen interaction by revacept.
Limitations
Our study has several limitations. First, the trial enrolled patients with SIHD undergoing elective PCI who were at very low risk of ischemic events. All patients routinely received standard antithrombotic therapy. Second, as a phase 2 trial, ISAR-PLASTER was not powered for hard clinical end points. The trial was only powered for detecting extremely large reductions in the primary end point represented by a surrogate of myocardial injury with little prognostic value.
Conclusions
In this first-in-class phase 2 trial on a competitive GPVI inhibitor, revacept did not reduce myocardial injury in patients with SIHD undergoing PCI. There were few bleeding events and no significant differences between treatment arms. The 160-mg dose of revacept had a small but statistically significant effect on collagen-induced but not ADP-induced platelet aggregation.
Trial Protocol
eAppendix 1. Committees and Investigators
eAppendix 2. Coordinating ISAResearch Center
eAppendix 3. Statistics
eTable 1. Angiographic and Interventional Characteristics
eTable 2. Concomitant Medication
eTable 3. Efficacy Endpoints at 30 Days
eTable 4. Laboratory Parameters at 48h
eTable 5. ECG Parameters at 48h
eTable 6. Baseline Patient Characteristics in Centers With vs. Without Planned Platelet Function Evaluation (Adenosine Diphosphate-Induced Platelet Aggregation)
eTable 7. Angiographic and Interventional Characteristics in Centers With vs. Without Planned Platelet Function Evaluation (Adenosine Diphosphate-Induced Platelet Aggregation)
eTable 8. Evaluation of ADP and Collagen-Induced Platelet Aggregation at 48h
eFigure 1. Kaplan–Meier Survival Curves of Major Adverse Cardiovascular Events (Left Panel) and BARC Type 2 to 5 Bleeding (Right Panel) at 30 Days.
eMethods.
Statistical Analysis Plan
Data Sharing Statement
References
- 1.Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. 2010;74(4):597-607. doi: 10.1253/circj.CJ-09-0982 [DOI] [PubMed] [Google Scholar]
- 2.Sibbing D, Aradi D, Alexopoulos D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12(16):1521-1537. doi: 10.1016/j.jcin.2019.03.034 [DOI] [PubMed] [Google Scholar]
- 3.Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group; ESC Committee for Practice Guidelines (CPG); ESC National Cardiac Societies . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39(3):213-260. doi: 10.1093/eurheartj/ehx419 [DOI] [PubMed] [Google Scholar]
- 4.Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72(23 Pt A):2915-2931. doi: 10.1016/j.jacc.2018.09.057 [DOI] [PubMed] [Google Scholar]
- 5.Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51(7):690-697. doi: 10.1016/j.jacc.2007.10.040 [DOI] [PubMed] [Google Scholar]
- 6.Buccheri S, Capodanno D, James S, Angiolillo DJ. Bleeding after antiplatelet therapy for the treatment of acute coronary syndromes: a review of the evidence and evolving paradigms. Expert Opin Drug Saf. 2019;18(12):1171-1189. doi: 10.1080/14740338.2019.1680637 [DOI] [PubMed] [Google Scholar]
- 7.Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost. 2011;9(suppl 1):92-104. doi: 10.1111/j.1538-7836.2011.04361.x [DOI] [PubMed] [Google Scholar]
- 8.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449-461. doi: 10.1182/blood-2002-12-3882 [DOI] [PubMed] [Google Scholar]
- 9.Massberg S, Gawaz M, Grüner S, et al. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med. 2003;197(1):41-49. doi: 10.1084/jem.20020945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massberg S, Konrad I, Bültmann A, et al. Soluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivo. FASEB J. 2004;18(2):397-399. doi: 10.1096/fj.03-0464fje [DOI] [PubMed] [Google Scholar]
- 11.Martins Lima A, Martins Cavaco AC, Fraga-Silva RA, Eble JA, Stergiopulos N. From patients to platelets and back again: pharmacological approaches to glycoprotein VI, a thrilling antithrombotic target with minor bleeding risks. Thromb Haemost. 2019;119(11):1720-1739. doi: 10.1055/s-0039-1695770 [DOI] [PubMed] [Google Scholar]
- 12.Schönberger T, Siegel-Axel D, Bussl R, et al. The immunoadhesin glycoprotein VI-Fc regulates arterial remodelling after mechanical injury in ApoE-/- mice. Cardiovasc Res. 2008;80(1):131-137. doi: 10.1093/cvr/cvn169 [DOI] [PubMed] [Google Scholar]
- 13.Bültmann A, Li Z, Wagner S, et al. Impact of glycoprotein VI and platelet adhesion on atherosclerosis: a possible role of fibronectin. J Mol Cell Cardiol. 2010;49(3):532-542. doi: 10.1016/j.yjmcc.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 14.Ungerer M, Rosport K, Bültmann A, et al. Novel antiplatelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation. 2011;123(17):1891-1899. doi: 10.1161/CIRCULATIONAHA.110.980623 [DOI] [PubMed] [Google Scholar]
- 15.Schönberger T, Ziegler M, Borst O, et al. The dimeric platelet collagen receptor GPVI-Fc reduces platelet adhesion to activated endothelium and preserves myocardial function after transient ischemia in mice. Am J Physiol Cell Physiol. 2012;303(7):C757-C766. doi: 10.1152/ajpcell.00060.2012 [DOI] [PubMed] [Google Scholar]
- 16.Goebel S, Li Z, Vogelmann J, et al. The GPVI-Fc fusion protein Revacept improves cerebral infarct volume and functional outcome in stroke. PLoS One. 2013;8(7):e66960. doi: 10.1371/journal.pone.0066960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borst O, Gawaz M. Glycoprotein VI: novel target in antiplatelet medication. Pharmacol Ther. 2021;217:107630. doi: 10.1016/j.pharmthera.2020.107630 [DOI] [PubMed] [Google Scholar]
- 18.Reimann A, Li Z, Goebel S, et al. Combined administration of the GPVI-Fc fusion protein Revacept with low-dose thrombolysis in the treatment of stroke. Heart Int. 2016;11(1):e10-e16. doi: 10.5301/heartint.5000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schüpke S, Hein-Rothweiler R, Mayer K, et al. ; ISAR-PLASTER-Trial Investigators . Revacept, a novel inhibitor of platelet adhesion, in patients undergoing elective PCI-design and rationale of the randomized ISAR-PLASTER trial. Thromb Haemost. 2019;119(9):1539-1545. doi: 10.1055/s-0039-1692423 [DOI] [PubMed] [Google Scholar]
- 20.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ; ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 21.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64(18):1929-1949. doi: 10.1016/j.jacc.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, Jaffe AS, et al. ; Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction; ESC Committee for Practice Guidelines (CPG) . Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551-2567. doi: 10.1093/eurheartj/ehs184 [DOI] [PubMed] [Google Scholar]
- 23.Cutlip DE, Windecker S, Mehran R, et al. ; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344-2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 24.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123(23):2736-2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 25.Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23(28):7199-7206. doi: 10.1200/JCO.2005.01.149 [DOI] [PubMed] [Google Scholar]
- 26.Moroi M, Jung SM, Okuma M, Shinmyozu K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J Clin Invest. 1989;84(5):1440-1445. doi: 10.1172/JCI114318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alshehri OM, Hughes CE, Montague S, et al. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126(13):1601-1608. doi: 10.1182/blood-2015-04-641654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mammadova-Bach E, Ollivier V, Loyau S, et al. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126(5):683-691. doi: 10.1182/blood-2015-02-629717 [DOI] [PubMed] [Google Scholar]
- 29.Lockyer S, Okuyama K, Begum S, et al. GPVI-deficient mice lack collagen responses and are protected against experimentally induced pulmonary thromboembolism. Thromb Res. 2006;118(3):371-380. doi: 10.1016/j.thromres.2005.08.001 [DOI] [PubMed] [Google Scholar]
- 30.Bynagari-Settipalli YS, Cornelissen I, Palmer D, et al. Redundancy and interaction of thrombin- and collagen-mediated platelet activation in tail bleeding and carotid thrombosis in mice. Arterioscler Thromb Vasc Biol. 2014;34(12):2563-2569. doi: 10.1161/ATVBAHA.114.304244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFadyen JD, Schaff M, Peter K. Current and future antiplatelet therapies: emphasis on preserving haemostasis. Nat Rev Cardiol. 2018;15(3):181-191. doi: 10.1038/nrcardio.2017.206 [DOI] [PubMed] [Google Scholar]
- 32.Voors-Pette C, Lebozec K, Dogterom P, et al. Safety and tolerability, pharmacokinetics, and pharmacodynamics of ACT017, an antiplatelet GPVI (glycoprotein VI) fab. Arterioscler Thromb Vasc Biol. 2019;39(5):956-964. doi: 10.1161/ATVBAHA.118.312314 [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Garcia HM, McFadden EP, von Birgelen C, et al. Impact of periprocedural myocardial biomarker elevation on mortality following elective percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12(19):1954-1962. doi: 10.1016/j.jcin.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Garcia HM, McFadden EP, Farb A, et al. ; Academic Research Consortium . Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Eur Heart J. 2018;39(23):2192-2207. doi: 10.1093/eurheartj/ehy223 [DOI] [PubMed] [Google Scholar]
- 35.Moussa ID, Klein LW, Shah B, et al. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J Am Coll Cardiol. 2013;62(17):1563-1570. doi: 10.1016/j.jacc.2013.08.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Committees and Investigators
eAppendix 2. Coordinating ISAResearch Center
eAppendix 3. Statistics
eTable 1. Angiographic and Interventional Characteristics
eTable 2. Concomitant Medication
eTable 3. Efficacy Endpoints at 30 Days
eTable 4. Laboratory Parameters at 48h
eTable 5. ECG Parameters at 48h
eTable 6. Baseline Patient Characteristics in Centers With vs. Without Planned Platelet Function Evaluation (Adenosine Diphosphate-Induced Platelet Aggregation)
eTable 7. Angiographic and Interventional Characteristics in Centers With vs. Without Planned Platelet Function Evaluation (Adenosine Diphosphate-Induced Platelet Aggregation)
eTable 8. Evaluation of ADP and Collagen-Induced Platelet Aggregation at 48h
eFigure 1. Kaplan–Meier Survival Curves of Major Adverse Cardiovascular Events (Left Panel) and BARC Type 2 to 5 Bleeding (Right Panel) at 30 Days.
eMethods.
Statistical Analysis Plan
Data Sharing Statement