Abstract
Background
There are limited data on the neutralizing activity of convalescent plasma (CP) administered in randomized controlled trials (RCT) of COVID‐19 infection.
Study Design and Methods
As part of an RCT, CP was collected per FDA guidelines from individuals recovered from COVID‐19 infection. CP donors had to have ≥145 optical density (OD) units (ideal target ≥300) using a semiquantitative, immunochromatographic test for IgG antibody to the nucleocapsid protein (NP) of SARS‐CoV‐2 (typical range 0–500 OD units). A random subset of samples [14 control plasma, 12 CP “medium‐anti‐NP” (145–299 OD units), and 13 CP “high” anti‐NP (≥300 OD units)] were tested for neutralizing antibodies using an established viral luciferase antibody inhibition assay to detect the infection of SARS‐CoV‐2 pseudovirus that encoded spike protein (SARS2‐Strunc) on a human immunodeficiency virus 1 vector (NL43dEnvNanoLuc), using ACE2‐expressing 293 T cells. The titer needed to neutralize 50% of viral activity (NT50) was calculated.
Results
The uptake of SARS‐CoV‐2 pseudovirus by 293TACE2 cells was inhibited by pretreatment with CP compared to control CP (p < .001) with control plasma having a median (IQR) 50% neutralization titer (NT50) of 1:28 (1:16,1:36) compared to 1:334 (1:130,1:1295) and 1:324 (1:244,1:578), for medium anti‐NP and high anti‐NP CP units, respectively. The neutralizing activity of CP met minimum FDA criteria with neutralizing antibody titers >1:80 in 100% of randomly selected samples, using a conservative approach that excluded non‐specific binding.
Discussion
Plasma from donors screened using an immunochromatographic test for IgG antibody to SARS‐CoV‐2 NP exhibited neutralizing activity meeting FDA's minimum standard in all randomly selected COVID‐19 CP units.
Keywords: FFP transfusion, immunology (other than RBC serology)
1. INTRODUCTION
The world‐wide SARS‐CoV‐2 (COVID‐19) pandemic has caused over 50 million confirmed cases and over 1 million deaths. Treatment of COVID‐19 continues to be a major objective of current research, however, few proven therapies exist. Convalescent plasma (CP) has shown some efficacy against other viruses, including the closely related SARS‐CoV, 1 and is therefore under investigation as a therapeutic agent for SARS‐CoV‐2. To date, only 2 peer‐reviewed randomized trials of CP for treatment of COVID‐19 infection have been published 2 , 3 and these yielded equivocal results.
An important aspect of treatment with CP is potential efficacy for treatment of patients early in the course of infection, before they have had time to generate their own humoral immune response. Another important methodological issue is that CP must have sufficient titers of neutralizing antibodies, presumably those that directly inhibit SARS‐CoV‐2 viral entry. 4 Some preliminary data suggest that the neutralizing capacity of CP does not directly correlate with antibodies to SARS‐CoV‐2. 5 Several clinically deployed serologic assays use nucleocapsid protein (NP), therefore, there is an unmet need to determine if the level of antibodies to SARS‐CoV‐2 NP correlates with neutralizing activity, as determined by SARS‐CoV‐2 pseudovirus neutralization assays. This is important to determine if CP has sufficient anti‐viral activity to have a therapeutic effect. Therefore, the current study aimed to identify the neutralizing capacity of CP collected for use in a double‐blind RCT.
2. STUDY DESIGN AND METHODS
We have been conducting a single‐center, double‐blind, randomized controlled trial (RCT) of CP as a potential treatment (vs. control plasma) for the treatment of hospitalized patients infected with SARS‐CoV‐2. The trial is approved by the local Institutional Review Board, registered at clinicaltrials.gov (NCT04344535), and has been conducted under an Investigational New Drug (IND) per FDA regulations.
CP was collected from COVID‐19 convalescent individuals who provided written informed consent. Consistent with FDA guidelines (April 8, 2020, April 13, 2020, May 1, 2020), a rigorous donor selection process screened individuals including PCR testing for active viral infection, routine donor health and transmittable disease testing (TDT), and serology testing for potential antibodies to SARS‐CoV‐2. Details of the donor screening and collection process and results are described elsewhere (manuscript under review).
We screened potential donors for antibodies using a semiquantitative, rapid immunochromatographic test for IgG antibody to the NP of SARS‐CoV‐2 (Chembio Diagnostic Systems Inc, Medford, NY). This test has a readout of approximately 0–500 OD units for the antibody/antigen band. While the diagnostic cutoff for positivity was only 25 IgG OD units, in order to target donors more likely to have high antibody titers, we targeted individuals with at least 145 OD units, and ideally >300 OD units. CP from fully qualifying individuals was collected in our hospital's accredited blood collection facility. This CP served as the treatment arm for the study. The control arm included plasma collected in the USA prior to January 2020, confirmed to be negative for SARS‐CoV‐2 NP IgG by the same test used for the treatment arm. Control plasma and CP were stored frozen in the Stony Brook University Hospital Blood Bank, per routine practice. While ≥145 OD units for IgG anti‐NP was used to qualify donors for our trial, we also measured IgM anti‐NP, as well as IgM and IgG anti‐Spike Protein using the same rapid immunochromatographic test with different antigen, for example, Spike, in the cassette. At this time (Spring 2020), FDA guidelines did not require any antibody testing for CP.
The virus neutralization assay is a high complexity, labor‐intensive test. A computer‐generated random sequence was used to randomly select a representative subset of control and CP units for testing. To further optimize the integrity of the results, the neutralizing antibody assay was performed by individuals blinded to plasma type.
Details for the assay are presented in Supplemental Content S1. Briefly, neutralizing activity was tested in vitro by measuring reduction in infectivity by NanoLuc‐expressing SARS‐CoV‐2 pseudovirus; the vector was provided by The Rockefeller University, NY (see Acknowledgements). 6 This strategy enables safer testing of neutralizing antibody activity, compared to the use of authentic, pathogenic virus. The assay is similar to other SARS‐CoV‐2 pseudovirus neutralization assays and plasma‐based inhibition assays that have been developed to measure the inhibitory effects of plasma. 7 , 8 , 9 Our system utilized pseudovirus of NanoLuc‐expressing human immunodeficiency virus 1 particles (HIV‐1NL4‐3‐∆Env‐NanoLuc) with the SARS‐CoV‐2 spike protein (SARS2‐Strunc). 6 This pseudovirus was used to infect 293 T ACE2‐expressing cells that have been a consistent and effective cell line for SARS‐CoV‐2 pseudovirus neutralization assays. 6 , 7 Viral infectivity was assessed via luciferase reporter activity for virus alone as well as control plasma, medium anti‐NP CP, and high anti‐NP CP. The blinded plasma samples were thawed and complement was inactivated by heating at 55°C for 30 min. Each of these heat inactivated samples was tested at seven dilutions, starting at 1:4, followed by 3‐fold serial dilutions. The inhibitory activity of CP to block pseudovirus infection for each group was determined by comparing to the average luciferase activity with control plasmas at 1:4 dilution. This represents a conservative approach and was done in response to observing inhibitory activity in control plasma. Consistent with previous publications, 10 , 11 each CP sample was run in duplicate experiments, each with two technical replicates.
Neutralization titers needed to neutralize 50% of viral activity (NT50) were interpolated using non‐linear regression in GraphPad Prism. Consistent with our planned analysis strategy and previously published studies by other groups, 10 , 11 the four values for each dilution level were inputted into GraphPad Prism, which used its algorithm to calculate a single NT50 value for each unique plasma sample. Our statistician then computed the median and IQR NT50 values for the three groups. Median NT50 values and corresponding dilution estimates were compared across the three groups using a Kruskal Wallis test with SAS © 9.4 software (Cary, NC). Post‐hoc tests for multiple comparisons were performed using Dunn's test at a reduced significance level of p = .017.
3. RESULTS
We randomly selected and tested 14 units of control plasma, 12 units of medium‐titer anti‐NP plasma (145–299 OD), and 13 units of high‐titer anti‐NP plasma (≥300 OD). All control plasma units were collected before January 2020 to reduce the likelihood of these containing SARS‐CoV‐2 reactive antibodies.
Clinical characteristics of the plasma are shown in Table 1. Limited demographic data were available for control plasma. For the CP from our qualified donors, males represented 75% and 85% in the medium anti‐NP and high anti‐NP groups, respectively. IgG anti‐Spike antibody levels, measured by the chromogenic assay, were also elevated in CP (Table 1).
TABLE 1.
Characteristics of control and convalescent plasma
| Control plasma IgG NP < 25 OD | Convalescent plasma IgG NP 145–299 OD | Convalescent plasma IgG NP ≥300 OD | |
|---|---|---|---|
| Number of units tested (% of total of 39) | 14 (36%) | 12 (31%) | 13 (33%) |
| Sex | ‐ | ||
| Male | ‐ | 9 (75%) | 11 (85%) |
| Female | ‐ | 3 (25%) | 2 (15%) |
| Blood type | ‐ | ||
| A | ‐ | 9 (75%) | 4 (31%) |
| B | ‐ | 2 (17%) | 1 (8%) |
| AB | ‐ | 1 (8%) | 0 (0%) |
| O | ‐ | 0 (0%) | 8 (61%) |
| Age (median, IQR) | ‐ | 45 (28, 54) | 52 (35, 56) |
| Duration illness, days (median, IQR) | ‐ | 12 (9, 20) | 12 (9, 14) |
| Symptom start to plasma donation, days (median, IQR) | ‐ | 42 (40, 49) | 49 (42, 58) |
| Symptom end to plasma donation, days (median, IQR) | ‐ | 29 (28, 34) | 33 (31, 43) |
| IgM NP OD units (median, IQR) | 5 (4, 6) | 55 (38, 67) | 118 (91, 186) |
| IgG NP OD units (median, IQR) | 4 (4, 9) | 212 (186, 274) | 349 (334, 354) |
| IgM spike OD units (median, IQR) | 54 (29, 81) | 76 (33, 113) | |
| IgG spike OD units (median, IQR) | 185 (131, 246) | 210 (164, 273) | |
| NT50 (median, IQR) a | 1:28 (1:16, 1:36) | 1:334 (1:130, 1:1295) | 1:324 (1:244, 1:578) |
Note: OD = optical density units for the antibody/antigen band formed for the four separate immunochromatrographic tests (ie, IgM/IgG antibodies to the Nucleocapsid Protein [NP] and IgM/IgG antibodies to the Spike protein). Cassettes with spike antigen were not available to test control plasma.
p < .001 (Kruskal Wallis) for comparison of all three study groups. No significant difference between the medium and high CP groups (Dunn's multiple comparison test).
Individual curves for virally induced luminescence at various dilutions of plasma are shown in Figure 1(A) (control), Figure 1(B) (medium anti‐NP CP), and Figure 1(C) (high anti‐NP CP). Aggregate curves for these three groups are shown in Figure 1(D). A dose‐response curve, needed for GraphPad Prism to calculate an NT50, was observed in 9, 11, and 12 of the control, medium‐ and high anti‐NP units, respectively. As shown in Table 1, control plasma exhibited significantly (p < .001) less inhibition of viral entry, that is, median (IQR, interquartile range) NT50 values detected were 1:28 (1:16, 1:36) compared to CP which had much higher median (IQR) NT50 values of 1:334 (1:130, 1:1295) and 1:324 (1:244, 1:578) for medium anti‐NP and high anti‐NP CP units, respectively. NT50 values were significantly different across the three groups (p < .001). Post hoc comparisons revealed that the control group was significantly different from both the medium and high antibody groups, but there were no significant differences between the medium and high groups. As shown in Figure 2, the neutralizing activity of CP exceeded the minimum FDA titer (>1:80) in 100% of randomly selected samples, based on a conservative approach that excluded non‐specific binding. There was not a trend toward a dose response with regard to neutralizing activity in medium versus high anti‐NP CP as it related to the “optimal” titer status initially stated by the FDA, with 58% (7 of 12) of NT50 values exceeding 1:320 for high anti‐NP units, and 55% (6 of 11) of NT50 values exceeding 1:320 for medium anti‐NP units.
FIGURE 1.
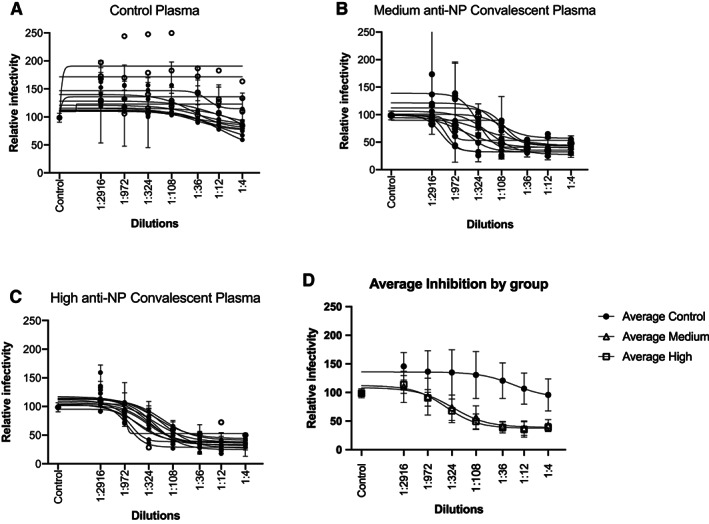
Inhibition of SARS‐CoV‐2 spike protein by control and convalescent plasma. Infectivity curves for SARS‐CoV‐2 pseudovirus incubated with control or convalescent plasma serially diluted between 1:4 and 1:2916. “Control” on the x‐axis represents the average luciferase activity with control plasmas at 1:4 dilution. (A) Depicts results from control plasma, 1(B) from the group of plasma possessing between 149 and 299 OD anti‐NP IgG (medium anti‐NP), and (C) from the group harboring >300 OD anti‐NP IgG (high anti‐NP). In (A)‐(C), plasma samples where an NT50 could be determined are marked with a filled in circle versus an open circle for samples for which an NT50 value could not be calculated. Aggregate curves (mean, SD) for these 3 groups are shown in (D)
FIGURE 2.
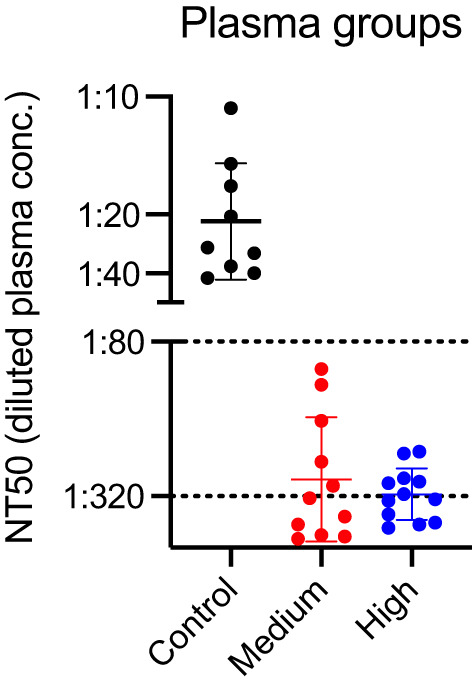
Neutralizing capacity (NT50) for control and convalescent plasma. NT50 for control and convalescent plasma (medium, high anti‐NP) of CP. Each dot represents the calculated NT50 value for an individual plasma sample. The dashed lines at 1:80 correspond with the minimum titer recommended by the FDA for CP, and with the “optimally greater than 1:320” titer stated by FDA in the initial (March 24, 2020) guidance document [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Using a SARS‐CoV‐2 pseudovirus to test for neutralizing activity against the SARS‐CoV‐2 spike protein we observed a clear antibody‐dependent reduction in viral infection of cells using CP from our double‐blind RCT. As expected, none of the control plasma units met FDA's minimum standard for CP, while all CP units met the FDA's minimum standard for neutralizing capacity for COVID‐19 CP.
Early in the COVID‐19 pandemic, FDA issued several guidance documents (March 24, 2020, April 8, 2020, April 13, 2020, May 1, 2020) for the selection of CP donors, including testing of donors and/or CP units for antibodies to SARS‐CoV‐2. Several aspects of these guidelines warrant discussion as they relate to the results of our study.
First, when we initiated our trial, no specific protocol for antibody testing was provided by FDA, and the minimum recommended titers were 1:80. Second, and more importantly, no clear protocol was provided for how these “titers” should be determined. For example, titers can be determined using a plaque reduction assay with authentic pathogenic SARS‐CoV‐2 (gold standard), however, this requires a BSL‐3 laboratory and is not feasible in most centers. On the other end of the spectrum, titers can be determined from in vitro binding studies testing whether a given dilution of plasma has “binding” activity to isolated spike protein. This method is less challenging but may have limited generalizability to inhibition of viral activity in vivo. We chose a third approach involving use of a pseudovirus, which is an established method for studying functional neutralizing activity in a safe manner. 12 , 13 The assay we used has been shown to correlate very well with neutralization results derived from authentic SARS‐CoV‐2 virus neutralization assays. 6 Furthermore, SARS‐CoV‐2 pseudovirus has been shown to be similarly sensitive to neutralization by antibodies in convalescent plasma as SARS‐CoV‐2. 6
Using a conservative analysis method of subtracting the effects of control plasma, for example, non‐specific binding, all of the CP met the FDA's minimum 1:80 titer for neutralizing antibody threshold. Our primary objective was to compare neutralizing activity in CP versus control plasma. We did not observe a dose response with regard to neutralizing activity in medium versus high anti‐NP CP, with 58% (7 of 12) of NT50 values exceeding 1:320 for high anti‐NP units, and 55% (6 of 11) of NT50 values exceeding 1:320 for medium anti‐NP units.
There are several possible reasons that we did not observe a statistically significant dose response for medium versus high NP samples on neutralizing activity. First, sample sizes were relatively small. It is interesting that there was more variability, including some lower neutralization titer values, in the medium NP samples compared with the high NP (Figure 2). Second, our study was not designed to rigorously assess “dose finding”, which would have required many more samples, and also more “dosing” groups. In contrast, this analysis focused on the following question: “Did selection of donors using an NP based test with two pre‐specified reflectance light unit levels (145–299, ≥300) result in collection of plasma with neutralizing activity to SARS‐CoV‐2 meeting FDA's minimum 1:80 titer?” We found that it did in all randomly selected samples.
We selected plasma donors using an NP‐based test for several reasons. First, early in the pandemic (March 2020) there were few testing options available for us to screen plasma donors. The test we had available in our area measured antibody to NP, and early reports suggested it was a good surrogate for a humoral response to COVID‐19 infection. It was only later, after we initiated our randomized trial, that other assays, for example, some spike‐based and other NP‐based assays, for example, Abbott Architect assay, became available. At this time, FDA Guidelines did not require antibody testing and instead used a successful recovery from COVID‐19 infection as a surrogate for the likely presence of neutralizing antibodies in collected plasma. Second, antibodies to both NP and spike are correlated, that is, are observed in the same patients across different series. 14 , 15 , 16 For example, To et al. reported that “Anti‐SARS‐CoV‐2‐NP or anti‐SARS‐CoV‐2‐RBD IgG levels correlated with virus neutralisation titre (R2>0·9)”. 16
It is important to note that neutralizing antibodies are not spike‐specific per se; they are receptor binding site‐specific, therefore, most antibodies to the spike protein would not be expected to functionally block the receptor‐binding domain. We did not find a significant correlation between levels of antibodies to spike protein and NT50 values within the medium and high groups, but given our small sample size we cannot rule out this possible association.
Limitations of this study include the relatively small numbers of plasma samples tested (25 total CP units and 14 control plasma units). The assay we used, however, is highly labor intensive so it was not practical to test all of our units. That being said, observed differences in neutralizing activity between control and CP were large enough to be statistically significantly different (p < .001), despite the small sample size. As described above, institutions are using different assay methods/platforms, therefore, we cannot strictly compare our results with other investigators. In addition, this was a single‐center experience, however, it is unlikely that the humoral immune response to SARS‐CoV‐2 infection varies dramatically from one geographic area to another. Finally, we are not surprised that an NT50 could not be calculated for 7 units. Calculation of the dilution at which there is a 50% reduction in neutralizing activity, requires a clear “dose‐dependent response” curve of decreasing neutralizing activity with upper and lower plateaus to define the curve. Nevertheless, we observed that these 7 units exhibited neutralizing capacity consistent with their respective groups (Figure 1).
Our study has several strengths. We randomly selected plasma units, thereby eliminating bias related to selection of test samples. In addition, this testing was performed by study team members who were blinded to plasma unit type, that is, use of masked samples prepared by an unblinded individual. Furthermore, samples were run in duplicate in two separate sets further reducing the impact of researcher technique or potential bias on results. Another strength of this study was the use of control plasma to ensure that results were specific to SARS‐CoV‐2 infection and not confounded by a non‐specific effect of the plasma in the assay. There is very little cross‐reactivity between antibodies to other common respiratory viruses and to SARS‐CoV‐2. 17 , 18 For example, it has been shown that even pre‐pandemic sera from individuals with recent seasonal coronavirus infection does not show SARS‐CoV‐2 neutralizing activity. 17 Samples collected from subjects who are positive for seasonal coronaviruses would be expected to have a higher chance of containing interfering antibodies, that is, to be cross‐reacting than samples from IAV‐, IBV‐, RSV‐, or HIV‐positive patients since seasonal coronaviruses are closely related to SARS‐CoV‐2 and might therefore be expected to elicit more similar adaptive immune responses.
In conclusion, an immunochromatographic test for IgG antibody to NP correlated with neutralizing efficacy of a SARS‐CoV‐2 pseudovirus in all randomly selected COVID‐19 CP units with every sample tested meeting minimum FDA criteria for use as a potential therapeutic agent.
CONFLICT OF INTEREST
No authors disclosed any conflicts of interest.
Supporting information
Appendix S1. Supplementary material.
ACKNOWLEDGMENTS
Paul Bieniasz, PhD (Professor and Investigator, Howard Hughes Medical Institute, Laboratory of Retrovirology) and Theodora Hatziioannou, PhD (Research Associate Professor, Laboratory of Retrovirology) at The Rockefeller University, NY, NY kindly provided us with the protocols, cell lines, and reagents. Luisa Escobar‐Hoyos PhD provided technical and scientific overview for the deployment of the SARS Cov‐2 neutralizing assay.
Freedenberg AT, Pan C‐H, Diehl WE, et al. Neutralizing activity to SARS‐CoV‐2 of convalescent and control plasma used in a randomized controlled trial. Transfusion. 2021;61:1363–1369. 10.1111/trf.16283
Trial Registration
ClinicalTrials.gov Identifier NCT04344535.
REFERENCES
- 1. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systematic review and exploratory meta‐analysis. J Infect Dis. 2015;211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid‐19 in adults in India: Open label phase II multicentre randomised controlled trial (PLACID trial). BMJ. 2020;371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: A randomized clinical trial. JAMA. 2020;324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rojas M, Rodríguez Y, Monsalve DM, Acosta‐Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid‐19: Possible mechanisms of action. Autoimmun Rev. 2020;19:102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robbiani DF, Gaebler C, Muecksch F, Lorenzi JC, Wang Z, Cho A, et al. Convergent antibody responses to SARS‐CoV‐2 in convalescent individuals. Nature. 2020;584:437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt F, Weisblum Y, Muecksch F, Hoffmann HH, Michailidis E, Lorenzi JC, et al. Measuring SARS‐CoV‐2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crawford KHD, Eguia R, Dingens AS, Loes AN, Malone KD, Wolf CR, et al. Protocol and reagents for pseudotyping lentiviral particles with SARS‐CoV‐2 spike protein for neutralization assays. Viruses. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. [DOI] [PubMed] [Google Scholar]
- 9. Yurkovetskiy L, Wang X, Pascal KE, Tomkins‐Tinch C, Nyalile TP, Wang Y, et al. Structural and functional analysis of the D614G SARS‐CoV‐2 spike protein variant. Cell. 2020;183:739–751.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muruato AE, Fontes‐Garfias CR, Ren P, Garcia‐Blanco MA, Menachery VD, Xie X, et al. A high‐throughput neutralizing antibody assay for COVID‐19 diagnosis and vaccine evaluation. Nat Commun. 2020;11:4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie X, Muruato AE, Zhang X, Lokugamage KG, Fontes‐Garfias CR, Zou J, et al. A nanoluciferase SARS‐CoV‐2 for rapid neutralization testing and screening of anti‐infective drugs for COVID‐19. Nat Commun. 2020;11:5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Q, Liu Q, Huang W, Li X, Wang Y. Current status on the development of pseudoviruses for enveloped viruses. Rev Med Virol. 2018;28:e1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu J, Gao Q, He C, Huang A, Tang N, Wang K. Development of cell‐based pseudovirus entry assay to identify potential viral entry inhibitors and neutralizing antibodies against SARS‐CoV‐2. Genes Dis. 2020;7:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS‐CoV‐2 in the Icelandic population. N Engl J Med. 2020;382:2302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lumley SF, O'Donnell D, Stoesser E, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS‐CoV‐2 infection in health care workers. N Engl J Med. 2020;384(6):533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: An observational cohort study. Lancet Infect Dis. 2020;20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poston D, Weisblum Y, Wise H, Templeton K, Jenks S, Hatziioannou T, et al. Absence of SARS‐CoV‐2 neutralizing activity in pre‐pandemic sera from individuals with recent seasonal coronavirus infection. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reche PA. Potential cross‐reactive immunity to SARS‐CoV‐2 from common human pathogens and vaccines. Front Immunol. 2020;11:586984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary material.


