Abstract
Background
The present study aims to verify the association between diabetes and thiamine deficiency in critically ill patients infected by severe acute respiratory syndrome coronavirus 2.
Methods
This is a descriptive cross‐sectional study, whose demographic, anthropometric, and laboratory data (arterial lactate, bicarbonate, and plasma thiamine) were obtained in the first hours of admission to the intensive care unit. Patients with diabetes were compared with individuals without diabetes, and the correlation was performed between thiamine and lactate levels. Thiamine levels <28 μg/L were considered as thiamine deficiency.
Results
Overall, 270 patients met the inclusion criteria; 51.1% were men, and the median age was 74 years (66.8‐81). The median value of thiamine was 54.0 μg/L (38‐72.3), and 15.6% had thiamine deficiency. Among patients with diabetes, 26.3% had thiamine deficiency, and 69.3% had hyperlactatemia. There was an association between thiamine deficiency and diabetes (odds ratio 4.28; 95% CI, 2.08‐8.81; P < .001). There was a strong negative correlation between thiamine and arterial lactate in patients with diabetes (r = −0.711, P < .001) and a moderate negative correlation in critically ill patients without diabetes (r = −0.489, P < .001).
Conclusions
The prevalence of thiamine deficiency in critically ill patients due to coronavirus disease 2019 is higher in patients with diabetes. There is a negative correlation between thiamine and arterial lactate levels, which is higher in people with diabetes.
Keywords: COVID‐19, diabetes, hyperlactatemia, intensive care, SARS‐CoV‐2, thiamine
Highlights
Thiamine is important for cellular metabolism and energy homeostasis. There are no data yet in the literature that have reported thiamine deficiency in coronavirus disease 2019.
Thiamine insufficiency may exist in critically ill patients not only due to reduced intake and very impaired absorptive processes during the critical stage but also due to the high consumption by hypermetabolism. Severe thiamine deficiency may cause lactic acidosis, hyperlactatemia, and even worsen diabetic ketoacidosis.
There is a negative correlation between thiamine levels and arterial lactate, especially among critically ill patients with diabetes.
摘要
背景
本研究旨在验证严重急性呼吸综合征冠状病毒2型感染的危重患者中, 糖尿病与硫胺素缺乏之间的关系。
方法
这是一项描述性横断面研究, 其人口学、测量学和实验室数据(动脉乳酸、碳酸氢盐和血浆硫胺素)在入住重症监护病房的第一个小时内获得。将糖尿病患者与非糖尿病患者进行比较, 并对硫胺素和乳酸水平进行相关性分析。硫胺素水平<28μg/L被定义为硫胺素缺乏症。
结果
总体上, 270名患者符合纳入标准, 51.1%为男性, 中位年龄为74岁(66.8‐81岁)。硫胺素中位数为54.0μg/L(38~72.3), 其中15.6%为硫胺素缺乏症。糖尿病患者中, 26.3%有硫胺素缺乏, 69.3%有高乳酸血症。硫胺素缺乏与糖尿病相关(优势比4.28, 95%可信区间2.08‐8.81, P<0.001)。糖尿病患者硫胺素与动脉血乳酸呈强负相关(r=−0.711, P<0.001), 无糖尿病危重患者硫胺素与动脉乳酸呈中度负相关(r=−0.489, P<0.001)。
结论
2019年冠状病毒病所致危重患者中, 糖尿病患者硫胺素缺乏症的患病率更高。硫胺素与动脉乳酸水平呈负相关, 糖尿病患者的血乳酸水平更高。
Keywords: 新型冠状病毒肺炎, 糖尿病, 高乳酸血症, 重症监护, SARS‐CoV‐2, 硫胺素
1. INTRODUCTION
The pandemic due to the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which causes coronavirus disease 2019 (COVID‐19), was declared a public health emergency of international importance by the World Health Organization (WHO) in 2020 because so far more than 9 million people have been affected worldwide. 1 Its clinical presentation is very diverse, ranging from asymptomatic patients to cases of viral pneumonia and severe acute respiratory distress syndrome. Some patients are considered to be at higher risk for developing serious infections, especially the elderly and those who have some chronic diseases such as hypertension, diabetes, cancer, and respiratory diseases. In these cases, COVID‐19 may progress in an unfavorable way with worsening of organic dysfunctions leading to respiratory failure, renal failure, shock, and death. 2
In the most severe cases of viral infection, there is an excessive release of pro‐inflammatory cytokines, activation of pro‐coagulation factors, and increased oxidative stress, which reflects changes in metabolic parameters such as hyperglycemia, increased energy expenditure, and loss of muscle proteins, in addition to the increased tissue demand for micronutrients. 3 , 4 Among them, thiamine, which is important for cellular metabolism and energy homeostasis, stands out. 5 Despite the lack of scientific evidence regarding serum micronutrient levels in critical situations, however, there is a consensus that thiamine insufficiency may exist not only due to reduced intake and very impaired absorptive processes during the critical stage but also due to the high consumption by hypermetabolism. 6 , 7
Thiamine, or vitamin B1, is a water‐soluble vitamin that performs primary functions in glucose and lactate metabolism pathways. It acts as a cofactor for the enzyme pyruvate‐dehydrogenase and facilitates the entry of pyruvate in the mitochondria, as well as its final product acetyl‐coenzyme A in the citric acid cycle. In thiamine insufficiency, the Krebs cycle is blocked, and pyruvate is diverted to anaerobic metabolism, which is an important mechanism of hyperlactatemia unrelated to tissue hypoxemia (type B lactic acidosis). 8 , 9 It was found that about 20% of critically ill patients have thiamine deficiency because of the low nutritional supply, which is insufficient to support the high metabolic demand, or because it was spoliated by diuretics and dialysis. 7 Clinically, it can lead to wet beriberi (whose main characteristic is severe cardiac dysfunction), dry beriberi (peripheral neuropathy manifestations), and delirium or mental confusion termed Wernicke's encephalopathy and Korsakoff's psychosis. 5 , 7
Thiamine deficiency is a well‐known situation among people with diabetes since the lack of insulin reduces the absorption of thiamine from the diet and reduces the reabsorption of thiamine by the proximal renal tubule. 10 Especially among individuals who have microalbuminuria, the low plasma levels of thiamine are explained by the increase in renal thiamine clearance. 11 In this case, a severe thiamine deficiency may cause lactic acidosis, hyperlactatemia, and even worsen diabetic ketoacidosis. 12
Therefore, the present study aims to assess the association between thiamine deficiency and hyperlactatemia in critically ill patients with diabetes infected by SARS‐CoV‐2.
2. MATERIAL AND METHODS
2.1. Study design and population
This is a descriptive cross‐sectional, single‐center study whose data were collected in the first 3 days of admission to the intensive care unit (ICU) due to COVID‐19 at Sancta Maggiore Hospital (Prevent Senior Private Health Operator, São Paulo, Brazil) between March and April 2020. The health care protocols for COVID‐19 started in the emergency department, and they were continued in the ICU. Patients were consecutively included in the study as they were admitted to the ICU and met the following inclusion criteria: (a) adults over 18 years old; (b) arterial blood gases, free plasma thiamine, and arterial lactate levels obtained in the first 3 days of admission to the ICU; (c) positive swab from the nasal cavity and oropharynx for detection of viral RNA for COVID‐19 using the reverse‐transcription polymerase chain reaction (RT‐PCR); (d) respiratory failure using mechanical ventilation; (e) and computed tomography scan of the chest showing bilateral pulmonary interstitial infiltrate with a typical “ground‐glass” pattern. The exclusion criteria were severe liver failure, chronic kidney failure on dialysis, and use of metformin in the last 3 days before hospital admission. Patients with a history of alcohol abuse were also excluded.
Participants were allocated to the group with diabetes or without diabetes according to the presence of a previous diagnosis of type 2 diabetes mellitus, defined by fasting glucose >126 mg/dL and/or glycosylated hemoglobin >6.5% and/or chronic use of medication for glycemic control. 13 All information was confirmed in the medical history and duly registered in the patient's medical record.
2.2. Data collection and procedures
Data were collected from electronic medical records during clinical patient care and according to institutional protocols for the treatment of critically ill patients with COVID‐19. The serum values of free plasma thiamine were obtained from whole blood within the first 72 hours of admission to the ICU. Arterial blood gases and serum lactate levels were collected in a paired way, and the first available value was considered after the procedure of orotracheal intubation and mechanical ventilation, as long as it occurred within 72 hours of admission to the ICU. The serum bicarbonate value was extracted from the same arterial blood gas. Other data were obtained from medical records: sex, age, weight, height, Simplified Acute Physiology Score III (SAPS III), 14 C‐reactive protein (CRP), presence or absence of type 2 diabetes mellitus diagnosed for at least 6 months, and other comorbidities.
The most current weight and height, as well as other data of interest that were not available in the medical record, were obtained from a survey with a family member or caregiver who lived with the patient. From these data, the body mass index (BMI) was calculated by dividing weight (in kilograms) by height (in meters) squared. The nutrition status classification for obesity was a BMI ≥30 kg/m2 according to the WHO (2004). 15
The procedures for dosing thiamine were performed using whole blood 16 in a tube filled with the anticoagulant EDTA and protected from light, and it was immediately frozen at −20°C and sent to the laboratory of the institution whose method of reading was liquid chromatography. This assay measures the concentration of thiamine diphosphate (TDP), the primary active form of vitamin B1, and deficiency was considered as TDP lower than 28 μg/L (or <78 nmol/L) determined according to laboratory standard values. Arterial blood gas was obtained from the collection of heparinized arterial whole blood, whose dosages of lactate and bicarbonate were obtained using the blood gas analyzer Rapidpoint 500 (Siemens Healthcare Diagnostics Inc, Tarrytown, NY, USA). The cutoff point for hyperlactatemia was above 2.0 mmol/L and for low bicarbonate levels below 20 mmol/L. 17
Ethical procedures: All patients admitted to the ICU with SARS‐CoV‐2 were vulnerable. Therefore, at the time of hospital admission, the responsible family members were invited to make the medical record data available for research and sign a consent form that protected the confidentiality of the data, which had previously been approved by the hospital company. Then, in order to allow access to the medical records, the research project was submitted to the local medical ethics committee (approval record number: CAAE 30608020.9.0000.8114), and the data were collected only after approval. All procedures were performed in accordance with the Declaration of Helsinki.
2.3. Outcomes
The main objective was to determine the association between diabetes and thiamine deficiency in critically ill patients infected by SARS‐CoV‐2. Secondary outcomes were to establish the correlation between the levels of thiamine, lactate, and bicarbonate in critically ill patients with diabetes.
2.4. Statistical analysis
First, for descriptive analysis, the variables were tested for normality using the Shapiro‐Wilk test (P > .05 for normality). The variables with nonparametric data were described as median and interquartile range (IQR) and categorical data expressed as a percentage of proportion. The comparison of percentage distribution of categorical variables was performed using Pearson's chi‐square test. The Mann‐Whitney U test was used for the comparison of continuous data that were not normally distributed.
Correlations between groups in quantitative variables were studied using Pearson's coefficient after logarithmic transformation to normalize nonparametric variables. Statistical significance was set at P < .05 and a 95% CI. Observational data were statistically analyzed using SPSS 24.0 software (version 24.0; SPSS Inc, Chicago, Illinois).
3. RESULTS
Of all selected patients, 270 critically ill patients met the inclusion criteria whose baseline characteristics are shown in Table 1. The data show that 51.1% were men, the median age was 74 years (IQR 66.8‐81), median weight 79 kg (IQR 70‐90), and median BMI 30.1 kg/m2 (IQR 24.8‐32.2). Overall, 51.9% of critically ill patients were classified as obese (BMI ≥30 kg/m2). The severity score of SAPS III on admission to the ICU was 69 (55‐87), which corresponds to a predicted mortality risk in the ICU of 68.9%. Of all patients in the sample, 15.6% had absolute thiamine deficiency with levels below 28 μg/L.
TABLE 1.
Baseline characteristics of critically ill patients infected by SARS‐CoV‐2 who required mechanical ventilation
| Variable | Patients(N = 270) |
|---|---|
| Age (y) | 74 (66.8‐81) |
| Sex (%) | |
| Female | 48.9 |
| Male | 51.1 |
| Weight (kg) | 79 (70‐90) |
| BMI (kg/m2) | 30.1 (24.9‐32.2) |
| Obesity (%) (BMI ≥30 kg/m2) | 51.9 |
| PaO2/FiO2 ratio (mm Hg) | 114.5 (92‐150) |
| SAPS III | 69 (55‐87) |
| CRP (mg/L) | |
| First day | 130.9 (68.1‐210.4) |
| Third day | 184.1 (110.3‐262.2) |
| Thiamine (μg/L) | 54 (38‐72.3) |
| Lactate (mmol/L) | 2.1 (1.4‐2.5) |
| Bicarbonate (mmol/L) | 22.5 (19.8‐25.1) |
| Comorbidities (%) | |
| Hypertension | 74.4 |
| Diabetes | 42.4 |
| COPD | 25.6 |
| CKD | 13.0 |
Note: Continuous values are expressed as median and interquartile range (IQR) and categorical data as percentage.
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; FiO2, fractional inspired oxygen; PaO2, arterial oxygen tension; SAPS III, Simplified Acute Physiology Score III; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Of the total participants, 114 had diabetes (42.4%), and of these, 30 (26.3%) had absolute thiamine deficiency and 79 (69.3%) had hyperlactatemia. Figure 1 shows the boxplots of plasma thiamine values between groups with diabetes and without diabetes.
FIGURE 1.
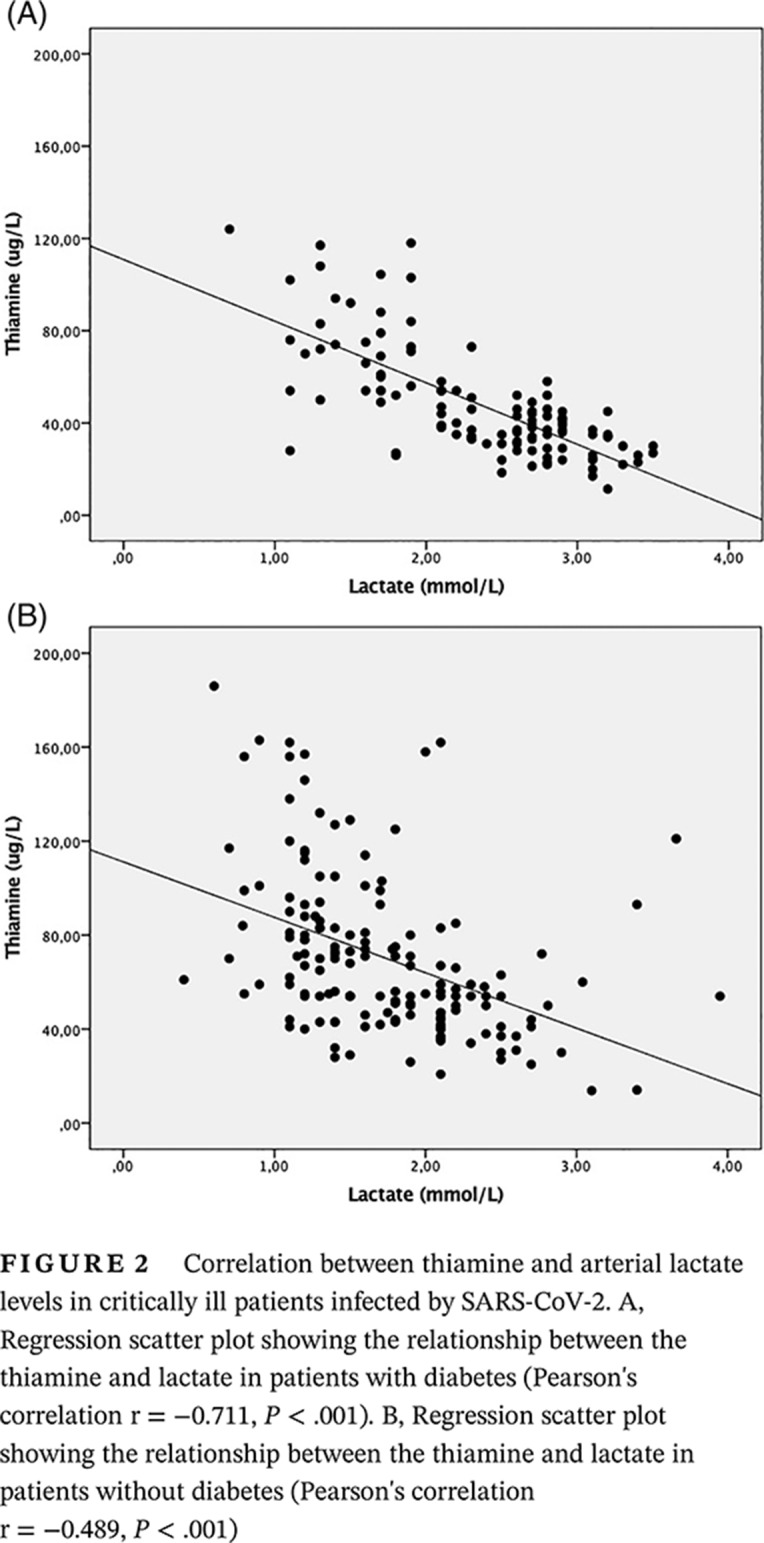
Boxplots comparing thiamine levels between critically ill patients with and without diabetes infected by SARS‐CoV‐2
According to the comparison between both groups, as shown in Table 2, there was an association between thiamine deficiency and diabetes (odds ratio [OR] 4.28; 95% CI, 2.08‐8.81; P < .001). The group without diabetes had a higher value of SAPS III and CRP on the first and third day of ICU stay.
TABLE 2.
Comparison between critically ill patients without and with diabetes infected by SARS‐CoV‐2
| Variable | Patients without diabetes (n = 156) | Patients with diabetes (n = 114) | P value |
|---|---|---|---|
| Age (y) | 74 (66‐81) | 74 (67‐81) | .695 |
| Sex (%) | |||
| F/M | 46.8/53.2 | 51.8/48.2 | .421 |
| Obesity (%) (BMI ≥30 kg/m2) | 55.1 | 47.4 | .208 |
| PaO2/FiO2 ratio (mm Hg) | 116 (93‐150) | 111(92‐154) | .922 |
| SAPS III | 75 (57‐88) | 67 (51‐82.3) | .020 |
| CRP (mg/L) | |||
| First day | 149.4 (82.6‐216.3) | 108.4 (52.9‐186.3) | .008 |
| Third day | 196.2 (128.4‐268‐9) | 159.8 (91.3‐248.4) | .033 |
| Thiamine (μg/L) | 60 (46‐84) | 41 (31‐56) | <.001 |
| Lactate (mmol/L) | 1.6 (1.2‐2.1) | 2.4 (2.1‐2.8) | <.001 |
| Bicarbonate (mmol/L) | 23.4 (20.7‐25.5) | 21.2 (19.1‐23.4) | <.001 |
| Thiamine deficiency (%) | 7.7 | 26.3 | <.001 |
Note: Continuous values are expressed as median and interquartile range (IQR) and categorical data as percentage.
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; F, female; FiO2, fractional inspired oxygen; M, male; PaO2, arterial oxygen tension; SAPS III, Simplified Acute Physiology Score III; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
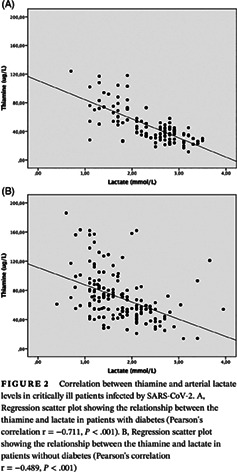
Among critically ill patients with diabetes, there was a strong negative correlation between thiamine and arterial lactate (r = −0.711, P < .001) and a moderate negative correlation for individuals without diabetes (r = −0.489, P < .001), as shown in Figure 2A and B. A weak negative correlation between the lactate and bicarbonate variables in the group with diabetes (r = −0.191, P = .04) and without diabetes was found (r = −0.338, P < .001). There was no significant correlation between plasma thiamine and bicarbonate between both groups.
FIGURE 2.

Correlation between thiamine and arterial lactate levels in critically ill patients infected by SARS‐CoV‐2. A, Regression scatter plot showing the relationship between the thiamine and lactate in patients with diabetes (Pearson's correlation r = −0.711, P < .001). B, Regression scatter plot showing the relationship between the thiamine and lactate in patients without diabetes (Pearson's correlation r = −0.489, P < .001)
4. DISCUSSION
In this study, we analyzed the presence of absolute thiamine deficiency, or TDP, in groups of critically ill patients with and without diabetes who were admitted to the ICU due to SARS‐CoV‐2. There are no data yet in the literature that have reported the prevalence of thiamine deficiency in patients with COVID‐19; however, the literature has already revealed analyses of thiamine deficiency in critically ill patients, 9 , 18 which ranges from 10% to 35% in septic patients. Similarly, our prevalence of thiamine deficiency in the whole sample was 15.6%, which is in line with the prevalence described for the critical patient population.
At the same time, data that have already been published on serum thiamine status in patients with diabetes revealed that there was a higher prevalence of thiamine deficiency in patients with microalbuminuria, which can vary from 20% to 90%. 10 , 11 , 19 In our study, we found that the prevalence of thiamine deficiency in critically ill patients with diabetes was 26.3% and only 7.7% in the group without diabetes, with an OR of 4.28 (95% CI, 2.08‐8.81; P < .001), which explains the hypothesis that diabetes increases the risk of deficiency of this micronutrient. We would like to emphasize that, as our prevalence of thiamine deficiency in critically ill patients was compatible with the literature, we can hypothesize that SARS‐CoV‐2 did not cause a change in the thiamine metabolism pathways.
In this study, we measured thiamine levels of whole blood, and not the transketolase activity, which is an indirect measure of TDP. Indeed, approximately 90% of vitamin B1 present in whole blood is TDP. Thiamine and thiamine monophosphate, which make up the remaining 10%, are not measured. 8 Whole blood is the preferred specimen for thiamine assessment because approximately 80% of thiamine present in whole blood is within the red blood cells. In addition, the direct measurement of the plasma thiamine level has a better correlation with transketolase activation 8 and erythrocyte thiamine phosphate measurement. 16 The active isoform of thiamine (TDP) has better clinical practicality, and it acts as the coenzyme for phosphorylation of carbohydrates. According to Lu et al, 16 plasma thiamine levels below 78 nmol/L have a stronger correlation with reduced activity of TDP.
We found a negative correlation between thiamine levels and lactate for both groups of patients, but it was stronger in the group with diabetes. A study conducted by Heming et al 20 demonstrated no correlation between thiamine and lactate, but in this study only 28 patients were included. In addition, the sample had only sepsis from any cause and did not include diabetes. However, Moskowitz et al 21 found a negative correlation between thiamine and lactate in a group of patients with diabetic ketoacidosis. Our study found the same strong negative correlation in patients with diabetes, and surprisingly they had less inflammatory activity and a lower severity profile on admission to the ICU when compared to the group without diabetes (see CRP and SAPS III values in Table 2), and even then lactate levels were higher. This raises the hypothesis that the presence of diabetes has a greater influence on thiamine levels than the critical disease.
Our study does not establish a causal relationship between the levels of thiamine and hyperlactatemia not only because of the cross‐sectional design but also because thiamine is involved in other metabolic pathways that can perpetuate tissue hypoperfusion and consequently increase lactate. 7 Thiamine, when insufficient in the body, reduces the energy available to the myocardial fiber and may decrease cardiac efficiency during critical illness, whose metabolic demand is much higher than in the normal state. 9 , 22 Cardiac dysfunction is a common event and an important cause of mortality in patients infected by SARS‐CoV‐2, 4 which explains the idea of measuring plasma thiamine in infected patients. Diabetes is known to be a comorbidity that greatly worsens the clinical outcomes of patients with COVID‐19, whose cardiac and kidney dysfunction is one of the main organic disorders in the context of critical illness 18 , 23 after respiratory failure. 24
Our study has some limitations, such as the measurement of only plasma thiamine, which may not reflect the tissue concentration of TDP, and also the lack of control of the dose of vasoactive drugs and the presence of previous microalbuminuria. However, our findings may be exploratory in order to understand the dynamic behavior between thiamine and lactate in COVID‐19, in addition to serving as a basis for the development of further studies with a prospective and interventionist design.
5. CONCLUSIONS
The prevalence of thiamine deficiency in critically ill patients due to COVID‐19 is higher in people with diabetes. There is a negative correlation between thiamine and arterial lactate levels in the population of critically ill patients with COVID‐19, which is stronger in patients with diabetes. However, further studies are needed to explore the significance of thiamine deficiency in COVID‐19 and diabetes, especially if thiamine deficiency is related to poor clinical outcomes.
DISCLOSURE
None of the authors of this manuscript has any financial or personal relationship with other people or organizations that could inappropriately influence this work. There is no conflict of interest regarding sponsorship and financing.
ACKNOWLEDGEMENTS
The authors wish to thank the volunteers for their participation. No funding received.
Gonçalves SEAB, Gonçalves TJM, Guarnieri A, Risegato RC, Guimarães MP, de Freitas DC. Association between thiamine deficiency and hyperlactatemia among critically ill patients with diabetes infected by SARS‐CoV‐2 . Journal of Diabetes. 2021;13:413–419. 10.1111/1753-0407.13156
REFERENCES
- 1. OMS declara emergência de saúde pública internacional para novo coronavírus — português (Brasil) . WHO declares international public health emergency for new coronavirus ‐ portuguese (Brazil). n.d. https://www.gov.br/pt‐br/noticias/saude‐e‐vigilancia‐sanitaria/2020/01/oms‐declara‐emergencia‐de‐saude‐publica‐internacional‐para‐novo‐coronavirus. (Accessed 13 August 2020).
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;6736:1‐9. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marini JJ, Gattinoni L. Management of COVID‐19 respiratory distress. JAMA. 2020;323:2329‐2330. 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 4. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID‐19): challenges and recommendations. Lancet Respir Med. 2020;8:506‐517. 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerns JC, Arundel C, Chawla LS. Thiamin deficiency in people with obesity. Adv Nutr. 2015;6:147‐153. 10.3945/an.114.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corcoran TB, Neill MPO, Webb SAR, Ho KM. Inflammation, vitamin deficiencies and organ failure in critically ill patients. Anaesth Intensive Care. 2009;37:740‐747. [DOI] [PubMed] [Google Scholar]
- 7. Collie JTB, Greaves RF, Jones OAH, Lam Q, Eastwood GM. Vitamin B1 in critically ill patients: needs and challenges. Clin Chem Lab Med. 2017;55:1652‐1668. 10.1515/cclm-2017-0054. [DOI] [PubMed] [Google Scholar]
- 8. Talwar D, Davidson H, Cooney J, St. JO'Reilly D. Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: comparison with erythrocyte transketolase activation assay. Clin Chem. 2000;46:704‐710. [PubMed] [Google Scholar]
- 9. Woolum JA, Abner EL, Kelly A, Bastin MLT, Morris PE, Flannery AH. Effect of thiamine administration on lactate clearance and mortality in patients with septic shock. Crit Care Med. 2018;46:1747‐1752. 10.1097/CCM.0000000000003311. [DOI] [PubMed] [Google Scholar]
- 10. Nix WA, Zirwes R, Bangert V, et al. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res Clin Pract. 2015;107:157‐165. 10.1016/j.diabres.2014.09.058. [DOI] [PubMed] [Google Scholar]
- 11. Valdés‐ramos R, Laura GA, Elina MB, Donají BA. Vitamins and type 2 diabetes mellitus. Endocr Metab Immune Disord‐Drug Targets. 2015;15:54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thornalley P. The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev. 2005;1:287‐298. 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes 2019. Diabetes Care. 2019;42:S13‐S28. 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 14. Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345‐1355. 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO/Europe | Nutrition ‐ Body mass index – BMI. n.d. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. (Accessed 15 August 2020).
- 16. Lu J, Frank EL. Rapid HPLC measurement of thiamine and its phosphate esters in whole blood. Clin Chem. 2008;54:901‐906. 10.1373/clinchem.2007.099077. [DOI] [PubMed] [Google Scholar]
- 17. Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA. 2016;315:801‐810. 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manzanares W, Hardy G. Thiamine supplementation in the critically ill. Curr Opin Clin Nutr Metab Care. 2011;14:610‐617. 10.1097/MCO.0b013e32834b8911. [DOI] [PubMed] [Google Scholar]
- 19. Waheed P, Naveed AK, Ahmed T. Thiamine deficiency and its correlation with dyslipidemia in diabetics with microalbuminuria. J Pak Med Assoc. 2013;63:340‐346. [PubMed] [Google Scholar]
- 20. Heming N, Salah A, Meng P, et al. Thiamine status and lactate concentration in sepsis: a prospective observational study. Medicine. 2020;99:1‐6. 10.1097/MD.0000000000018894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moskowitz A, Graver A, Giberson T, et al. The relationship between lactate and thiamine levels in patients with diabetic ketoacidosis. J Crit Care. 2014;29:182.e5‐182.e8. 10.1016/j.jcrc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eshak ES, Arafa AE. Thiamine deficiency and cardiovascular disorders. Nutr Metab Cardiovasc Dis. 2018;28:965‐972. 10.1016/j.numecd.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 23. Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID‐19. Intensive Care Med. 2020;46:1339‐1348. 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]


