Abstract
To clarify the association between lifestyle changes as a result of coronavirus disease 2019 containment measures and changes in metabolic and glycemic status in patients with diabetes, a cross‐sectional, single‐center, observation study was carried out. A self‐reported questionnaire was provided to ascertain the frequency of various lifestyle activities before and after the coronavirus disease 2019 containment measures in Japan. Among 463 patients, change in glycated hemoglobin was significantly associated with change in bodyweight. After stratification by age 65 years, binary logistic regression analysis showed that increased frequency of snack eating increased bodyweight (odds ratio 1.709, P = 0.007) and glycated hemoglobin (odds ratio 1.420, P = 0.025) in the younger group, whereas in the older patients, reduced walking activities resulted in weight gain (odds ratio 0.726, P = 0.010). In conclusion, changes in eating behavior and physical activity increased bodyweight and reduced glycemic control among diabetes patients, but by different processes depending on age under the coronavirus disease 2019 containment measures in Japan.
Keywords: COVID‐19, Eating behavior, Physical activity
We investigated lifestyle changes during the coronavirus disease 2019 containment measures, and its relationship with glycemic control and body weight change.

INTRODUCTION
Coronavirus disease 2019 (COVID‐19), which is caused by severe acute respiratory syndrome coronavirus 2, has become a worldwide pandemic; containment measures or lockdown (stay‐at‐home order) were enacted to prevent further spread of the infection1, 2, 3, 4. The Japanese Government implemented a declaration of a state of emergency (DSE) on 7 April 2020, which was at first adapted to local areas and then implemented nationwide on 16 April 2020, exhorting the Japanese people to refrain from leaving home, and to restrict their use of public transportation and facilities5, and afterward it was extended to 25 May. Although healthy eating and adequate physical activity is essential for patients with diabetes to achieve their glycemic targets, it is reported that containment measures for COVID‐19 negatively affect lifestyle in diabetes patients, who are at increased risk of mortality from COVID‐196, 7, 8, 9, 10. However, it is not known how lifestyle changes as a result of COVID‐19 measures affect metabolic status, such as bodyweight (BW) change and glycemic control, in diabetes patients, especially under the less stringent Japanese DSE. The aim of the present study was to clarify the association between lifestyle alteration during COVID‐19 countermeasures and change in metabolic and glycemic status in diabetes patients in Japan.
MATERIALS AND METHODS
This was a cross‐sectional, single‐center, observational study. Participants were patients with diabetes who visited Kansai Electric Power Hospital between 8 June and 18 June 2020. The study was approved by the Ethical Committees of Kansai Electric Power Medical Research Institute, and carried out in accordance with the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare of Japan. A self‐reported questionnaire was provided to collect information on how often patients carried out various activities before and after the DSE: (i) eating out; (ii) having takeaway meals; (iii) having snacks; (iv) drinking alcohol; (v) going for a walk; (vi) going out routinely, such as commuting and/or shopping; (vii) exercising, such as gym training and playing sports; (viii) failing to take medicines and/or inject insulin for diabetes treatment; (ix) failing to take medicines for diseases other than diabetes; and (x) sleeping time. The patients were asked to mark whether each activity was ‘decreased’, ‘not changed’, ‘increased’ or ‘not applicable (NA)’ during the DSE. The type of diabetes, age, sex and medication use (oral agents, insulin and glucagon‐like peptide‐1 receptor agonist) were also noted. BW and glycated hemoglobin (HbA1c) before and after the DSE were measured. The primary outcome of the present study was to explore lifestyle‐related factors associated with change in BW and HbA1c during the period.
Continuous variables with normal distribution were expressed as the mean ± standard deviation, and those with non‐normal distribution as the median (interquartile range), unless otherwise stated. The difference between variables in diabetes was analyzed using the Mann–Whitney U‐test. Differences between variables before the DSE and after the DSE were analyzed using the Wilcoxson signed‐rank test. Associations between change in HbA1c and change in BW were analyzed using the Kruskal–Wallis test with Bonferroni’s post‐hoc analysis. Binary logistic regression analysis was carried out to explore factors associated with changes in HbA1c and BW. All statistical analyses were carried out using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P < 0.05.
RESULTS
Questionnaires were collected from 463 (men/women: 333/130) diabetes. Clinical characteristics are shown in Table 1. The mean BW and HbA1c before and after DSE were 69.6 ± 15.2 kg and 69.5 ± 15.3 kg (P < 0.01), and 7.7 ± 1.1% and 7.5 ± 1.2% (P < 0.01), respectively. We found that 55.8% of the patients reduced the frequency of eating out, 21.0% increased the frequency of eating snacks, 51.2% reduced the number of steps per day, 44.9% reduced the frequency of going out routinely and 17.4% increased sleeping time (Table 2). HbA1c in patients in the lowest quartile (Q1) of BW change (median −3.63%BW, interquartile range 2.67%BW) was significantly improved compared with that in patients in the highest quartile (Q4; median 1.91%BW, interquartile range 1.82%BW; −0.29 ± 0.78% vs −0.08 ± 0.86%, respectively, P = 0.001; Figure 1). We divided the diabetes patients into two groups at the age of 65 years, as most Japanese people retire from their job and start to receive the public pension at 65 years. Among these participants, 32.2% of those aged <65 years (non‐elderly) and 29.3% of those ≥65 years (elderly) showed an increase in their HbA1c (>0.0%; P = 0.541). In the same manner, 36.7% of non‐elderly and 35.7% of elderly patients showed an increase in their BW (>0.0kg; P = 0.842). Binary logistic regression analysis was carried out to explore factors attributable to change in HbA1c and BW, which showed that the lifestyle changes that affected HbA1c and BW differed in the age groups (Table 3).
Table 1.
Clinical characteristics of the study participants
| n (men/women) | 463 (333/130) |
| Age (years) | 65 (17) |
| Type of diabetes |
Type 1/type 2/other 31/425/7 |
| Bodyweight, before DSE (kg) | 68.7 (18.9) |
| Bodyweight, after DSE (kg) | 68.5 (18.4) |
| HbA1c, before DSE (%) | 7.5 (1.3) |
| HbA1c, after DSE (%) | 7.3 (1.3) |
| Total alcohol consumption, before DSE (g/week)† | 84.0 (113.0) |
| Total alcohol consumption, after DSE (g/week)† | 70.0 (119.0) |
| Steps per day, before DSE (steps/day) | 6,000 (4,000) |
| Steps per day, after DSE (steps/day) | 4,000 (5,000) |
Data are presented as the median (interquartile range) or number. †Data were calculated only in current drinkers. DSE, declaration of a state of emergency; HbA1c, glycated hemoglobin.
Table 2.
Results of the self‐reported questionnaire to collect how often patients carried out activities before and after declaration of a state of emergency
| Decreased | No change | Increased | Not applicable | |
|---|---|---|---|---|
| 1) Eating out (times per week) | 55.8 | 19.4 | 2.2 | 22.6 |
| 2) Having takeaway meals (times per week) | 13.1 | 36.2 | 25.8 | 24.9 |
| 3) Having snacks (times per week) | 12.0 | 41.8 | 21.0 | 25.2 |
| 4) Drinking alcohol (g of ethanol/week) | 12.3 | 29.5 | 8.7 | 49.5 |
| 5) Going for a walk (steps/day) | 51.2 | 39.4 | 8.5 | 0.9 |
| 6) Going out routinely such as commuting and shopping (times/week) | 44.9 | 44.5 | 3.9 | 6.7 |
| 7) Going out for exercise, such as gym training and playing sports (times/week) | 26.5 | 13.8 | 4.2 | 55.5 |
| 8) Failure to take medication and/or inject insulin for diabetes treatment (times/week) | 1.8 | 53.6 | 4.2 | 40.4 |
| 9) Failure to take medication for diseases other than diabetes (times/week) | 0.7 | 47.5 | 3.2 | 48.6 |
| 10) Sleeping time (h/day) | 9.2 | 73.4 | 17.4 | 0.0 |
Data are presented as percentage.
Figure 1.
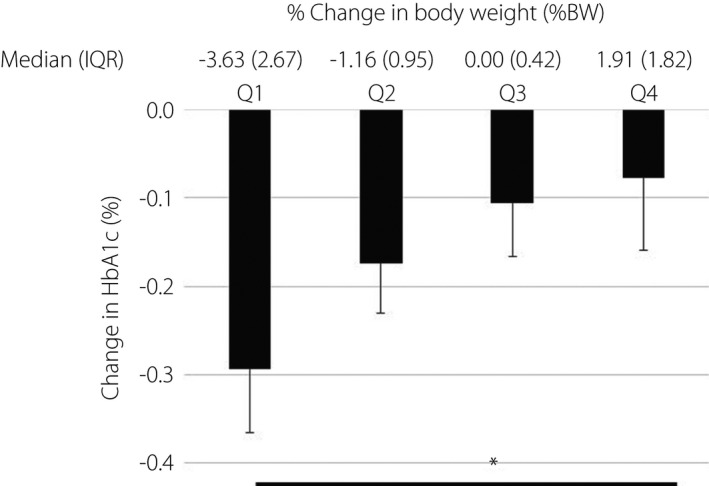
The association between quartiles of percentage change in bodyweight (%BW) and change in glycated hemoglobin (HbA1c) during the declaration of a state of emergency among patients with diabetes is shown. Values are the mean ± standard error. The median bodyweight change (interquartile range [IQR]) from quartile 1, 2, 3 and 4 was −3.63 (2.67), −1.16 (0.95), 0.00 (0.42) and 1.91 (1.82), respectively. *P < 0.05.
Table 3.
Association of factors with lifestyle change with change in glycated hemoglobin and bodyweight
| HbA1c | <65 years | ≥65 years | ||||
|---|---|---|---|---|---|---|
| Lifestyle alteration | B | OR | 95% CI | B | OR | 95% CI |
| 1) Eating out (times/week) | 0.351* | 1.420 | 1.046, 1.928 | −0.140 | 0.870 | 0.603, 1.256 |
| 2) Having takeaway meals (times/week) | 0.034 | 1.034 | 0.789, 1.356 | −0.230 | 0.795 | 0.564, 1.120 |
| 3) Having snacks (times/week) | 0.536* | 1.709 | 1.155, 2.528 | 0.039 | 1.039 | 0.732, 1.476 |
| 4) Drinking alcohol (g of ethanol/week) | 0.001 | 1.001 | 0.997, 1.005 | 0.005 | 1.005 | 0.996, 1.014 |
| 5) Going for a walk (×1,000 steps/day) | −0.024 | 0.976 | 0.850, 1.122 | −0.130 | 0.878 | 0.708, 1.089 |
| 6) Going out routinely (times/week) | −0.230 | 0.795 | 0.631, 1.002 | 0.009 | 1.009 | 0.750, 1.357 |
| 7) Exercising (times/week) | −0.047 | 0.954 | 0.805, 1.130 | −0.065 | 0.937 | 0.818, 1.073 |
| 10) Sleeping time (h/day) | −0.568* | 0.567 | 0.323, 0.994 | 1.092* | 2.981 | 1.481, 5.998 |
| Sex (male) | 0.468 | 1.596 | 0.495, 5.152 | −0.078 | 0.925 | 0.306, 2.792 |
| BW change (kg) | 0.164 | 1.178 | 0.908, 1.528 | 0.546* | 1.726 | 1.215, 2.450 |
| BW | <65 years | ≥65 years | ||||
|---|---|---|---|---|---|---|
| Lifestyle | B | OR | 95% CI | B | OR | 95% CI |
| 1) Eating out (times/week) | 0.281* | 1.325 | 1.007, 1.744 | −0.023 | 0.977 | 0.687, 1.390 |
| 2) Having takeaway meals (times/week) | −0.051 | 0.95 | 0.715, 1.263 | 0.076 | 1.079 | 0.743, 1.566 |
| 3) Having snacks (times/week) | 0.411* | 1.508 | 1.048, 2.168 | 0.643* | 1.903 | 1.166, 3.105 |
| 4) Drinking alcohol (g of ethanol/week) | 0.002 | 1.002 | 0.998, 1.006 | −0.002 | 0.998 | 0.991, 1.006 |
| 5) Going for a walk (×1,000 steps/day) | −0.118 | 0.888 | 0.769, 1.026 | −0.320* | 0.726 | 0.568, 0.927 |
| 6) Going out routinely (times/week) | 0.035 | 1.036 | 0.831, 1.292 | 0.526* | 1.693 | 1.089, 2.632 |
| 7) Exercising (times/week) | 0.126 | 1.134 | 0.935, 1.375 | −0.128 | 0.880 | 0.764, 1.014 |
| 10) Sleeping time (h/day) | −0.324 | 0.723 | 0.432, 1.212 | −0.058 | 0.943 | 0.588, 1.150 |
| Sex (male) | −1.077* | 0.341 | 0.121, 0.958 | −0.942 | 0.390 | 0.138, 1.101 |
| Change in HbA1c (%) | −0.078 | 0.925 | 0.494, 1.734 | 0.630 | 1.878 | 0.759, 4.649 |
*P < 0.05. Binary logistic regression analysis was carried out to investigate whether lifestyle alteration during the declaration of a state of emergency was associated with a change in glycated hemoglobin (HbA1c) and bodyweight (BW). This analysis was separately carried out according to age group of <65 years and that of ≥65 years. B, partial regression coefficient; CI, confidence interval; OR, odds ratio.
DISCUSSION
The present study showed that diabetes patients altered their lifestyles toward a more sedentary mode during the DSE. In contrast, weight reduction was associated with improvement of glycemic control, suggesting the importance of weight control during a DSE. As lifestyle differs greatly according to employment status, we analyzed factors associated with change in BW and HbA1c stratified by age 65 years. As expected, an increase in the frequency of eating snacks affected BW and glycemic control in both age groups. In non‐elderly patients, a lower frequency of eating out was associated with HbA1c and BW reduction. In contrast, reduced steps per day in elderly diabetes patients was related to an increase in BW, whereas regular daily activities were positively associated with an increase in BW, but these findings should be interpreted carefully. Although more active elderly patients might show reduced steps per day, which could increase their BW, more sedentary elderly patients might tend to reduce their regular daily activities to eventual loss of skeletal muscle mass. These results suggest that younger diabetes patients should be aware of weight gain from snacking, whereas older diabetes patients should ensure they exercise to prevent weight gain or weight loss which may lead to sarcopenia. Further investigation is necessary to clarify whether lifestyle changes during containment measures for COVID‐19 affect skeletal mass in the older population. A limitation of the present study is that reduced HbA1c might partly be due to seasonal fluctuation of HbA1c, as reported in previous studies11, 12.
To our knowledge, this is the first study to investigate the impact of COVID‐19 pandemic countermeasures on lifestyle changes, metabolic status and glycemic control among patients with diabetes. The present study showed that patients with diabetes who reduced their BW during the DSE improved their glycemic control. This change during the DSE might be unique to Japan, as weight gain associated with deteriorated glycemic control during confinement measures or lockdown was generally reported in other countries13, 14. Recently, another published report from Japan showed that 50.9% of patients having diabetes with deteriorated glycemic control by DSE, in contrast to the 35.3% having improved glycemic control, reported decreased physical activity levels15. These results are generally consistent with the results of the present study, but the studies did not evaluate the change in relation to BW during the DSE.
The limitations of the present study must be considered carefully. First, this study was carried out in a single center; reproducibility of the study needs to be established in a larger, multicenter study. Second, the findings of this study are based on self‐reported questionnaire, and misreporting is possible. Finally, the duration of data collection varied among study participants, and depended on the duration of the individual hospital visits.
In conclusion, the present study showed that COVID‐19 containment measures carried out in Japan have an impact on lifestyle in individuals with diabetes. Change in bodyweight is associated with change in HbA1c. In addition, increased eating of snacks drastically affects metabolic and glycemic status in diabetes patients, whereas sedentary lifestyle induces weight gain in younger patients, but possible sarcopenic change in elderly patients. Where COVID‐19 has not been brought under control, the present study provides guidance for vulnerable populations, such as diabetes patients, for keeping healthy during containment measures.
DISCLOSURE
N Tanaka received speaker fees from Taisho Pharmaceutical, Nippon Becton‐Dickinson Co. Ltd. and Terumo. Y Hamamoto received speaker fees from Novo Nordisk Pharma Ltd., and also received research grants from Sumitomo‐Dainippon Pharma Co. Ltd. and Nippon Boehringer Ingelheim. H Kuwata received research grants from Taisho Pharmaceutical Co. Ltd, Novo Nordisk Pharma and Ono Pharmaceutical Co. Ltd. T Hyo received consulting or speaker fees from Arklay, AstraZeneca, Kissei Pharmaceutical, LifeScan Japan, Medtronic Japan, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Sanofi and Terumo. Y Yamada received consulting or speaker fees from MSD, Novo Nordisk Pharma, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Sanofi, Daiichi Sankyo and Mitsubishi Tanabe Pharma. Y Yamada also received clinically commissioned/joint research grants from Novo Nordisk Pharma, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Daiichi Sankyo and Mitsubishi Tanabe Pharma. T Kurose received speaker fees from Taisho Pharmaceutical and Ono Pharmaceutical. Y Seino received consulting or speaker fees from Eli Lilly Japan, Sanofi, Novo Nordisk Pharma, GlaxoSmithKline, Taisho Pharmaceutical, Taisho Pharmaceutical, Astellas Pharma, BD, Nippon Boehringer Ingelheim, Johnson & Johnson and Takeda Pharmaceutical. Y Seino also received clinically commissioned/joint research grants from Nippon Boehringer Ingelheim, Eli Lilly, Taisho Pharmaceutical, MSD, Ono Pharmaceutical, Novo Nordisk Pharma, Arklay and Terumo. Y Kurotobi, Y Yamasaki, S Nakatani, M Matsubara, T Haraguchi, Y Yamaguchi, K Izumi and Y Fujita declare no conflict of interest.
Acknowledgments
The authors are grateful to Kazumi Yamauchi, Hiromi Fujikawa, Maika Wada, Ayumi Nakane and Miki Mori for their excellent assistance and contribution to the implementation of this study. The authors also thank the patients and colleagues who contributed to this study.
J Diabetes Investig. 2021; 12: 1718–1722
References
- 1.Shi Q, Zhang X, Jiang F, et al. Clinical characteristics and risk factors for mortality of COVID‐19 patients with diabetes in Wuhan, China: a two‐center retrospective study. Diabetes Care 2020; 43: 1382–1391. [DOI] [PubMed] [Google Scholar]
- 2.Hwang Y, Khasag A, Jia W, et al. Diabetes and COVID‐19: IDF perspective in the Western Pacific region. Diabetes Res Clin Pract 2020; 166: 108278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabinet Secretariat Government of Japan . Basic Policies for Novel Coronavirus Disease Control by the Government of Japan (Summary) March 28, 2020 (Revised on May 25, 2020).
- 6.Sardu C, D'Onofrio N, Balestrieri ML, et al. Outcomes in patients with hyperglycemia affected by COVID‐19: Can we do more on glycemic control? Diabetes Care 2020; 43: 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab 2020; 31: 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holman N, Knighton P, Kar P, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol 2020; 8: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Zhong NS. Clinical characteristics of covid‐19 in China. Reply. N Engl J Med 2020; 382: 1861–1862. [DOI] [PubMed] [Google Scholar]
- 10.Team CC‐R . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 ‐ United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng CL, Brimacombe M, Xie M, et al. Seasonal patterns in monthly hemoglobin A1c values. Am J Epidemiol 2005; 161: 565–574. [DOI] [PubMed] [Google Scholar]
- 12.Sakura H, Tanaka Y, Iwamoto Y. Seasonal fluctuations of glycated hemoglobin levels in Japanese diabetic patients. Diabetes Res Clin Pract 2010; 88: 65–70. [DOI] [PubMed] [Google Scholar]
- 13.Ghosal S, Arora B, Dutta K, et al. Increase in the risk of type 2 diabetes during lockdown for the COVID19 pandemic in India: a cohort analysis. Diabetes Metab Syndr 2020; 14: 949–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Renzo L, Gualtieri P, Pivari F, et al. Eating habits and lifestyle changes during COVID‐19 lockdown: an Italian survey. J Transl Med 2020; 18: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto M, Ishikawa T, Odawara M. Behavioral changes in patients with diabetes during the COVID‐19 pandemic. Diabetol Int 2020. 10.1007/s13340-020-00467-1 [DOI] [PMC free article] [PubMed] [Google Scholar]


