Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) can be transmitted via respiratory droplets, aerosols, and to a lesser extent, fomites. Defining the factors driving infectivity and transmission is critical for infection control and containment of this pandemic. We outline the major methods of transmission of SARS‐CoV‐2, focusing on aerosol transmission. We review principles of aerosol science and discuss their implications for mitigating the spread of SARS‐CoV‐2 among children and adults.
Keywords: aerosol, COVID‐19, face mask, SARS‐CoV‐2, transmission
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was first described in Wuhan, China and rapidly spread across the world within weeks. 1 , 2 , 3 Defining the factors driving the infectivity and transmission of SARS‐CoV‐2 has become critical for infection control and containment of this pandemic. Like other respiratory viruses, SARS‐CoV‐2 can be transmitted in three ways: droplets, aerosols, and contact with surfaces (fomites).
2. PRINCIPLES OF AEROSOL SCIENCE
The first two modes of transmission involve exhalation of viral particles from the respiratory tract (see Figure 1). Large particles (droplets) are subject to gravitational forces and typically land within a few feet of the subject and removed from the air quickly, while smaller particles (aerosols) remain suspended in air for longer and can travel much further. When inhaled, larger particles are trapped in the upper airways, while smaller particles deposit in the lower respiratory tract. 4 The World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) classify particles with a diameter greater than 5 μm as droplets and those ≤5 μm as aerosols. 5 , 6 However, particles up to 20 μm with favorable aerodynamic characteristics (density, shape, etc.) and low settling velocity can linger for longer periods, behaving as suspended aerosols. 7 , 8 , 9 Even large particles emitted from the nose or mouth can evaporate very quickly, decreasing their size and transforming into aerosols (a “droplet nucleus”). 8
Figure 1.
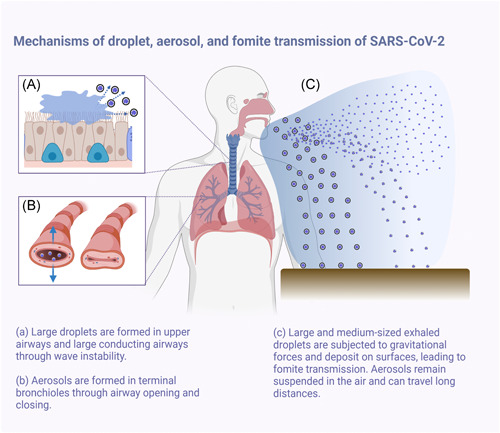
Mechanisms of droplet, aerosol, and fomite transmission of SARS‐CoV‐2. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2 [Color figure can be viewed at wileyonlinelibrary.com]
The characteristics of the indoor environment (i.e., size/volume, ventilation rate, and heating ventilation and air conditioning systems) into which aerosols are dispersed are also critical. In confined spaces with limited ventilation, the concentration of aerosols can remain high, facilitating transmission. Understanding droplet and aerosol dynamics and their link to built environment characteristics is critical in mitigating the spread of airborne infectious diseases. 10
Much of the early work on aerosolized viral transmission dates to the early 20th century, when the spread of respiratory infections among school children and workers was investigated. As early as 1897, the German bacteriologist Carl Flügge noted that respiratory pathogens were present in expiratory droplets that settled around an individual, a finding that led to the development of surgical gauze masks. 11 In 1945, J. P. Duguid studied characteristics of droplets and aerosols from humans during expiratory activities; he found that nasal exhalation sheds primarily droplets, with a minimal amount of aerosols. By contrast, talking, coughing, and sneezing produced more aerosols than droplets, primarily from the mouth. 12
The mechanism of droplet production differs between the upper and lower airways, and these airway dynamics vary with age. In the upper airway, airflow and shear stress sweep excess airway lining fluid into a wave, and the resulting surface instability at the mucus‐air interface breaks the fluid wave into smaller droplets. Formation of wave instability (and therefore droplet production) depends on mucus thickness, viscoelastic properties, surface tension at the mucus‐air interface, and attaining a critical airspeed. 13 Coughing and sneezing generate high airspeeds and can cause droplet formation via wave instability. During tidal breathing, however, the shear force provided by the low‐velocity airflow is insufficient to create droplets via wave instability. However, droplets can be formed during tidal breathing during the closing and reopening of collapsed terminal airways at low lung volumes; these small particles (<1 μm) are formed in the terminal bronchioles and are exhaled as aerosols. 14
These mechanisms have special relevance to transmission of SARS‐CoV‐2 by children. Though children can carry a SARS‐CoV‐2 viral load that is greater than that of adults 15 , 16 and can transmit infection, 17 , 18 they may be less efficient transmitters than adults. Young children have a simpler airway structure (fewer alveoli and terminal bronchioles), lower exhaled airspeed, and less airway collapse than older children and adults. 19 , 20 Among adults, a positive association has been demonstrated between breath aerosol concentration and age, likely related to age‐related airway collapse. 21 , 22 Understanding the differences in droplet and aerosol formation across the lifecourse is a critical knowledge gap that requires further study.
Once exhaled, the fate of aerosols and droplets is affected by evaporation, Brownian motion, electrostatic forces, inertial forces, the temperature, and humidity of the surrounding air, and gravity. 10 , 23 The risk of infection from aerosols is directly related to particle concentration, which is affected by the balance between production (affected by biological factors and patient density in a room) and elimination. Exhaled particles are subjected to two competing forces, gravity downwards and air friction upwards, and the balance of the two and cross‐flows determine the buoyancy of a particle. 24 , 25 Factors, such as doors, windows, human movement, ceiling fans, and heating, ventilation and air conditioning systems, as well as the direction of ventilation, can affect the buoyancy and travel of particles.
3. TRANSMISSION OF SARS‐CoV‐2 VIA RESPIRATORY DROPLETS AND AEROSOLS
As the major reservoir of the SARS‐CoV‐2 virus, the airway and nasal passages of infected individuals shed infectious droplets and aerosols that are not only created by “aerosol‐generating procedures,” 26 but also by coughing, sneezing, breathing, or even simply talking. 27 , 28 Factors affecting potential for transmission include droplet size, viral load per droplet, and quantity and duration of droplet exposure. Large droplets fall nearby under the influence of gravity and contaminate surfaces, leading to fomite transmission. 29 , 30 Smaller droplets form aerosols that linger in the air for prolonged periods of time with the potential to travel greater distances, posing a greater risk of spreading COVID‐19.
The minimum infectious dose (ID50, the dose that causes infection in 50% of hosts) for SARS‐CoV‐2 has not been established, though the ID50 for SARS‐CoV (the original SARS virus) is estimated at 280 plaque forming units. 31 Each SARS‐CoV‐2 virion is approximately 50–200 nm in diameter; aerosolized particles of less than 5 µm (1 μm, or micron equals 1000 nm) potentially harbor several viral particles and can remain airborne for several hours. A laboratory study of nebulized SARS‐CoV‐2 demonstrated that the virus retains structural integrity for up to 16 h in respirable‐sized aerosols, longer than either SARS‐CoV or Middle East respiratory syndrome coronavirus 32 ; another laboratory study demonstrated that viable virus can be recovered for at least three hours from aerosols sprayed into a closed non‐ventilated room. 33 Early in the pandemic, droplets were thought to be the dominant method of disease transmission, despite research suggesting the original SARS‐CoV outbreak was spread by aerosols. 34 , 35 Multiple reports of aerosolized spread of COVID‐19 have been published since then, leading both the WHO and CDC to highlight the importance of aerosol transmission. 10 , 36 , 37 , 38
One of the first studies to suggest aerosol transmission of SARS‐CoV‐2 came from Inner Mongolia where a case of COVID‐19 appeared to result from regularly passing by the door of an asymptomatic patient. 36 Airborne transmission was also likely responsible for an outbreak during a bus ride in China, in which one individual infected 23 of 67 passengers, including those seven rows behind the index case. 39 Multiple outbreaks among adjacent tables in restaurants have also been reported. 40 , 41 Within the healthcare environment, one study found SARS‐CoV‐2 on air exhaust vent surfaces in patient rooms. 42 A second study sampled air throughout inpatient wards, at least 2 m from patient beds, and recovered viral particles in 2 of 14 samples. 43 A third study collected air samples in hospital rooms of COVID‐19 patients, and isolated viable virus in aerosols in the absence of aerosol generating procedures between 2 and 4.8 m from patients' beds. 44 Additional outbreaks have been reported related to choir rehearsals, consistent with increased production of aerosols due to loud speech and singing. 45 , 46 , 47 Taken together, these data suggest that infectious aerosols are commonly produced by patients infected with SARS‐CoV‐2, and that exposure to aerosolized virus in an indoor space for a prolonged period may carry significant risk which is underappreciated. 48
Many routine pulmonary procedures have the potential of aerosol generation. Nebulized therapies are classified as a “possible aerosol generating procedure” by the CDC based on conflicting data from the SARS‐CoV epidemic. 49 , 50 Given this uncertainty, many hospitals and clinics treat nebulizer therapy as an aerosol generating procedure to protect healthcare providers from SARS‐CoV‐2 infection. 51 Pulmonary function testing and spirometry are not currently classified as aerosol generating procedures by the CDC, 49 though the American Thoracic Society and the American Academy of Asthma, Allergy, and Immunology recognize that pulmonary function testing has the potential to generate aerosols. 4 , 5 , 6 , 52 , 53 , 54 A small study of five adults undergoing pulmonary function testing demonstrated aerosol generation with tidal breathing, the forced vital capacity maneuver, and maximum voluntary ventilation, though additional studies are needed. 55
4. FOMITE TRANSMISSION
Fomite transmission requires contact with a contaminated surface followed by contact with a port of entry (eyes, mouth, nose). Viable SARS‐CoV‐2 can be recovered for at least 3 days after surfaces exposed to infected individuals. 33 , 42 , 56 , 57 , 58 Though respiratory viruses can be transmitted via contact with surfaces, 59 the relative contribution of fomites to SARS‐CoV‐2 transmission remains unknown. Two meta‐analyses of handwashing interventions before the COVID‐19 pandemic demonstrate that while hand hygiene is effective at reducing transmission of a range of respiratory viruses (including coronaviruses), it is less effective than mask use, providing indirect evidence that this is not the dominant mode of transmission for most respiratory viruses. 60 , 61
5. MITIGATING RISK OF TRANSMISSION
Simple measures are likely to significantly reduce the spread of droplets and aerosols. Masks (cotton, surgical, and N95) are very effective at limiting respiratory particle transmission during speaking and coughing (90% and 74%, respectively). 62 , 63 Masks reduce both inhalation and exhalation of droplets and aerosols, and are thus critical for interrupting transmission. 64 , 65 , 66 , 67 The efficacy of masks in reducing transmission depends on a variety of factors, including fit, time worn, and filtration efficacy. Higher filtration efficacy masks (e.g., N95 masks) are much more effective at filtration of aerosols than lower‐efficiency masks (e.g., surgical masks), 68 , 69 , 70 but these do not currently exist in sizes for children and are poorly tolerated for long periods of time.
The widely used spacing of 2 m is based on work by W. F. Wells, who pioneered the concept of aerosol‐transmitted infections in the 1930 s. Wells noted that large droplets (>100 μm) fall to the floor within 2 m from the source and are dispersed depending on the exhalation air velocity and relative humidity of the air. New models, though, demonstrate that with the flow rates of coughing and sneezing, droplets can travel up to 6 m, 71 and patients with respiratory illness can generate a droplet cloud spanning up to 8 m. 72 , 73 Thus, social distancing is appropriate but no replacement for masks.
6. CONCLUSION
Droplets and aerosols are generated by tidal breathing, coughing, and sneezing. SARS‐CoV‐2 can be transmitted by all these methods and is viable in particles that remain suspended in air. Understanding the behavior of virus‐laden droplets and aerosols in confined spaces is urgently needed for schools and workplaces to open safely. This makes the usage of masks, social distancing, and adequate room ventilation critical for limiting the aerosol spread of SARS‐CoV‐2.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Peter Moschovis: conceptualization (equal); writing original draft (lead); writing review & editing (lead). Lael M Yonker: writing review & editing (supporting). Jhill Shah: writing review & editing (supporting). Dilpreet Singh: methodology (equal). Philip Demokritou: methodology (lead); writing original draft (supporting); writing review & editing (supporting). T Bernard Kinane: conceptualization (lead); writing original draft (equal); writing review & editing (equal).
ACKNOWLEDGMENTS
The figure above was created using https://BioRender.com. The authors report having received support from the National Institutes of Health (K23ES030399 [PPM], K08HL143183 [LMY], T32HL007427 [JS], U24ES026946 [PD]) and the Cystic Fibrosis Foundation (LMY).
Moschovis PP, Yonker LM, Shah J, Singh D, Demokritou P, Kinane TB. Aerosol transmission of SARS‐CoV‐2 by children and adults during the COVID‐19 pandemic. Pediatric Pulmonology. 2021;56:1389–1394. 10.1002/ppul.25330
REFERENCES
- 1. Zhouou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhuu N, Zhang D, Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC COVID Response Team , Jorden MA, Rudman SL, Villarino E, et al. Evidence for limited early spread of COVID‐19 within the United States, January–February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(22):680‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knight V. Airborne transmission and pulmonary deposition of respiratory viruses. Paper presented at: Airborne transmission and airborne infections. 6th International symposium on aerobiology; 1973.
- 5. WHO Guideline Committee . Infection Prevention and Control of Epidemic‐ and Pandemic‐Prone Acute Respiratory Infections in Health Care. World Health Organization; 2014. [PubMed] [Google Scholar]
- 6. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory C. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65‐S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gralton J, Tovey E, McLaws ML, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2(3):143‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J R Soc Interface. 2009;6(Suppl 6):S783‐S790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16(5):335‐347. [DOI] [PubMed] [Google Scholar]
- 11. Flügge C. Ueber luftinfection. Zeitschrift für Hygiene und Infektionskrankheiten. 1897;25(1):179‐224. [Google Scholar]
- 12. Duguid JP. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinb Med J. 1945;52:385‐401. [PMC free article] [PubMed] [Google Scholar]
- 13. Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44(9 Suppl):S102‐S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Almstrandlmstrand AC, Bake B, Ljungström E, et al. Effect of airway opening on production of exhaled particles. J Appl Physiol. 2010;108(3):584‐588. [DOI] [PubMed] [Google Scholar]
- 15. Yonkeronker LM, Neilan AM, Bartsch Y, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heald‐Sargent T, Muller WJ, Zheng X, Rippe J, Patel AB, Kociolek LK. Age‐related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) levels in patients with mild to moderate coronavirus disease 2019 (COVID‐19). JAMA Pediatr. 2020;174:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartzchwartz NG, Moorman AC, Makaretz A, et al. Adolescent with COVID‐19 as the source of an outbreak at a 3‐week family gathering—four states, June–July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1457‐1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castagnoliastagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882‐889. [DOI] [PubMed] [Google Scholar]
- 19. Herring MJ, Putney LF, Wyatt G, Finkbeiner WE, Hyde DM. Growth of alveoli during postnatal development in humans based on stereological estimation. Am J Physiol Lung Cell Mol Physiol. 2014;307(4):L338‐L344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riediker M, Morawska L. Low Exhaled Breath droplet formation may explain why children are poor SARS‐CoV‐2 transmitters. Aerosol Air Qual Res. 2020;20(7):1513‐1515. [Google Scholar]
- 21. Johnson GR, Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm Drug Deliv. 2009;22(3):229‐237. [DOI] [PubMed] [Google Scholar]
- 22. Anthonisen NR, Danson J, Robertson PC, Ross WRD. Airway closure as a function of age. Respir Physiol. 1969;8(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 23. Baron PA, Willeke K. Respirable droplets from whirlpools: measurements of size distribution and estimation of disease potential. Environ Res. 1986;39(1):8‐18. [DOI] [PubMed] [Google Scholar]
- 24. Wells WF. On air‐borne infection. Study II. Droplets and droplet nuclei. Am J Hyg. 1934;20:611‐618. [Google Scholar]
- 25. Atkinson J, Chartier Y, Pessoa‐Silva CL, Jensen P, Li Y, Seto WH, eds. Natural Ventilation for Infection Control in Health‐Care Settings. World Health Organization; 2009. [PubMed] [Google Scholar]
- 26. Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol‐generating medical procedures. Viruses. 2019;11(10):940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma J, Qi X, Chen H, et al. COVID‐19 patients in earlier stages exhaled millions of SARS‐CoV‐2 per hour. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS‐CoV‐2 transmission. Proc Natl Acad Sci USA. 2020;117(22):11875‐11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grayson SA, Griffiths PS, Perez MK, Piedimonte G. Detection of airborne respiratory syncytial virus in a pediatric acute care clinic. Pediatr Pulmonol. 2017;52(5):684‐688. [DOI] [PubMed] [Google Scholar]
- 30. Liu L, Wei J, Li Y, Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2017;27(1):179‐190. [DOI] [PubMed] [Google Scholar]
- 31. Watanabe T, Bartrand TA, Weir MH, Omura T, Haas CN. Development of a dose‐response model for SARS coronavirus. Risk Anal. 2010;30(7):1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fearsears AC, Klimstra WB, Duprex P, et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. 2020;26(9):2168‐2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Doremalenan Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olsenlsen SJ, Chang HL, Cheung TYY, et al. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349(25):2416‐2422. [DOI] [PubMed] [Google Scholar]
- 35. McKinney KR, Gong YY, Lewis TG. Environmental transmission of SARS at Amoy gardens. J Environ Health. 2006;68(9):26‐30. [PubMed] [Google Scholar]
- 36. Wang J, Du G. COVID‐19 may transmit through aerosol. Ir J Med Sci. 2020;189(4):1143‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. World Health Organization . Transmission of SARS‐CoV‐2: implications for infection prevention precautions: scientific brief. 2020. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautionsexternalicon
- 38. Centers for Disease Control and Prevention . Scientific brief: SARS‐CoV‐2 and potential airborne transmission. 2020. https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html. Accessed October 15, 2020.
- 39. Shenhen Y, Li C, Dong H, et al. Community outbreak investigation of SARS‐CoV‐2 transmission among bus riders in Eastern China. JAMA Intern Med. 2020;180:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Qian H, Hang J, et al. Evidence for probable aerosol transmission of SARS‐CoV‐2 in a poorly ventilated restaurant. medRxiv. 2020. [Google Scholar]
- 41. Luu J, Gu J, Li K. COVID‐19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26(7):1628‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ongng SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) from a symptomatic patient. JAMA. 2020;323(16):1610‐1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kenarkoohienarkoohi A, Noorimotlagh Z, Falahi S, et al. Hospital indoor air quality monitoring for the detection of SARS‐CoV‐2 (COVID‐19) virus. Sci Total Environ. 2020;748:141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lednickyednicky JA, Lauzardo M, Fan ZH, et al. Viable SARS‐CoV‐2 in the air of a hospital room with COVID‐19 patients. Int J Infect Dis. 2020;100:476‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alsvedlsved M, Matamis A, Bohlin R, et al. Exhaled respiratory particles during singing and talking. Aerosol Sci Technol. 2020;54(11):1245‐1248. [Google Scholar]
- 46. Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 2019;9(1):2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamneramner L, Dubbel P, Capron I, et al. High SARS‐CoV‐2 attack rate following exposure at a choir practice—Skagit county, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69(19):606‐610. [DOI] [PubMed] [Google Scholar]
- 48. Morawska L, Milton DK. It is time to address airborne transmission of coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2020;71(9):2311‐2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention . Clinical questions about COVID‐19: questions and answers | CDC. 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html. Accessed January 12, 2021.
- 50. Tran K, Cimon K, Severn M, Pessoa‐Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLOS One. 2012;7(4):e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sethi S, Barjaktarevic IZ, Tashkin DP. The use of nebulized pharmacotherapies during the COVID‐19 pandemic. Ther Adv Respir Dis. 2020;14:1753466620954366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. American Academy of Allergy AaI . Spirometry during COVID. 2020. https://www.aaaai.org/ask-the-expert/spirometry. Accessed January 12, 2021.
- 53. American Thoracic Society . Pulmonary function laboratories: advice regarding COVID‐19. 2020. https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/pulmonary-function-laboratories.php. Accessed January 12, 2021.
- 54. Pasnick S, Carlos WG, Dela Cruz CS, Gross JE, Garrison G, Jamil S. SARS‐CoV‐2 transmission and the risk of aerosol‐generating procedures. Am J Respir Crit Care Med. 2020;202(4):P13‐P14. [DOI] [PubMed] [Google Scholar]
- 55. Helgeson SA, Lim KG, Lee AS, Niven AS, Patel NM. Aerosol generation during spirometry. Ann Am Thorac Soc. 2020;17(12):1637‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yamagishiamagishi T, Ohnishi M, Matsunaga N, et al. Environmental sampling for severe acute respiratory syndrome coronavirus 2 during a COVID‐19 outbreak on the diamond princess cruise ship. J Infect Dis. 2020;222(7):1098‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yungung CF, Kam K, Wong MSY, et al. Environment and personal protective equipment tests for SARS‐CoV‐2 in the isolation room of an infant with infection. Ann Intern Med. 2020;173(3):240‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Riddell S, Goldie S, Hill A, Eagles D, Drew TW. The effect of temperature on persistence of SARS‐CoV‐2 on common surfaces. Virol J. 2020;17(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Julian TR, Leckie JO, Boehm AB. Virus transfer between fingerpads and fomites. J Appl Microbiol. 2010;109(6):1868‐1874. [DOI] [PubMed] [Google Scholar]
- 60. Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta‐analysis. Am J Public Health. 2008;98(8):1372‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jeffersonefferson T, Del Mar CB, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst Rev. 2011;2011(7):CD006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fischer EP, Fischer MC, Grass D, Henrion I, Warren WS, Westman E. Low‐cost measurement of face mask efficacy for filtering expelled droplets during speech. Sci Adv. 2020;6(36):eabd3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Asadi S, Cappa CD, Barreda S, Wexler AS, Bouvier NM, Ristenpart WD. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci Rep. 2020;10(1):15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chanhan JFW, Yuan S, Zhang AJ, et al. Surgical mask partition reduces the risk of non‐contact transmission in a golden Syrian hamster model for coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2020;71:2139‐2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chuhu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person‐to‐person transmission of SARS‐CoV‐2 and COVID‐19: a systematic review and meta‐analysis. Lancet. 2020;395(10242):1973‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID‐19: reducing the inoculum of SARS‐CoV‐2 to protect the wearer. J Gen Intern Med. 2020;35:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hendrix MJ, Walde C, Findley K, Trotman R. Absence of apparent transmission of SARS‐CoV‐2 from two stylists after exposure at a hair salon with a universal face covering policy—Springfield, Missouri, May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(28):930‐932. [DOI] [PubMed] [Google Scholar]
- 68. Yin X, Wang X, Xu S, He C. Comparative efficacy of respiratory personal protective equipment against viral respiratory infectious diseases in healthcare workers: a network meta‐analysis. Public Health. 2020;190:82‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kimim MC, Bae S, Kim JY, et al. Effectiveness of surgical, KF94, and N95 respirator masks in blocking SARS‐CoV‐2: a controlled comparison in 7 patients. Infect Dis. 2020;52(12):908‐912. [DOI] [PubMed] [Google Scholar]
- 70. Uekieki H, Furusawa Y, Iwatsuki‐Horimoto K, et al. Effectiveness of face masks in preventing airborne transmission of SARS‐CoV‐2. mSphere. 2020;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xie X, Li Y, Chwang AT, Ho PL, Seto WH. How far droplets can move in indoor environments—revisiting the wells evaporation‐falling curve. Indoor Air. 2007;17(3):211‐225. [DOI] [PubMed] [Google Scholar]
- 72. Bourouiba L. Images in clinical medicine. A Sneeze. N Engl J Med. 2016;375(8):e15. [DOI] [PubMed] [Google Scholar]
- 73. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID‐19. JAMA. 2020;323(18):1837‐1838. [DOI] [PubMed] [Google Scholar]


