Abstract
Background
In March 2020, a state of emergency was declared to facilitate organized responses to the coronavirus disease 2019 (COVID‐19) pandemic in British Columbia, Canada. Emergency blood management committees (EBMCs) were formed regionally and provincially to coordinate transfusion service activities and responses to possible national blood shortages.
Study Design and Methods
We describe the responses of transfusion services to COVID‐19 in regional health authorities in British Columbia through a collaborative survey, contingency planning meeting minutes, and policy documents, including early trends observed in blood product usage.
Results
Early strategic response policies were developed locally in collaboration with members of the provincial EBMC and focused on three key areas: utilization management strategies, stakeholder engagement (collaboration with frequent users of the transfusion service, advance notification of potential inventory shortage plans, and development of blood triage guidance documents), and laboratory staffing and infection control procedures. Reductions in transfusion volumes were observed beginning in mid‐March 2020 for red blood cells and platelets relative to the prepandemic baseline (27% and 26% from the preceding year, respectively). There was a slow gradual return toward baseline beginning one month later; no product shortage issues were experienced.
Conclusion
Provincial collaborative efforts facilitated the development of initiatives focused on minimizing potential COVID‐19–related disruptions in transfusion services in British Columbia. While there have been no supply issues to date, the framework developed early in the pandemic should facilitate timely responses to possible disruptions in future waves of infection.
Keywords: blood management, blood center operations, transfusion service operations
Abbreviations
- COVID‐19
coronavirus disease 2019
- EBMC
emergency blood management committee
- EOC
emergency operations center
- NAC
Canadian National Advisory Committee on Blood and Blood Products
- PBCO
Provincial Blood Coordinating Office
1. INTRODUCTION
The World Health Organization declared the coronavirus disease 2019 (COVID‐19) outbreak a public health emergency of international concern in late January 2020 and, subsequently, a pandemic in early March 2020. 1 The severe acute respiratory syndrome epidemic of 2002‐2004 was associated with significant reductions in blood donation, with some centers requiring import of blood products from other regions to sustain clinical capacity unless there were comparable reductions in demand for products. 2 , 3 , 4 Similarly, significant disruptions in social and institutional functions resulting from global spread of the severe acute respiratory syndrome coronavirus 2 may potentially threaten blood supplies worldwide. 5 Multiple jurisdictions have reported decreasing donations leading to decreased blood supply, offset in some settings by declining demand for transfusion in the short term. 6 , 7 , 8 , 9 , 10
Contingency planning for supply shortages in a national blood supply system requires a nationally and locally coordinated approach. The Canadian National Advisory Committee on Blood and Blood Products (NAC) is an expert advisory group to the Provincial and Territorial Blood Liaison Committee and to the Ministers of Health that is tasked to advise on blood product utilization management and transfusion practice in Canada, exclusive of Quebec. The governors of the Canadian blood supply have previously directed the NAC to develop a framework for managing blood shortages in the country. Relevant policies are documented in the National Plan for Management of Shortages of Labile Blood Components (The Plan), which includes the recognition of the National Emergency Blood Management Committee (EBMC), which is mandated to support coordinated responses to critical national blood shortages. 11 The National EBMC consists of provincial government officials, provincial transfusion medicine leads, and leads from the national blood supplier Canadian Blood Services (serving all parts of Canada except for Quebec). The Plan references the Emergency Framework for rationing blood for massively bleeding patients during a Red Phase shortage that was developed with multidisciplinary and specialist society contribution, and primarily includes a description of advisory phases for the national blood inventory 11 , 12 :
Green Phase:
Normal component inventory
Green Phase Advisory:
Low inventory for at least some components
Requires local evaluation of stock
Amber Phase:
National inventory insufficient to proceed with routine transfusion practices
Requires reductions in blood usage
Red Phase:
Critical inventory shortages
Insufficient to ensure availability of transfusions for all nonelective indications
Recovery Phase:
Inventory increasing toward Green Phase
In the province of British Columbia, Canada, the third known Canadian case of COVID‐19 was reported in late January 2020, the first instance of community transmission on March 5, and the first death resulting from COVID‐19 on March 9. 13 , 14 , 15 On March 17, a provincial state of emergency was declared to support coordinated responses to COVID‐19, and the national EBMC released a Green Phase Advisory on all fresh blood components and plasma protein products, predicting possible shortages due to significant blood donation cancellations. 16 , 17 Regional and provincial EBMCs were convened in accordance with the National Shortage Plan to facilitate concerted recommendations and actions from transfusion services. The provincial EBMC of British Columbia includes transfusion medicine leadership from nine regional health authorities and the national blood supplier. This report reviews transfusion policy changes implemented in our health authority (encompassing the largest tertiary care center in British Columbia) and other regional health authorities participating in the provincial EBMC to plan for the potential development of shortages in the national blood inventory, as well as trends observed in provincial blood product usage in the first three months of the COVID‐19 pandemic.
2. METHODS
Data regarding changes in transfusion laboratory policy and practice, stakeholder engagement, document distribution, and established initiatives were extracted from sources including minutes of local, provincial, and national EBMCs; locally maintained catalogs of action items, projects, and outcomes arising from meetings; and documentation generated for transfusion laboratory use or distribution resulting from these initiatives. Detailed blood product usage data were collected from the authors' center from the laboratory information system. Surveys were distributed and completed by members of the provincial EBMC inquiring about changes in blood supply and demand during the pandemic, strategies for utilization management and stakeholder engagement, and approaches for laboratory infection control (Supplementary Material S1). Descriptive statistics were used to summarize usage trends.
3. RESULTS
3.1. Stakeholder engagement for contingency planning
Successful implementation of utilization management strategies and maintenance of clinical service required engagement with stakeholders in clinical, laboratory, and government settings. The national EBMC met weekly during the pandemic to review national inventory levels, blood product “days on hand” for each of the provinces, and the overall advisory phase in Canada; medical specialty professional groups were invited to meetings for further coordination. The British Columbia EBMC would also meet weekly to coordinate provincial approaches and to discuss issues related to the pandemic and its effects. Health authority–specific EBMCs maintained ongoing communication at provincial and national levels, harmonizing policies and actions in transfusion service responses to the pandemic. In many health authorities in British Columbia, regional emergency operations centers (EOCs) were created to monitor the ongoing response to the pandemic. These EOCs allowed transfusion medicine services to advance specific issues, such as formation of health authority–specific EBMCs, through communications to senior leadership in Situation, Background, Assessment, Recommendation format.
Examples of local communications included memos regarding Green Phase Advisories and evidence‐based recommendations in transfusion practice. As part of the British Columbia Blood Contingency Planning Toolkit, developed by the British Columbia Provincial Blood Coordinating Office (PBCO), notifications were also developed provincially for Amber and Red Phases to be distributed in the event of escalation. Transfusion service representatives liaised with high‐volume clinical users to determine approaches to ongoing transfusion in the setting of inventory shortages and contributed to the development of national guidelines for transfusion support in patients with hemoglobinopathies to minimize blood supply impacts. Additional activities have included advocating to health agencies to maintain availability of intravenous iron infusions during the pandemic as an essential service to emphasize patient blood management and engagement with palliative care services to facilitate out‐of‐hospital transfusions (if feasible under Health Canada Blood Regulation standards). Participating health authorities have not reported challenges with access to intravenous iron infusion, and decreased overall usage during the early stages of the pandemic was observed in several facilities owing to cancellation of elective surgeries and enhanced screening measures for infusion prescription (in one health authority when comparing the period of March to October 2020 to the same period in 2019, the average numbers of patients receiving iron infusions monthly were 103 ± 20.7 compared to 150 ± 16.6, respectively).
In anticipation of a possible severe blood shortage and at the direction of the NAC National Shortages Plan, the EBMCs at several health authorities developed interim guidance documents to form a triage team for allocation of blood products to patients requiring high‐volume transfusion support. The EBMC in our health authority, having broad clinical representation, collaborated with health authority communications, risk management, and ethics to create the framework. Trauma team, anesthesia, and critical care representatives were chosen to lead the triage team along with the transfusion medicine director to help coordinate the local and provincial/national inventories. The National Shortages plan also outlines blood triage exclusion criteria, which were adapted to local triage team documents: these provide guidance to the triage team (who must not be directly involved in care of the patient receiving transfusion) to exclude patients from receiving blood products if their predicted mortality risk is high (listed at https://www.nacblood.ca/resources/shortages-plan/emergency-framework-final.pdf). Figure 1 depicts a schematic of the Vancouver Coastal Health framework for blood triage scenarios and the agreed‐upon ideal triage team composition. Establishment of these documents included examination and approval by EOC leadership as interim guidance, with critical care and ventilator exclusion criteria to be incorporated when developed and approved provincially.
FIGURE 1.
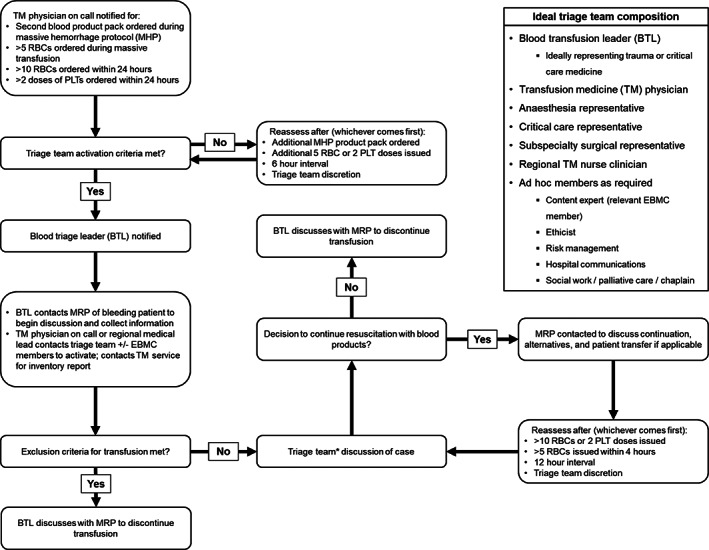
Interim framework for Vancouver Coastal Health blood triage scenarios (for appropriate allocation of blood product support in massive transfusion situations in the event of severe blood product shortage). EBMC, emergency blood management committee; MRP, most responsible physician; PLT, platelets. *Triage team case discussions require a minimum of three team members, including the blood triage leader and transfusion medicine physician
3.2. Usage mitigation strategies
In anticipation of blood shortages, utilization management strategies were developed and implemented during early stages of the pandemic. Meetings of the provincial EBMC continued weekly to facilitate early detection of inventory or logistic issues and enable cooperative solutions to shortages. To reduce unnecessary usage, a provincial infographic emphasizing evidence‐based restrictive practices for transfusion and guidance regarding consequences and avoidance of iatrogenic anemia was developed and distributed to clinical departments and personnel involved in transfusion. The EOCs provided a valuable method to disseminate essential communications to clinical stakeholders.
Practices intended to directly limit unnecessary transfusions were developed, including best practice alerts in electronic order entry systems for red blood cell (RBC) transfusion orders and laboratory screening guidelines for prospective review of plasma or platelet orders falling outside of usual ordering practices for stable inpatients. Guidelines for blood usage to be distributed to clinical services during times of Amber and Red Phases of blood product inventory were created for future use, incorporating the developed framework for blood product triage during severe blood shortages. The British Columbia Transparent Blood Inventory, a Web‐based tool developed by the PBCO, provides British Columbia transfusion medicine stakeholders with detailed hospital blood inventories updated hourly and blood supplier inventory data updated once daily. It was reemphasized during a provincial EBMC meeting to inform requirements for future emergency response activities in real time during Amber and Red Phases. Specifically, the toolʼs Emergency Management dashboard contains data on RBC and platelet units, days to expiry for units, and 90‐day trend lines for both hospital inventory and blood supplier RBC days on hand.
Strategies implemented in other transfusion services represented in the provincial EBMC varied, with the majority planning or undertaking communications to clinical services, increased laboratory screening of orders, and decreased on‐hand inventory (Figure 2). While deployment of contingency strategies has not been required, a recent prospective Canadian study of blood bank technologist screening of RBC orders (before COVID‐19) suggests blood product savings of approximately 9%, and a similar retrospective Canadian audit suggests the potential for savings of up to 35% of ordered RBCs when deferring those ordered for patients with hemoglobin values greater than 70 g/L. 18 , 19
FIGURE 2.
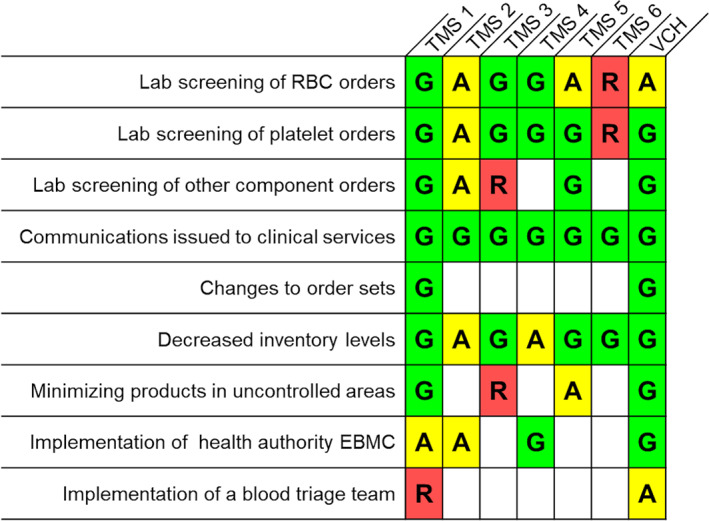
Strategies implemented by the authors' health authority (Vancouver Coastal Health [VCH]) and those reported to be in use in transfusion medicine services (TMS) represented in the provincial emergency blood management committee (EBMC) with regards to utilization management. G denotes Green Phase Advisory strategies (currently in use), A indicates strategies to be implemented with Amber Phase Advisory or above (insufficient inventory for routine transfusion practices), and R indicates strategies to be implemented in the event of Red Phase Advisory (critical inventory shortages) [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Laboratory staffing and testing management, including infection prevention and control
Plans were developed to limit the transmission of infection associated with blood product distribution from or return to the transfusion service, including general infection control guidelines for laboratory staff, and policies were established to limit product contamination in patient care areas, including restricting pneumatic delivery tube materials from entering patient rooms and allowing only single units of blood components or plasma protein products intended for imminent transfusion to be provided at the bedside.
Hospital porter services were asked to adapt their routine practices to minimize unnecessary interactions in the transfusion laboratory, and only porters were allowed to pick up blood in lieu of clinical staff. Contingency plans to ensure emergency health personnel continued to be capable of collecting required blood products for prehospital emergency transfusions were put in place. Physical plexiglass barriers were installed at some accessioning sites.
Processes were developed in partnership with immunologists to reduce exposures for high‐risk individuals requiring outpatient infusion of immune globulin products for immunodeficiency while meeting accreditation standards for traceability and transport of product. In some regions, a concerted effort was made to switch patients, where appropriate, to subcutaneous immune globulin to limit future hospital exposures. Throughout British Columbia, transfusion services developed processes to allow patients receiving outpatient blood products to arrange for curbside pickup of their products or to authorize a designate to retrieve products from the transfusion laboratory on their behalf (in all cases with strict infection control measures).
To minimize the risk of viral transmission by surface contamination, protocols for disinfection of transport cooler surfaces and the distribution and return of blood components and products were instituted in collaboration with infection control. All components and products were distributed in overwrap bags securely sealed using cable ties or tape, and on return to the transfusion service the integrity of the seal was evaluated by laboratory personnel. The disposition of blood products returned without an intact seal was determined by the time until unit expiry based on published data regarding viral persistence on surfaces and is outlined in Figure 3. 20 To date, resulting excess product wastage has been minimal (five units of RBCs and three of platelets). As the prevalence of COVID‐19 initially decreased in the province, components and products were distributed in overwrap bags only to clinical wards designated for patients with COVID‐19.
FIGURE 3.
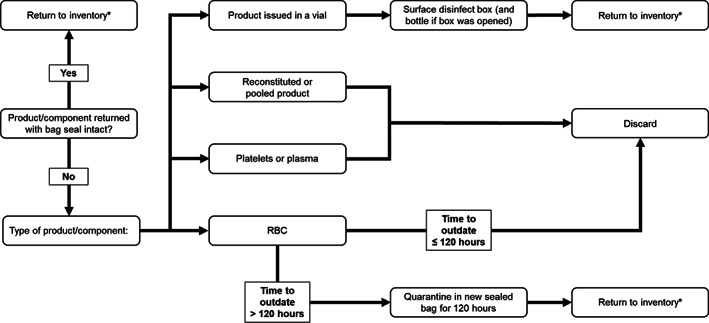
Protocol for disposition of products returned to the transfusion medicine laboratory in the authors' center. Products are distributed in sealed overwrap bags and integrity of the seal is evaluated on return to the laboratory. *Provided standard criteria for return to inventory are met
Measures implemented by other services in the provincial EBMC (Figure 4) included similar processes, with mixed approaches to surface contamination control (including product overwrapping and/or surface disinfection).
FIGURE 4.
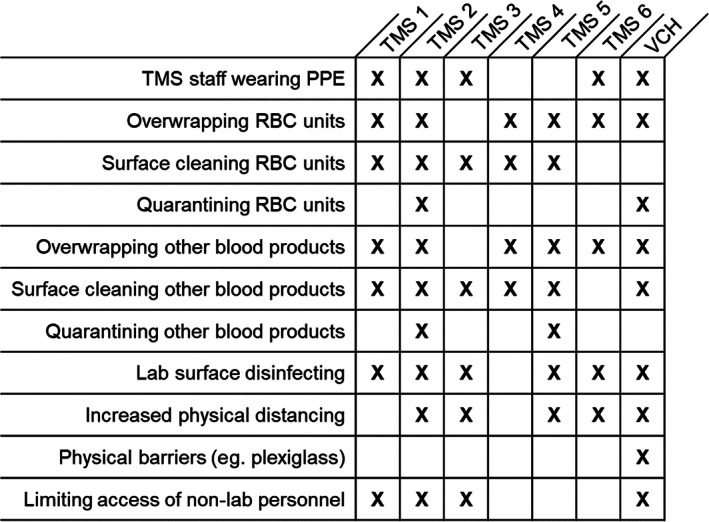
Strategies implemented by the authors' health authority (Vancouver Coastal Health [VCH]) and those reported to be in use in transfusion medicine services (TMS) represented in the provincial emergency blood management committee (EBMC) with regards to reduction of viral transmission in the transfusion laboratory and managing risks related to surface contamination of returned blood products. X indicates a strategy is in use
3.4. Changes in blood product supply and demand during the pandemic
In the weeks following the declaration of a pandemic, blood collection centers experienced a surge in donors despite overall reductions in capacity, and the decrease in the number of blood donors was less than anticipated. 21 , 22 At the same time, a provincial public health emergency was declared, and elective procedures were halted in British Columbia on March 16 to prepare for a possible surge in COVID‐19–related hospitalizations. 23 Emergency department patient volumes decreased dramatically in the same time period. 24 Accordingly, significant declines in transfusion rates were observed in the authors' center (Figure 5A): Average daily RBC transfusion rates fell by approximately 30% from preceding months through late March and April, with similar trends in platelet transfusion rates (decreasing from 679 and 758 platelet transfusions in the two months preceding the pandemic declaration to 450 and 425 platelet transfusions in the two months following). On 8 April 2020, the Green Phase Advisory was lifted for all non–plasma protein products, delayed in part by the perceived risk of platelet shortages should donation rates fall and clinical volumes increase. By 6 May 2020, the Green Phase Advisory was discontinued, replaced by a Recovery Phase Advisory (corresponding with “reopening” and transition to normal demand within hospitals). The anticipated increase in demand was reflected in transfusion trends observed in the authors' center, which at the time of writing are approaching prepandemic rates. Plasma protein products did not fall into shortage during this time, but there are concerns regarding their future supply. An interim immune globulin product plan is being developed to define advisory phases for immune globulin inventory levels and actions to be taken at each level of inventory shortage.
FIGURE 5.
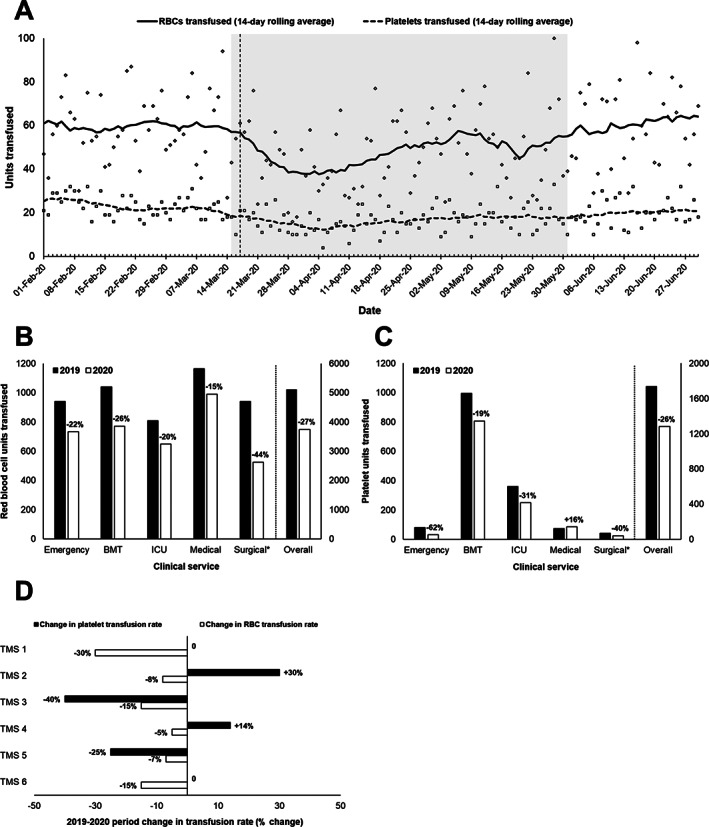
A. RBC and platelet utilization over time at Vancouver General Hospital. Solid lines indicate 14‐day rolling average rates and icons indicate daily rates (diamond: RBC; square: Platelet). The dashed vertical line indicates the date a provincial state of emergency was declared. The shaded area denotes the time period reviewed in panels (B‐D). B‐C, RBC (B) and platelet (C) transfusion rates by clinical service and overall at Vancouver General Hospital between 15 March‐31 May, 2019 and 2020. Relative changes in transfusion rates observed in 2020 relative to 2019 are indicated. BMT, bone marrow transplant; ICU, intensive care unit. D, Observed changes in RBC and platelet transfusion rates between 15 March‐31 May 2019 and 2020, as reported by members of the provincial emergency blood management committee for their local transfusion services. Decreases in RBC transfusion rates were most pronounced in emergency, trauma, and surgical areas in most centers. Reported increases in platelet transfusion were accounted for by unusually high use (4‐fold increase) in intensive care at one center and one patient requiring large numbers of transfusions for hypoproliferative thrombocytopenia at another. *Surgical transfusion data exclude intraoperative transfusions, which are not captured in the laboratory information system
When compared to the same period in 2019, transfusion rates of both RBCs (Figure 5B) and platelets (Figure 5C) between March 15 and May 31 decreased by approximately one quarter, with reduced rates in most hospital services for both (except for increased platelet usage in medical services, accounted for by oncology and other outpatient transfusions), particularly in surgical services. Other members of the provincial EBMC reviewing the same period (Figure 5D) reported decreases in RBC transfusion rates in all sites and variable changes in platelet transfusion rates across sites. Most sites reported the greatest reductions in transfusion rates in surgical and emergency/trauma services. The most remote health authorities tended to be most affected. Flights and other transportation options dwindled during the pandemic, complicating blood transportation from the blood supplier to remote hospitals and in some cases requiring collaboration between health authorities, the national blood supplier, and local couriers for alternative arrangements. However, no difficulties in accessing blood products were reported.
4. DISCUSSION
Early in the COVID‐19 pandemic, in the face of an uncertain trajectory of infections in British Columbia and the threat of potential blood product inventory shortages, coordinated local and provincial approaches were implemented to help mitigate developing shortages and minimize transfusion laboratory‐associated infections. Simultaneously, contingency strategies were developed for deployment in the event of severe blood shortages. No shortages have been reported from members of the British Columbia provincial EBMC during the pandemic to date, and transfusion rates generally decreased in the early stages of the pandemic, with a subsequent gradual return to prepandemic levels over several weeks. Relative to the same period in 2019, RBC transfusion rates were consistently lower in 2020 provincially, while changes in platelet transfusion rates varied significantly (often stable or in some cases increased). Internationally, similar trends in blood product supply and demand during early phases of the pandemic have been described. Pagano et al 8 observed a sharp decline in inpatient transfusions in a hospital in Washington, while outpatient transfusions remained relatively stable. Similarly, Grandone et al 10 in southern Italy reported early reductions in RBC and plasma inpatient transfusions, which began to rebound by five weeks, while platelet transfusion rates remained stable. Groups in Illinois and Saudi Arabia have described similar observations. 6 , 9
Previous published discussions of essential transfusion considerations during a pandemic have emphasized the importance of a multifaceted response, including the need for clear communication between all groups involved and mitigation or management of supply shortages. 2 The response of transfusion medicine services to the threat to the blood supply posed by COVID‐19 in our and other provincial health authorities in British Columbia exemplifies a multicenter collaborative approach to blood management in a time of crisis, including strengthening of utilization management efforts; active engagement with a diverse group of stakeholders to promote best transfusion practices and ensure that clinical needs are met; and rigorous infection control measures in the laboratory and associated clinical sectors. At the time of writing, new daily cases of COVID‐19 have gradually increased in British Columbia; national blood inventory levels are adequate; and resumption of elective procedures is ongoing across the province. Although active cases are currently on the rise and a “second wave” of infections is underway, with potentially unpredictable impacts on the national supply of and demand for blood products, we anticipate these early efforts and ongoing collaboration to facilitate early responses to a rapidly evolving situation.
CONFLICT OF INTEREST
E.M., R.J.G., K.M.M., B.B., R.C., V.M., D.M., R.O., L.S., J.T., and M.W. have no conflicts of interest to declare. A.W.S. is a consultant for Octapharma Canada, has participated in an advisory board for CSL Behring, and has received an unrestricted educational grant from Hemerus Medical, LLC.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors recognize the hard work from all of the members of the BC EBMC who continue to guide the province through the pandemic, including Ministry of Health representation (Wendy Vowles, Katherine Leong, and Jennifer Brooke), the British Columbia PBCO (Brenda Jackson, Aimee Beauchamp, Elena Tavaszi, Ursula Maeser, and Pam Danesin), Canadian Blood Services British Columbia & Yukon (Dr. Tanya Petraszko, Dr. Mark Bigham, Judy Hrytzak, Irena Gordon, Ed Yee, Cynthia Calinisan, Leanna Vinogradov, David Bregani, and James Pattulo), Fraser Health Authority (Dr. Matthew Yan and Darlene Mueller), Interior Health Authority (Dr. Jason Doyle, Dr. Nick Sunderland, Maureen Wyatt, Katie Gabanowicz, Kelly Bradley, and Barbara Zielke), Northern Health Authority (Christorina Taruc and Julius Valido), Provincial Health Services Authority (Dr. Kate Chipperfield, Dr. Nick Au, Dr. Suzanne Vercauteren, Clare OʼReilly, and Graham Parke), Vancouver Coastal Health Authority (Dr. Kristine Roland, Margaret Roche, Shelley Feenstra, and Lawrence Sham), Providence Health Care (Dr. Hamish Nicolson, Dr. Mohammad Bahmanyar, Dr. Karen Dallas, Tina Jacobucci, Colleen Chan, and Crystal Brunk), Vancouver Island Health Authority (Dr. Zohra Daw, Dr. Wei Xu, Dr. Annemarie de Koker, Sharon Sutherland, Melissa Dougherty), and Yukon Territory (Chad Milford and Gregory Shaw). Finally, we would like to thank the tireless work of the medical laboratory technologists throughout British Columbia for their resilience and professionalism during these challenging times.
McGinnis E, Guo RJ, Marcon KM, et al. Adaptations of transfusion systems to the COVID‐19 pandemic in British Columbia, Canada: Early experiences of a large tertiary care center and survey of provincial activities. Transfusion. 2021;61:1102–1111. 10.1111/trf.16265
REFERENCES
- 1. World Health Organization . WHO Director‐General's opening remarks at the media briefing on COVID‐19 ‐ 11 March 2020, 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar]
- 2. Teo D. Blood supply management during an influenza pandemic. ISBT Sci Ser. 2009;4:293–298. [Google Scholar]
- 3. Lee C. Impact of severe acute respiratory syndrome on blood services and blood in Hong Kong in 2003. Transfus Med. 2020;30:169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shan H, Zhang P. Viral attacks on the blood supply: The impact of severe acute respiratory syndrome in Beijing. Transfusion. 2004;44:467–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar S, Azim D, Nasim S, Hashmi S. Dwindling blood reserves: An ominous downside of COVID‐19 pandemic. Transfus Apher Sci. 2020;59(5):102818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gniadek T, Mallek J, Wright G, et al. Expansion of hospital‐based blood collections in the face of COVID‐19 associated national blood shortage. Transfusion. 2020;60:1470–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Han W, Pan L, et al. Impact of COVID‐19 on blood centres in Zhejiang province China. Vox Sang. 2020;115(6):502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pagano MB, Hess JR, Tsang HC, et al. Prepare to adapt: Blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID‐19) pandemic affecting Washington State. Transfusion. 2020;60:908–911. [DOI] [PubMed] [Google Scholar]
- 9. Yahia AIO. Management of blood supply and demand during the COVID‐19 pandemic in King Abdullah Hospital. Bisha, Saudi Arabia: Transfusion and Apheresis Science, 2020. [In press, corrected proof]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grandone E, Mastroianno M, Caroli A, Ostuni A. Blood supply and transfusion support in southern Italy: Findings during the first four weeks of the SARS‐CoV‐2 pandemic. Blood Transfus. 2020;18:230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Advisory Committee on Blood and Blood Products and Canadian Blood Services . The national plan for management of shortages of labile blood components. 2020. https://www.nacblood.ca/resources/shortages-plan/index.html [Google Scholar]
- 12. National Advisory Committee on Blood and Blood Products . Emergency framework for rationing of blood for massively bleeding patients during a red phase of a blood shortage. 2012. https://nacblood.ca/resources/shortages-plan/emergency-framework-final.pdf [Google Scholar]
- 13. Weeks C, Woo A, Kirkup K. Canadaʼs third likely case of coronavirus reported in B.C. The Globe and Mail, 28 Jan 2020. https://www.theglobeandmail.com/canada/article-bc-reports-first-presumptive-case-of-coronavirus-man-recovering-at/
- 14. Woo A, Grant K. B.C. records first‐known community transmission case of coronavirus. The Globe and Mail, 5 Mar 2020. https://www.theglobeandmail.com/canada/article-bc-records-first-known-community-transmission-case-of-coronavirus/
- 15. Grant K, Woo A, Weeks C BC records Canadaʼs first coronavirus death The Globe and Mail: @globeandmail, 9 Mar 2020. https://www.theglobeandmail.com/canada/article-bc-records-canadas-first-coronavirus-death/
- 16. British Columbia Public Safety and Solicitor General . Province declares state of emergency to support COVID‐19 response. BC Gov News, 18 Mar 2020. https://news.gov.bc.ca/releases/2020PSSG0017-000511
- 17. National Emergency Blood Management Committee . National inventory advisory: Green phase advisory. 17 Mar 2020. https://www.blood.ca/sites/default/files/Green%20Advisory_2020_03_17.pdf
- 18. Nahirniak S. Allocation of red cells during a blood shortage. Blood Matters Conference, 6 Nov 2015.
- 19. Tron A, Collins A, Cserti‐Gazdewich C, et al. A prospective interventional study of blood bank technologist screening of red blood cell orders. AABB Annual Meeting, 3–5 Oct 2020.
- 20. van Doremalen N, Bushmaker T, Morris D, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382:1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leung W. Canadian Blood Services sees “dramatic” surge in donations after making public appeal. The Globe and Mail, 26 Mar 2020.
- 22. Dunham J. PM Trudeau urges Canadians to donate blood during COVID‐19 pandemic. CTV News, 19 Mar 2020.
- 23. Stueck W. Hospitals postpone surgeries, treatments to make space for potential surge in coronavirus patients. The Globe and Mail, 27 Mar 2020.
- 24. Shore R. ER doctors worry people with serious health concerns are avoiding the hospital. Vancouver Sun, 21 Apr 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


