Abstract
The coronavirus disease 2019 (COVID‐19) pandemic has grown to be a global public health crisis with no safe and effective treatments available yet. Recent findings suggest that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the coronavirus pathogen that causes COVID‐19, could elicit a cytokine storm that drives edema, dysfunction of the airway exchange, and acute respiratory distress syndrome in the lung, followed by acute cardiac injury and thromboembolic events leading to multiorgan failure and death. Mesenchymal stem cells (MSCs), owing to their powerful immunomodulatory abilities, have the potential to attenuate the cytokine storm and have therefore been proposed as a potential therapeutic approach for which several clinical trials are underway. Given that intravenous infusion of MSCs results in a significant trapping in the lung, MSC therapy could directly mitigate inflammation, protect alveolar epithelial cells, and reverse lung dysfunction by normalizing the pulmonary microenvironment and preventing pulmonary fibrosis. In this review, we present an overview and perspectives of the SARS‐CoV‐2 induced inflammatory dysfunction and the potential of MSC immunomodulation for the prevention and treatment of COVID‐19 related pulmonary disease.
Keywords: coronavirus, COVID‐19, cytokine storm, immunomodulation, mesenchymal stem cells, SARS‐CoV‐2
Potential mechanism of MSC action in COVID‐19 infected patients. SARS‐CoV‐2 enters cells through receptor‐mediated endocytosis via interactions with cell surface protein angiotensin‐converting enzyme II (ACE2) receptor with the assistance of transmembrane protease serine 2 (TMPRSS2) protease, thus triggering a complex immune response involved in T cells, dendritic cells, natural killer cells and macrophages. Engineering MSCs with immunomodulatory molecules enhance the efficacy of homing to damaged tissues or cells and attenuate the cytokine storm, ultimately improving patients' outcome.
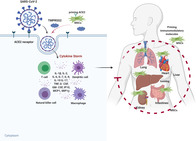
Significance statement.
This study provides the cutting edge knowledge on the emerging role of mesenchymal stem cell in our fight against COVID‐19, and will have implications on developing innovative therapies for COVID‐19 infected patients
1. COVID‐19 INDUCED DISEASE AND INFLAMMATORY DYSFUNCTION
Coronavirus disease 2019 (COVID‐19), a newly emerged respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has recently become a pandemic leading to innumerable deaths across the globe. SARS‐CoV‐2 is a novel beta‐coronavirus (a large RNA virus), that utilizes the viral S spike protein which interacts with human angiotensin‐converting enzyme 2 (ACE2) receptor to gain entry to cells. 1 , 2 The internalization of SARS‐CoV‐2 virus is assisted by transmembrane protease serine 2 (TMPRSS2) protease. 2 SARS‐CoV‐2 exhibits enhanced ACE2 receptor binding affinity and stability for fast dissemination among diverse cells including type II pneumocytes, bronchial cells, macrophages, monocytes, and enteric cells. 3 Consequently, the localization pattern of ACE2 receptors strongly relates to tissues affected by SARS‐CoV‐2 leading to symptoms and organ dysfunction. 1 In particular, SARS‐CoV‐2 exhibits a high replication rate and infectivity in the human oral pharynx and upper airway, due to high expression of ACE2 receptor in these areas. Most patients with COVID‐19 exhibit mild to moderate symptoms, and 73% infected patients are men. 4 Approximately 15% to 20% progress to severe pneumonia and about 5% eventually develop acute respiratory distress syndrome (ARDS), septic shock, and/or multiple organ failure. 1 , 4 Therefore, a comprehensive understanding of how these severe symptoms develop could reveal promising strategies for therapeutic intervention.
Severe COVID‐19 infection is characterized by pneumonia, lymphopenia, and a cytokine storm. 5 This latter phenomenon is characterized by an excessive inflammatory response, caused by a dysregulated immune system, which begins at a local site and then spills over to the systemic circulation, affecting multiple organs. Cytokine storm is not specific to SARS‐CoV‐2 infection; it has been also observed initially in the context of graft‐vs‐host disease, as well as other infectious diseases caused by cytomegalovirus, Epstein‐Barr virus, streptococcus, influenza virus, variola virus, and SARS‐CoV. 6 Moreover, it was also observed in noninfectious diseases and the corresponding therapeutic interventions. 7
SARS‐CoV‐2 infection induces the activation of both innate and adaptive immune responses, 5 , 8 leading to massive production of an array of inflammatory factors, resulting in uncontrolled ramping up of the immune response. 9 , 10 Most severe COVID‐19 patients exhibit elevated serum levels of pro‐inflammatory cytokines including interleukin (IL)‐1β, IL‐2, IL‐6, IL‐7, IL‐8, IL‐17, granulocyte‐colony stimulating factor, granulocyte‐macrophage colony‐stimulating factor, interferon γ‐induced protein 10 (IP10; CXCL10), monocyte chemoattractant protein 1 (MCP1, CCL2), macrophage inflammatory protein 1‐α (MIP1α), and tumor necrosis factor‐α (TNF‐α). 4 , 5 , 8 , 9 A retrospective study of 150 COVID‐19 patients indicated that elevated ferritin and IL‐6 due to hyperinflammation might be involved in mortality risk. 11 Substantially, high levels of cytokines may lead to cytokine release syndrome (CRS), causing shock and tissue damage in vital organs. 5 , 10 In particular, cytokine storm in COVID‐19 patients has been shown to aggravate acute cardiac injury comprising of myocarditis, acute heart failure, and cardiac arrest. 12
These markers of inflammation detected in the serum, are most likely the end result of a chain of events starting in the lung itself. This local immunological cascade is generally crucial to immunopathology of other severe infections affecting deep tissues of the respiratory tract, such as avian influenza virus or severe primary influenza virus infections. 13 , 14 Specifically, the cytokine storm in the lung is a hallmark of SARS‐CoV‐2 pathogenesis, resulting in extensive pulmonary pathology including increased permeability of lung endothelial and epithelial cells, interstitial edema, diffuse alveolar damage, dysfunction of air‐exchange, and ARDS (Figure 1). 5 , 15 , 16 Usually, CRS is initiated by macrophages, dendritic cells (DCs), natural killer (NK) cells and T cells, owing to pathogen‐associated molecular pattern activation. 17 Indeed, it has been reported that bronchoalveolar fluid from patients with severe COVID‐19 had high concentrations of chemokines CCL2 and CCL7, which are potent monocyte recruiters, thus suggesting a role for those cells in the pathophysiology of the disease. Notably, mononuclear phagocytes represented 80% of bronchoalveolar fluid cells in severe COVID‐19 cases, 60% in mild cases, and only 40% in healthy controls. In severe COVID‐19 patients, there was also depletion of tissue macrophages, an increase in inflammatory monocyte derived macrophages 18 , 19 and a general increment in the number of neutrophils and leukocytes. Moreover, the prominent lymphopenia, which develops in most COVID‐19 patients, indicates an impairment of immune system and further supports the evidence of innate immunity as initiator of the cytokine storm. 20 Nonetheless, adaptive immune cells, namely T lymphocytes, may drive inflammation at later disease stages, 21 thus secondary hemophagocytic lymphohistiocytosis may be another pathophysiological feature of notable attention. 9 , 21 However, while the general concept of an excessive or uncontrolled release of pro‐inflammatory cytokines is well known, there remains lack of good understanding of the molecular events that unleash the cytokine storm as well as its respective prevention and therapeutic strategies. 6
FIGURE 1.
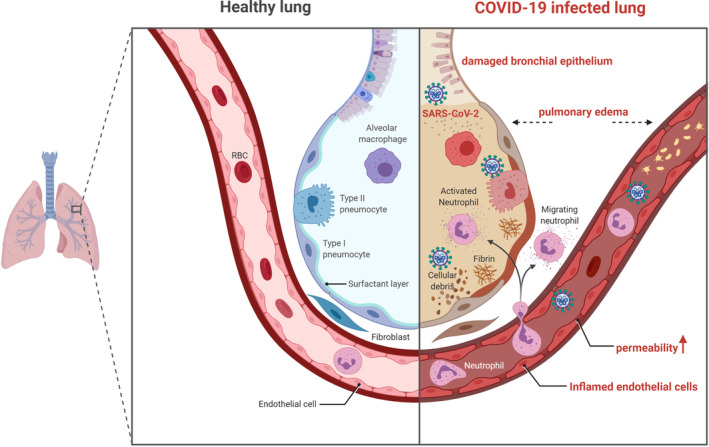
Schematic showing pathophysiological features of COVID‐19 infected lung. SARS‐CoV‐2 enters target cells and induces extensive pulmonary pathology including increased permeability of lung endothelial and epithelial cells, pulmonary edema, diffuse alveolar damage, and dysfunction of air‐exchange. Figure drawn with BioRender (https://biorender.com/). SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
It has been proposed that SARS‐CoV‐2 binds to the Toll‐like receptors (TLRs) leading to the synthesis and release of pro‐inflammatory IL‐1, IL‐6, and TNF‐α as main mediators of the inflammation pathway. 22 , 23 Indeed, after the binding to the TLR, myeloid differentiation primary response 88 (MyD88) or TIR domain‐containing adapter‐inducing interferon‐β (TRIF) activates tumor necrosis factor receptor associated factor 6 (TRAF6), which in turn stimulates caspase 1, inducing the cleavage of pro‐IL‐1 and the activation of the inflammasomes as well as tumor growth factor‐β‐activated kinase and I kappa B kinase (IKK). This cascade ultimately initiates the activation of nuclear factor‐kappa B in the nucleus. Subsequently, pro‐inflammatory cytokines, such as TNF‐α, IL‐6, and IL‐1β, initiate the inflammatory processes in the lung, giving rise to the classic COVID‐19 symptoms. 22 , 23 Moreover, in a proposed model based on SARS‐CoV‐1, viral protein encoded by ORF8b directly interacts with inflammasome NLRP3 (nucleotide‐binding domain leucine‐rich repeat and pyrin domain containing receptor 3), which activates the adaptor protein apoptosis‐associated speck‐like protein containing CARD (ASC) and caspases 4, 5, and 11. This leads to a disruption of the cell membrane and the release of the cell content to the extracellular space, causing local inflammation. 24 Furthermore, the binding of SARS‐CoV‐2 to the ACE2 on the cell surface impairs its enzymatic activity, thus resulting in increased levels of Angiotensin II (AngII) in the extracellular space which, in turns, leads to higher levels of TNF‐α and IL‐6 in the cell that upregulate NF‐κβ, ultimately activating the inflammasome. 23 Finally, recruitment of macrophages to the site of injury and phagocytosis of dead cells results in the release of ATP, which binds to the P2X purinoceptor 7 (P2RX7) giving another important contribution to the activation of the inflammasome. 25 Also, the increased calcium levels caused by the viral proteins results in lysosomal damage, thereby releasing cathepsins that, again, activate the inflammasome. 22
Several strategies to suppress the degree of immune storm have been attempted to treat critically ill patients with COVID‐19. In this respect, as serum IL‐6 plays an important role in CRS which correlates with respiratory failure, ARDS, elevated serum C‐reactive protein (CRP), and poor clinical outcomes, 26 , 27 , 28 tocilizumab, a IL‐6 receptor blocker, has been tested and shown to improve respiratory function in 21 patients with severe COVID‐19. 5 These encouraging results suggest that neutralizing monoclonal antibodies against IL‐6 or other pro‐inflammatory cytokines such as IL‐1 and their receptors may provide a novel strategy for counteracting the cytokine storm in COVID‐19 patients. A great deal of effort has been devoted to targeting the host response with a variety of anti‐inflammatory drugs and adjunct approaches, including corticosteroids, nonsteroidal anti‐inflammatory drugs, 29 neutralizing monoclonal antibodies such as Lenzilumab and Tocilizumab, 30 , 31 statins, 32 chloroquine and hydroxychloroquine, complement inhibitors, 33 and others. 34 However, these therapeutic approaches possess safety concerns due to their low specificity to lung immunopathology. 35
Despite multiple therapeutic strategies have been attempted, the mortality of severely ill COVID‐19 patients remains high and warrants an urgent need of identifying a better therapeutic strategy against the cytokine storm. The use of living cells as effectors offers intrinsic advantages from a pharmacokinetic and pharmacodynamic point of view, compared to the administration of conventional drugs. Cell therapies render the effect localized at the site where the cell resides, reducing the possibility of side effects at a distant site. Moreover, cells have the possibility to persist in the tissue, allowing a longer‐lasting effect. Furthermore, their mechanism of action is often multifaceted and involves different pathways simultaneously. 36 , 37 Related to this, cell‐based therapies can offer additional mechanisms to dampen lung inflammation by regulating the activation of specific cell types. For example, CD200R expression on alveolar macrophages helps in resolving lung inflammation during influenza virus infection by restraining macrophage activity. 38 Moreover, the production of anti‐inflammatory cytokines, mainly IL‐10 by macrophages and certain types of T (Th2 and regulatory T cells) and B cells, represents another mechanism involved in regulating pro‐inflammatory responses. 7 In this respect, mesenchymal stem cells (MSCs) demonstrate a remarkable immune‐modulatory capacity in different settings and represent an attractive option for the treatment of COVID‐19 related cytokine storm.
2. MSC MEDIATED IMMUNOMODULATION
In the last decade, cell‐based therapies have emerged as promising therapeutic options for many incurable diseases. Among different cell platforms, MSCs raised a particular interest due to easy sourcing and attractive features of plasticity, tropism for inflamed tissues, and a high immunomodulatory potential. 39 MSCs are characterized phenotypically by the expression of certain cell surface markers, including CD73, CD90, and CD105, and lack of expression of CD11b, CD14, CD34, CD45, CD79, or HLA‐DR, and functionally by the ability to differentiate into mesenchymal lineages. 40 Preclinical studies and clinical trials have provided compelling evidence supporting their immunomodulatory functions in the context of various conditions, including graft‐vs‐host disease, autoimmune diseases, inflammatory illnesses, myocardial infarction, lung injuries, liver cirrhosis, diabetes, and cancer. 41 , 42 , 43 MSCs modulate both innate and adaptive immunity, influencing a wide range of effectors, including T cells, B cells, macrophages, neutrophils, NK cells, and DCs (Figure 2). The mechanisms underlying anti‐inflammatory and immunomodulatory effects which ultimately lead to improvement of the aforementioned conditions are exerted either via cell‐to‐cell contact or paracrine activity, affecting recruitment, differentiation, proliferation, activation, and survival of effector cells or promoting their polarization into anti‐inflammatory subtypes. 43 , 44 Experimental evidence suggests that cell contact is involved in the control of immune cells survival through Fas/FasL signaling, their blockade through the stimulation of programmed cell death protein 1 (PD‐1)/ programmed‐death ligand 1 (PD‐L1) axis, the suppression of DC generation by activation of the Notch pathway, and abrogation of NK cells functions by the activation of the inhibitory TLR4 receptor. 43 , 44 On the other side, MSCs exert paracrine action by releasing biologically active molecules, and shedding extracellular vesicles, or exosomes, that contain a broad spectrum of cytokines, chemokines, and growth factors. 43 In a preclinical setup, MSC‐derived exosomes have demonstrated aptitude as an acellular alternative to cell‐based therapy, against ARDS. 45 Among these, the main immunomodulatory players are prostaglandin E2 (PGE‐2), indoleamine 2,3‐dioxygenase (IDO), TNF, nitric oxide (NO), IL‐1Rα, HLA‐G, and IL‐10. 43 , 46 , 47 Finally, MSCs can be manipulated ex vivo to integrate and empower their features. In particular, it has been demonstrated experimentally that MSCs preconditioned by hypoxia, oxidative stress, heat shock, nutrient starvation, or genetic manipulation with immunomodulatory molecules have the ability to increase their survival and potency, and thereby enhancing the therapeutic efficacy. 48 , 49 Although MSCs possess a marked immune‐regulatory effect against inflammatory and autoimmune disorders, it is widely acknowledged that they are not immunosuppressive in nature. Instead, MSCs may have different immunoregulatory effects depending on the immune milieu and disease setting. For example, while MSCs are able to suppress compromising antiviral responses needed for disease control, 50 , 51 they can also exert differential effects on alloantigen and virus specific T cells that retain the ability to proliferate and kill the virus‐infected cells. 52 Moreover, an increased risk to (opportunistic) infections has never been reported, as described in a recent meta‐analysis on almost 2700 patients treated by MSC for different indications. 53
FIGURE 2.
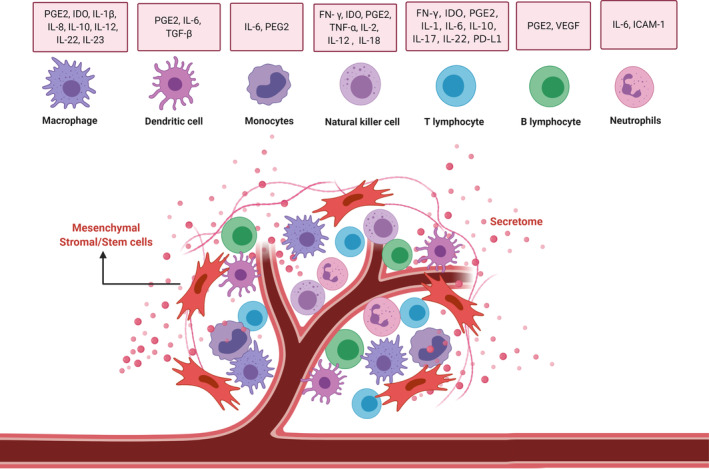
Immunomodulatory interactions between mesenchymal stem cells (MSCs) and immune cells. MSCs exert immunomodulatory functions mainly via interactions with immune cells (including T cells, B cells, NK cells, macrophages, monocytes, dendritic cells, and neutrophils) through cell‐to‐cell contacts and paracrine activity (mainly by secretome). Figure drawn with BioRender (https://biorender.com/). ICAM‐1, intercellular adhesion molecule‐1; IDO, indoleamine‐pyrrole 2,3‐dioxygenase; IFN, interferon; IL, interleukin; NK, natural killer; PD‐L1, programmed death ligand 1; PD‐L2, programmed death ligand 2; PGE2, prostaglandin E2; TGF‐β, transforming growth factor‐β; TNF‐α, tumor necrosis factor‐α; VEGF, vascular endothelial growth factor
A number of studies have demonstrated the preliminary safety and efficacy of both MSCs and exosomes in alleviating comorbidities associated with COVID‐19. 54 , 55 , 56 MSC‐shed exosomes, owing to their ability to endogenously repair and decrease the inflammatory reactions involved in the morbidity and mortality of COVID‐19, present a promising potential to be used following clinical evaluation. Thus, MSCs represent a valuable and versatile cell platform which is able to modulate the immune response at different levels. However, the interplay between MSC and various elements of the immune system is not fully understood and may result in controversial outcomes depending on MSC sources, target cells, and the microenvironment. 43 , 44 A further advantage of applying MSCs is the possibility to use an allogeneic source, thus circumventing the time lag needed to produce a clinical grade product. Most importantly, the low levels of expression of HLA class I molecules and lack of HLA class II and costimulatory molecules 57 enable their administration across HLA barriers without preventive immune‐ablative treatment. 58 However, when sought, measurable humoral alloimmunization in human subjects receiving mismatched MSCs has been detected, 59 although it does not seem to affect their therapeutic efficacy. In this regard, it should be pointed out that MSCs are now considered immune‐evasive and not immune‐privileged as previously thought. 60 Notwithstanding, after more than a decade of clinical application of MSCs, an overwhelming safety profile has been repeatedly documented, 53 including the absence of malignant transformation since they do not engraft host tissues. Indeed, their mechanism of action does not depend on their differentiation into specific end‐organ cells, but rather on the creation of an appropriate microenvironment, called “quasi niche” 61 where both resident and immune cells are aided in re‐establishing tissue homeostasis. 62 Finally, it was recently found that human MSCs are SARS‐CoV‐2 infection resistant, likely due to the low ACE2 and TMPRSS2 expression on their surface, and that they keep their IDO‐1 production capacity in the presence of SARS‐CoV‐2. 63 This represents a unique strength when evaluating new treatment strategies in the specific clinical setting of COVID‐19. All these properties, together with the absence of ethical controversies, make MSCs very attractive for cell‐based therapy.
3. MSCs CAN ATTENUATE COVID‐19 RELATED CYTOKINE STORM AND COAGULOPATHY
One of the most harmful consequences of SARS‐CoV‐2 infection is the excessive and aberrant host immune response, accompanied by a cytokine storm and the subsequent ARDS, resulting in multiple organ failure and death. By virtue of their powerful immunomodulatory ability, MSCs offer a promising innovative strategy for attenuating the cytokine storm and ultimately improving patients' outcome (Figure 3). 10 After intravenous infusion, MSCs get trapped in the inflamed lung and exert immunomodulatory function via direct interaction with respiratory epithelial cells and immune cells, or release of a wide variety of soluble mediators, ultimately reducing the inflammation and protecting the alveolar epithelial cells. 64 , 65 , 66 As discussed, it is likely that the local immunological events in the deep tissues of the lungs are crucial for the initiation of the cytokine storm and thus a localized treatment might be, in theory more effective to stop the cascade. 18 , 19 In this regard, it should be emphasized that MSC administration may be performed by using different routes: intravenously with access from a peripheral or central vein, intra‐arterial depending on the target organ, but also directly in the damaged tissue as in fistulizing Crohn's disease where local injections have proved successful in inducing closure of fistula tracks refractory to standard treatment. 67 Additionally, delivery routes such as intrathecal, intra‐articular, and intradermal have been shown feasible in patients suffering from amyotrophic lateral sclerosis, osteoarthritis, and scleroderma, respectively. 68 , 69 , 70 It is conceivable therefore, that also in the case of COVID‐19 patients, intratracheal administration might work better. Nonetheless, all the clinical trials so far use intravenous route of MSC administration (Table 1) and feasibility and effectiveness of intratracheal/bronchial administration of MSCs is unknown. To date, preclinical studies have provided robust evidence for the effect of MSCs on treating lung injury and ARDS, even though in vivo models still fail to elucidate their precise mechanism of action. A common observation is that MSC exposure results in a decline of pro‐inflammatory cytokines including IL‐1α, IL‐1β, IL‐6, IFN‐γ, and TNF‐α and an increase in anti‐inflammatory cytokines such as IL‐4, IL‐5, and IL‐10. The restored, noninflamed microenvironment aids in the repair of pulmonary epithelial cell damage and promotes alveolar fluid clearance, thus restoring lung function with enhanced alveolar air‐space volume, reduced alveolar thickening, and decreased markers of inflammation. 5 , 40 Noticeably, ex vivo manipulation of MSCs can result in improved function. In particular, preconditioning MSC with ARDS serum has been shown to significantly increase the expression of IL‐10, IL‐1Rα, and reduce the expression of pro‐inflammatory mediators. 71 In addition, adipose tissue‐derived MSCs transduced to express soluble IL‐1 receptor‐like‐1 or bone marrow‐derived MSCs expressing ACE‐2 significantly reduced lung injury index and levels of pro‐inflammatory cytokines in a murine model of non‐SARS‐CoV‐2 acute lung injury. 72 , 73 Interestingly, apart from the infusion of viable cells, MSC secretome is emerging as a promising cell‐free therapeutic strategy for the treatment of acute and chronic lung diseases. 45 , 74 , 75 , 76 , 77 A growing number of reports account for the use of MSC derivatives, such as conditioned medium or extracellular vesicles, demonstrating some initial positive effects which, if confirmed in more extensive studies, might further extend the versatility of MSCs to treat inflammatory lung conditions (Table 2). 45
FIGURE 3.
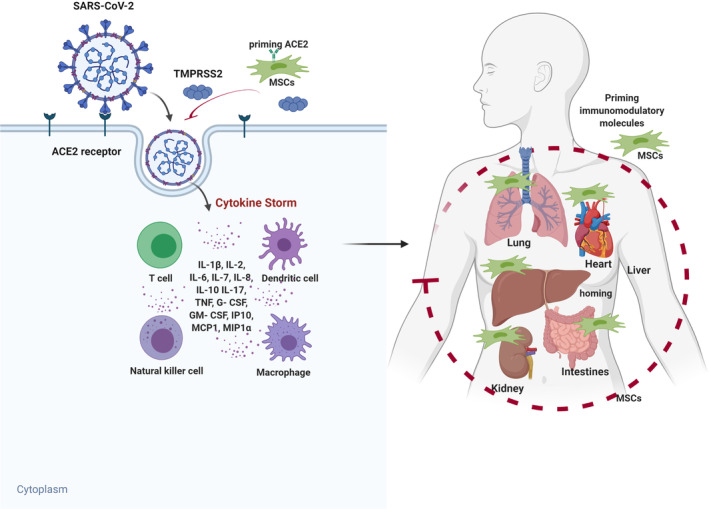
Potential mechanism of MSC action in COVID‐19 infected patients. SARS‐CoV‐2 enters cells through receptor‐mediated endocytosis via interactions with cell surface protein angiotensin‐converting enzyme II (ACE2) receptor with the assistance of TMPRSS2 protease, thus triggering a complex immune response involved in T cells, dendritic cells, NK cells, and macrophages. These cells release high amounts of cytokines and chemokines responsible for the cytokine storm, leading to symptoms and major organ dysfunction. Engineering MSCs with immunomodulatory molecules enhance the efficacy of homing to damaged tissues or cells and attenuate the cytokine storm, ultimately improving patients' outcome. Figure drawn with BioRender (https://biorender.com/). G‐CSF, granulocyte‐colony stimulating factor; GM‐CSF, granulocyte‐ macrophage colony‐stimulating factor; IL, interleukin; IP10, interferon gamma‐induced protein 10; MCP1, monocyte chemoattractant protein 1; MIP1α, macrophage inflammatory protein 1‐alpha.; MSCs, mesenchymal stem cells; NK, natural killer; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TNF, tumor necrosis factor
TABLE 1.
Active MSC clinical trials for COVID‐19 as listed at www.ClinicalTrials.gov (accessed on September 30, 2020)
| ClinicalTrials.gov identifier | Study title | Stem cell type | Dosing regimen | Injection route | MSC status | Clinical phase | Status |
|---|---|---|---|---|---|---|---|
| NCT04313322 | Treatment of COVID‐19 patients using Wharton's jelly‐mesenchymal stem cells | Wharton's jelly MSC | 1 × 106 cells/kg | IV | Native | I | Recruiting |
| NCT04428801 | Autologous adipose‐derived stem cells (AdMSCs) for COVID‐19 | Adipose MSC | 200 × 106 | IV | Native | II | Not yet recruiting |
| NCT04336254 | Safety and efficacy study of allogeneic human dental pulp mesenchymal stem cells to treat severe COVID‐19 patients | Dental pulp MSC | 3 × 107 | IV | Native | I/ II | Recruiting |
| NCT04429763 | Safety and efficacy of mesenchymal stem cells in the management of severe COVID‐19 pneumonia | Bone marrow MSC | 1 × 106 cells/kg | IV | Native | II | Not yet recruiting |
| NCT04416139 | Mesenchymal stem cell for acute respiratory distress syndrome due to COVID‐19 | Bone marrow MSC | 1 × 106 cells/ kg | IV | Native | II | Recruiting |
| NCT04315987 | NestaCell mesenchymal stem cell to treat patients with severe COVID‐19 pneumonia | NestaCell | 20 × 106 cells | IV | Modified | II | Not yet recruiting |
| NCT04348435 | A randomized, double‐blind, placebo‐controlled clinical trial to determine the safety and efficacy of hope biosciences allogeneic mesenchymal stem cell therapy (HB‐adMSCs) to provide protection against COVID‐19 | Adipose MSC | 200 × 106 cells | IV | Modified | II | Enrolling by invitation |
| NCT04366323 | Clinical Trial to assess the safety and efficacy of intravenous administration of allogeneic adult mesenchymal stem cells of expanded adipose tissue in patients with severe pneumonia due to COVID‐19 | Bone marrow MSC | 80 × 106 cells | IV | Native | II | Recruiting |
| NCT04349631 | A Clinical Trial to determine the safety and efficacy of hope biosciences autologous mesenchymal stem cell therapy (HB‐adMSCs) to provide protection against COVID‐19 | Adipose MSC | Not mentioned | IV | Native | II | Enrolling by invitation |
| NCT04302519 | Novel Coronavirus induced severe pneumonia treated by dental pulp mesenchymal stem cells | Dental pulp MSC | 1 × 106 cells /kg | IV | Native | I | Not yet recruiting |
| NCT04252118 | Mesenchymal stem cell treatment for pneumonia patients infected with COVID‐19 | Bone marrow MSC | 3 × 107 cells | IV | Native | I | Recruiting |
| NCT04346368 | Bone marrow‐derived mesenchymal stem cell treatment for severe patients with coronavirus disease 2019 (COVID‐19) | Bone marrow MSC | 1 × 106 cells/kg | IV | Native | I/ II | Not yet recruiting |
| NCT04288102 | Treatment with human umbilical cord‐derived mesenchymal stem cells for severe corona virus disease 2019 (COVID‐19) | Umbilical cord MSC | 4 × 107 cells | IV | Native | II | Active, not recruiting |
| NCT04273646 | Study of human umbilical cord mesenchymal stem cells in the treatment of severe COVID‐19 | Umbilical cord MSC | 5 × 106 cells/kg | IV | Native | Not applicable | Not yet recruiting |
| NCT04437823 | Efficacy of intravenous infusions of stem cells in the treatment of COVID‐19 patients | Bone marrow MSC | 5 × 105 cells/ kg | IV | Native | II | Recruiting |
| NCT04339660 | Clinical research of human mesenchymal stem cells in the treatment of COVID‐19 pneumonia | Umbilical cord MSCs | 1 × 106 cells/kg | IV | Native | I/ II | Recruiting |
| NCT04382547 | Treatment of COVID‐19 associated pneumonia with allogenic pooled olfactory mucosa‐derived mesenchymal stem cells | Olfactory mucosa MSC | Not mentioned | IV | Native | I/ II | Enrolling by invitation |
| NCT04371601 | Safety and effectiveness of mesenchymal stem cells in the treatment of pneumonia of coronavirus disease 2019 | Bone marrow MSC | 1 × 106 cells /kg | IV | Native | I | Active, not recruiting |
| NCT04366063 | Mesenchymal stem cell therapy for SARS‐CoV‐2‐related acute respiratory distress syndrome | Bone marrow MSC | 100 × 106 MSCs and extracellular vesicles (EVs) | IV | Native | II/III | Recruiting |
| NCT04355728 | Use of UC‐MSCs for COVID‐19 patients | Umbilical cord MSC | 100 × 106 cells | IV | Native | I/ II | Completed |
| NCT04362189 | Efficacy and safety study of allogeneic HB‐adMSCs for the treatment of COVID‐19 | Adipose MSC | 100 × 106 cells | IV | Native | II | Not yet recruiting |
| NCT04392778 | Clinical use of stem cells for the treatment of COVID‐19 | Adipose MSC | 3 × 106 cells/kg | IV | Native | I/ II | Recruiting |
| NCT04299152 | Stem cell educator therapy treat the viral inflammation in COVID‐19 | Cord blood stem cells | Not mentioned | IV | Native | II | Not yet recruiting |
| NCT04390152 | Safety and efficacy of intravenous Wharton's jelly derived mesenchymal stem cells in acute respiratory distress syndrome due to COVID‐19 | Wharton's jelly MSC | 50 × 106 | IV | Native | I/ II | Not yet recruiting |
| NCT04293692 | Therapy for pneumonia patients infected by 2019 novel coronavirus | Bone marrow MSC | 0.5 × 106/kg | IV | Native | Not applicable | Withdrawn |
| NCT04348461 | Battle against COVID‐19 using mesenchymal stromal cells | Bone marrow MSC | 1.5 × 106/kg | IV | Native | II | Not yet recruiting |
| NCT04331613 | Safety and Efficacy of CAStem for severe COVID‐19 associated with/without ARDS | Embryonic stem cells | 3, 5 or 10 × 106 cells/kg | IV | Native | I/ II | Recruiting |
| NCT04377334 | MSCs in inflammation‐resolution programs of coronavirus disease 2019 (COVID‐19) induced ARDS | Bone marrow MSC | Not mentioned | IV | Native | II | Not yet recruiting |
| NCT04371393 | MSCs in COVID‐19 ARDS | Bone marrow MSC | 2 × 106/kg | IV | Native | III | Recruiting |
| NCT04341610 | ASC therapy for patients with severe respiratory COVID‐19 | Adipose MSC | 100 × 106 | IV | Native | I/II | Withdrawn |
| NCT04390139 | Efficacy and safety evaluation of mesenchymal stem cells for the treatment of patients with respiratory distress due to COVID‐19 | Wharton‐Jelly MSC | 1 × 106 cells/Kg | IV | Native | I/ II | Recruiting |
| NCT04400032 | Cellular immuno‐therapy for COVID‐19 acute respiratory distress syndrome – Vanguard | Bone marrow MSC | 75 × 106 | IV | Native | II | Not yet recruiting |
| NCT04398303 | ACT‐20 in patients with severe COVID‐19 pneumonia | Umbilical cord MSC | 1 × 106 cells/kg | IV | Native | I/ II | Not yet recruiting |
| NCT04397796 | Study of the safety of therapeutic Tx with immunomodulatory MSC in adults with COVID‐19 infection requiring mechanical ventilation | Bone marrow MSC | Not mentioned | IV | Modified | I | Not yet recruiting |
| NCT04393415 | Using PRP and cord blood in treatment of COVID‐19 | Umbilical cord MSC | Not mentioned | IV | Native | Not applicable | Recruiting |
| NCT03042143 | Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) (COVID‐19) | Umbilical cord MSC | 400 × 106 | IV | Native | I/ II | Recruiting |
| NCT04345601 | Mesenchymal stromal cells for the treatment of SARS‐CoV‐2 induced acute respiratory failure (COVID‐19 Disease) | Bone marrow MSC | 2 × 106 cells/kg | IV | Native | I | Not yet recruiting |
| NCT04361942 | Treatment of severe COVID‐19 pneumonia with allogeneic mesenchymal stromal cells (COVID_MSV) | Bone marrow MSC | 1 × 106/kg | IV | Modified | II | Recruiting |
| NCT04269525 | Umbilical cord (UC)‐derived mesenchymal stem cells (MSCs) treatment for the 2019‐novel coronavirus pneumonia | Umbilical cord MSC | 3.3 × 1067 | IV | Native | II | Recruiting |
| NCT04333368 | Cell therapy using umbilical cord‐derived mesenchymal stromal cells in SARS‐CoV‐2‐related ARDS | Umbilical cord MSC | 1 × 106/kg | IV | Native | I/ II | Not yet recruiting |
| NCT04389450 | Double‐blind, multicenter, study to evaluate the efficacy of PLX PAD for the treatment of COVID‐19 | Placental MSC | Not mentioned | IV | Modified | II | Recruiting |
| NCT04367077 | MultiStem administration for COVID‐19 induced ARDS (MACoVIA) | Multistem MSC | Not mentioned | IV | Modified | II/III | Recruiting |
| NCT04276987 | A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia | MSCs‐derived exosomes | 2 × 108 nano vesicles | IV | Native | I | Completed |
Abbreviations: ARDS, Acute respiratory distress syndrome; ASC, Apoptosis‐associated speck‐like protein containing a CARD; MSC, mesenchymal stem cells; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
TABLE 2.
Studies testing the effects of MSC derivatives on inflammation
| Disease models | Origin of MSCs | MSC derivatives | Inflammatory mediators | References |
|---|---|---|---|---|
| Acute respiratory distress syndrome (ARDS) | BM‐MSCs | EVs | Increased expression of interleukin (IL)‐10 | 119 |
| Acute lung injury (ALI) | BM‐MSCs | EVs | Increased IL‐10 secretion, decreased pro‐inflammatory cytokine‐TNF‐α | 120 |
| ARDS and ALI | BM‐MSCs | Conditioned medium, EVs | Decreased secretion of TNF‐α and IL‐8 | 121 |
| ALI | BM‐MSCs | EVs | Decreased TNF‐α and increased IL‐10 | 122 |
| Lung ischemia | BM‐MSCs | Exosomes | Downregulated IL‐8, IL‐1 β, IL‐17, TNF‐α, and IL‐6 | 123 |
| ALI | BM‐MSCs | Exosomes overexpressing miR 30b‐3p | Decreased expression of serum amyloid A3 (SAA3) | 124 |
| ARDS | BM‐MSCs | Culture medium | Increased expression of interleukin (IL)‐10 and IL‐1 receptor antagonist | 103 |
| Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) | Engineered A‐MSCs overexpressing soluble IL‐1 receptor‐like‐1 | Culture medium | Increased IL‐10 expression, prevented IL‐33, TLR‐4, IL‐1β, and IFN‐γ induction, decreased pro‐inflammatory cytokines (TNF‐α, IL‐6, and macrophage inflammatory protein 2) | 65 |
| Cecal ligation and puncture model of sepsis | WJ‐MSCs | Culture medium | Decreased expression of nuclear factor κB and of cytokines; increased expression of eNOS, vascular endothelial growth factor | 66 |
| Severe COVID‐19 pneumonia) | MSCs | Clinical grade MSCs | Decreased TNF‐α and increased IL‐10 | 54 |
| ALI | Modified MSCs with ACE2 | Culture medium | Downregulated ICAM‐1, VCAM‐1, TNF‐α, and IL‐6 | 102 |
| ALI | Modified UC‐MSCs with ACE2 | Culture medium | Decreased expression of MDA, GSSG, TNF‐α, IFN‐γ, TGF‐β, IL‐1, IL‐2, IL‐6, collagen type 1 mRNA, MMPs and TIMPs as well as hydroxyproline concentration, and upregulation of SOD, GSH and ACE2 and IL‐10. | 103 |
Abbreviations: A‐MSCs, adipose MSC; BM‐MSC, bone marrow MSC; eNOS, endothelial nitric oxide synthase; EVs, extracellular vesicles; MSC, mesenchymal stromal cells; UC‐MSC, umbilical cord MSC; WJ‐MSC, Wartons jelly MSC.
The encouraging preclinical data indicating the suitability of MSCs in controlling inflammatory responses in the lungs as well as in other organs have led to their application in early stage clinical trials. These trials have confirmed the ability of MSC to improve disease‐associated parameters in ARDS subjects 78 with anti‐inflammatory and antiapoptotic effects. 78 Furthermore, MSC administration protects and reduces morphological and multiple organ dysfunction. 78 , 79 A phase IIa study including 60 patients with moderate to severe ARDS showed intravenous infusion of MSC was safe but did not improve the rate of 28‐day mortality (30% vs 15%), suggesting that studies with larger patient cohorts are necessary. 80 A recently published paper reported a clinical study for transplantation of allogeneic menstrual‐blood‐derived MSCs for treating influenza H7N9‐induced ARDS in 17 patients. 28 Results showed that MSC transplantation significantly lowered the mortality compared to control group (17.6% vs 54.5%) without harmful effects. 5 , 28 Given that H7N9 and COVID‐19 share similar complications (including ARDS, lung failure, and multiorgan dysfunction), MSCs offer an innovative therapy for treating COVID‐19. 4 , 28 While no firm conclusions may be drawn on the efficacy and safety of MSC administration in ARDS, the encouraging results of these trials have paved the way for using MSC‐based immunomodulation treatment as a suitable therapeutic approach for COVID‐19.
The endothelial damage and the activation of blood coagulation are emerging as common phenomena in critical COVID‐19 patients. The virus appears to bind to the endothelial cells causing damage especially in the microcirculation leading to platelet aggregation associated with organ failure. 81 , 82 In the context of MSC‐based therapy, this aspect needs careful consideration. Indeed, MSCs isolated from different sources largely differ in their hemocompatibility depending on the expression of tissue factor TF/CD142, a key trigger of the extrinsic coagulation pathway, and receptors for complement activation products (eg, C3a, C5a). 83 , 84 , 85 More in depth, compared to Bone Marrow MSCs (BM‐MSCs), AT‐ and PT‐derived products demonstrate higher TF expression and reduced hemocompatibility, with substantial donor variation and culture passage‐dependent TF induction. 83 , 86 Specifically, a thromboembolic risk becomes evident when using MSCs into systemically activated/pro‐inflammatory patients who do not receive anticoagulation treatment at the time of cell infusion. 87 Moreover, freeze‐thawed MSCs trigger a strong instant blood‐mediated inflammatory reaction when washed with buffer containing human blood type AB plasma instead of human serum albumin. 88 It is strongly recommended to use regular low‐dose anticoagulants and, possibly, to perform standardized hemocompatibility testing in those MSC products that are intended for intravascular delivery. Nevertheless, the largest meta‐analysis on MSC in clinical settings did not report increased thromboembolic risk. 53 On the contrary, data demonstrated that MSCs have the ability to reduce platelet adhesion and aggregation in a rat model of a vascular graft, which depended on cell‐surface heparan sulfate proteoglycans. 89 In addition, MSC can inhibit platelet activation and aggregation through CD73 ectonucleotidase activity, 90 one of key MSC membrane markers. Hence, MSCs might be able to play a crucial role in dampening both inflammation and hypercoagulopathy status during SARS‐CoV‐2 related severe pneumonia.
4. MSC MEDIATED EPITHELIAL AND VASCULAR PROTECTION
Recent studies carried out in both in vitro and in vivo models of influenza respiratory infections have shown the protective effects of MSCs on the alveolar‐capillary barrier, the functional entity responsible for gas exchange that undergoes disruption during pathogen‐induced ARDS. 91 MSCs were able to improve the dysregulated alveolar fluid clearance and protein permeability induced by H5N1 and H7N9 influenza viruses, by releasing soluble mediators that upregulated sodium and chloride transporters. 92 A single intravenous infusion of BM‐derived MSCs in H9N2‐induced lung injury mice led to reduction of mortality, lung edema, histologic injury, and chemokines and cytokines levels in bronchoalveolar lavage (BALF) and serum. 93 Additionally, umbilical cord mesenchymal stem cells (UC‐MSCs) were more effective than human BM‐derived MSCs at restoring impaired alveolar fluid clearance and permeability in vitro airway epithelial cell models. 94 These effects seemed largely mediated by secretion of an array of bioactive molecules, such as angiopoietin‐1 (Ang‐1), keratinocyte growth factor, and hepatocyte growth factor, that act as antiapoptotic, pro‐regenerative, and proangiogenic factors. 95
5. CLINICAL EVIDENCE OF MSCs THERAPY FOR COVID‐19 INDUCED INFLAMMATORY DYSFUNCTION
Currently, the www.ClinicalTrials.gov database, as accessed on September 30, 2020, reports 2251 active COVID‐19 clinical studies in different phases of clinical testing and recruitment. Of these, 44 studies involve the use of MSCs for the management of COVID‐19 (Table 1). A multicenter phase‐III study is currently being conducted in the United States using a MSC industrial preparation, Remestemcel‐L plus standard of care to treat ARDS associated with SARS‐CoV2 infection (NCT04371393). This study is based on preclinical and clinical evidence indicating that MSCs migrate to the lung and respond to the pro‐inflammatory lung environment by releasing anti‐inflammatory factors reducing the production of pro‐inflammatory cytokines while modulating regulatory T cells and macrophages to promote resolution of inflammation. Mesoblast reported an 83% survival in ventilator‐dependent COVID‐19 patients with moderate/severe ARDS, and 75% have successfully come off ventilator following Remestemcel‐L administration (Table 3). 96
TABLE 3.
Cytokines and cytokine receptors elevated in the plasma/serum of COVID‐19 patients
| Immune modulator | N (number of patients) | References |
|---|---|---|
| Pro‐inflammatory cytokines | ||
| IL‐6 | 452, 25, 21 | 111, 125, 126 |
| IL‐1β | 41 | 4 |
| TNF‐α | 452 | 125 |
| VEGF | 41 | 4 |
| Inflammatory cytokines | ||
| IL‐2 | 25 a | 124 |
| IL‐4 | 31 b | 124 |
| IL‐10 | 452, 41, 25 b | 111, 125, 126 |
| IL‐7 | 41 | 4 |
| IL‐9 | 41 | 4 |
| Chemokines | ||
| MCP‐1/CCL2 | 41 | 4 |
| IL‐8 | 452, 41 | 4, 126 |
| MIP‐1a | 41 | 4 |
| MIP‐1b | 41 | 4 |
| Inflammatory cytokine receptors | ||
| Il‐1Ra | 41 | 4 |
| IL‐2R | 452 | 8 |
Severe.
Mild/moderate.
In the United Kingdom, the REALIST trial is in phase II and is testing a single infusion of Orbcel‐C, a human umbilical cord stem cell product enriched with CD362 expressing cells for the management of ARDS. 54 In the completed part of the phase I REALIST trial, an infusion of 400 million cells was achieved without any dose limiting toxicity at day 7 and has been chosen as the dose for phase II clinical testing. The MACoVIA study is a multicenter phase II/III clinical study aimed at evaluating the safety and efficacy of Multistem MSC product for the management of moderate to severe ARDS in COVID‐19 patients. MultiStem is a cell therapeutic isolated from adult bone marrow approved for clinical use in patients with ischemic stroke. 97
MSC from further sources are also being tested for clinical COVID‐19 management. The earliest of the trials embarked on by Stemcells Arabia in Amman, Jordan, utilized intravenous infusion of Wharton's Jelly (WJ) derived MSCs. This phase I study, slated to complete in September 2020, enrolled five patients with COVID‐19 illness and a dose of 1 × 106 body weight WJ‐MSC/kg was administered for three times 3 days apart. Renmin Hospital of Wuhan University in China is currently evaluating the safety and efficacy of dental pulp derived MSCs as a part of a phase I/II randomized controlled clinical study. 98 Time to clinical improvement is the main outcomes indicator and is considered as the time taken to reduce disease severity by two stages following treatment. 98 The Wellness trial in Mexico utilizes autologous adipose tissue‐derived MSCs for COVID‐19 management. A three‐dose regimen of Celltex‐AdMSC will be administered, about 200 million cells per dose; the safety, tolerability, and efficacy will be evaluated as a part of this multicenter study.
A recently conducted clinical study by Leng et al at the Beijing YouAn Hospital, China, demonstrated the use of bone marrow‐derived MSCs for the management of pneumonia associated with COVID‐19. Ten patients with worsening respiratory condition were recruited with seven patients assigned to the treatment arm and three to the placebo arm. 10 , 99 , 100 A dose of 1 × 106 MSC/kg body weigh was used, and results report a reduction of symptoms within 2 to 4 days postinfusion and a majority of patients showed negative for the SARS‐CoV‐2 nucleic acid test 2 days after MSC administration with no apparent treatment‐related adverse effects. 99 This small study provided evidence that the intervention was feasible, safe, and has the potential to improve functional outcomes. It is conceivable that the main mechanisms of MSC action underlying clinical improvement was linked to the immunosuppressive capacities of MSCs to attenuate over‐activated cytokine‐secreting immune cells. 99 , 100 Indeed, this process was accompanied by an increased number of peripheral lymphocytes as well as of both regulatory DCs and T cells, decrease of CRP, and regression of over‐activated cytokine‐secreting immune cells. 99 MSCs decreased the levels of TNF‐α and increased the concentration of anti‐inflammatory protein IL‐10. 99 , 100 RNA‐sequencing revealed that the infused MSCs in patients resulted in downregulating expression of ACE2 and TMPRSS2. 99 This pilot study demonstrated that MSC therapy inhibited the overactivation of the immune system and promoted endogenous repair by improving the lung microenvironment after the SARS‐CoV‐2 infection. 99 , 100 Hope Biosciences has recently obtained clearance from the Food and Drug Administration (FDA) to initiate a phase 2 study to evaluate the efficacy and safety of allogeneic adipose tissue‐derived MSCs (HB‐adMSCs) as treatment for patients with COVID‐19. The study purpose is to evaluate the safety and efficacy of four intravenous infusions of either placebo or HB‐adMSCs in subjects with COVID‐19. HB‐adMSC have been previously approved for clinical use in patients with traumatic brain injury. 101
Another case report described the recovery of a COVID‐19 patient with severe inflammation symptoms after treated with allogenic human umbilical cord MSCs (hUCMSCs). 102 A dose of 5 × 107 hUCMSCs was administered to the patient three times with a 3‐day interval. Four days after the end of the treatment, the patient was released from ICU with the remission of the inflammation symptoms and without developing side effects. 102 A growing number of MSC‐based clinical trials will continue to be carried out to validate the therapeutic utility of MSCs in COVID‐19 management. The basic rationale for all MSC‐related therapies seems to be dependent on the potential to alleviate the cytokine storm caused by SARS‐CoV2 infection and early evidence from the data published by Leng et al 99 and Liang et al 102 supported the remission of inflammation symptoms in patients with COVID‐19.
6. PERSPECTIVES AND FUTURE DIRECTIONS
COVID‐19 has emerged as a global health care crisis and several studies have demonstrated that hyperinflammation associated with high levels of circulating cytokines induced by SARS‐CoV‐2 is a major contributing factor of disease severity and death in patients. 9 , 10 , 11 By virtue of their immunomodulatory and anti‐inflammatory features, MSCs appear to be a promising therapeutic candidate for COVID‐19 management. While waiting for outcomes from impending clinical studies, MSCs could be used as a compassionate strategy for managing critically ill COVID‐19 patients with the intent to reduce morbidity and mortality. 10 , 64 , 103 Despite encouraging early clinical data, there remain scientific concerns regarding the use of MSCs as a therapeutic intervention for COVID‐19, as detailed here below.
The main sources of MSCs in active clinical trials range from umbilical cord (Wharton's jelly, UC), umbilical cord blood, adipose tissue, bone marrow, menstrual blood, and dental pulp. 15 Although initial clinical results spark enthusiasm for adipose tissue‐derived MSCs as a therapeutic option in COVID‐19 linked respiratory conditions, 104 the best MSC source is yet to be determined. One key aspect in deciding the most suited MSC option could be their vulnerability to SARS‐CoV‐2 once infused into a patient with an ongoing infection, as well as how this would affect the potential beneficial effects. Two studies have demonstrated that overexpression of ACE2 in murine BM‐MSCs offered additional anti‐inflammatory and endothelial‐protective effects against endotoxin‐induced lung injury in mice. 73 , 105 Importantly, both studies showed detectable basal levels of cellular and secreted ACE2 by unmodified BM‐MSCs as measured by polymerase chain reaction, Western blot, and enzyme‐linked immunosorbent assay. Similarly, lentiviral‐transduced ACE2‐overexpressing human UC‐MSCs were more effective than constitutively ACE2‐expressing UC‐MSCs in a rat acute lung ischemia reperfusion injury model. 106 Hence, a real dilemma of choosing the best MSCs is to determine a fine line between an optimal level of ACE2 for lung protection and the minimum vulnerability to SARS‐CoV‐2, which will require well‐designed preclinical studies.
Developing MSCs as a potential treatment option for COVID‐19 patients is still challenging due to the lack of suitable preclinical models. 107 To fill this gap, a number of studies are currently being developed. Moreover, it is imperative to better understand the mechanisms underlying MSCs modulation of the process of COVID‐19 infection 108 before translating it into the clinic. Preclinical studies could also resolve the queries related to the anti‐inflammatory function of MSCs, either acting directly against viral infection or by stimulating antiviral T‐cell actions. 109 Preclinical data generated in relevant mouse models of COVID‐19 infection may help to better stratify the patients to appropriate MSC dosing and administration schedule. 110 Considering that animal models may be difficult to fully reproduce the complicated pathophysiological processes and internal environment changes in severe COVID‐19 patients, the data from early clinical studies are crucial. However, a longer time is needed to accurately monitor MSC‐treated patients with respect to safety and efficacy in ongoing clinical studies, as well as accounting the emerging coagulation issues in these patients. For instance, a recent clinical report described an emerging chronic tissue injury related to COVID‐19 111 which could motivate the use MSCs as treatment due to its antifibrotic properties. 36
MSC can also act through secreted factors 43 which might offer a new therapeutic approach in treating COVID‐19 patients due to their broad pharmacological effects. 74 MSC secretome offers the ease of manipulation at relatively lower costs. 74 MSCs can also be genetically modified to release specific bioactive molecules able to counteract a pathological situation. For instance, as the viral receptor ACE2 and TMPRSS2 contribute to COVID‐19 progression, engineering MSCs with immunomodulatory molecules to target these receptors in the process of SARS‐CoV‐2 internalization may provide an alternative therapeutic option. In this regard, given that the IFN/TNF‐related apoptosis‐inducing ligand (TRAIL) axis is subjected to a complex modulation during the process of acute respiratory viral infection including coronaviruses, 112 MSCs could be genetically modified to therapeutically target IFN/TRAIL signaling. Previous studies have shown that viral infection can upregulate TRAIL receptors and enhance sensitivity to TRAIL‐induced apoptosis, 113 , 114 whereas the increase of TRAIL may induce lymphocyte apoptosis in SARS‐CoV infection. 114 Furthermore, an alternative mechanism of action of TRAIL is the induction of autophagy, which contributes to viral clearance by transferring viral materials to cellular endosomes and subsequently activating adaptive immune system. 115 Therefore, engineering MSCs to express TRAIL may present promise for future clinical applications in COVID‐19 management, 116 as it has previously been reported in cancer therapy. 117 , 118 Alternative options have been conceived using MSCs delivering leukemia inhibitory factor to antagonize COVID‐19 cytokine storm. 16
Taken together, we discussed the nature of the inflammatory responses that have been detected in patients with COVID‐19 and outlined the scientific rationale as well as several preliminary findings to corroborate that MSCs emerge as a promising therapeutic candidate to manage this condition. To ensure that MSC intervention reaches its full potential, research and development will need to focus on understanding the complexity of COVID‐19 pathophysiology and precise therapeutic mechanisms of action to optimize the therapeutic procedures and stratify patient cohorts. Multidisciplinary and vigorous efforts by interventional physicians, basic scientists, and other health care professionals could realize the promise of MSCs as a valuable asset for COVID‐19 treatment.
Although further investigations are needed on the use of wild‐type and gene‐modified MSCs for the treatment of COVID‐19, the preliminary findings highlight therapeutic benefit. The use of MSCs for ARDS associated with COVID‐19 is highly experimental. Patients suffering from SARS‐CoV‐2 severe pneumonia reported amelioration of respiratory function as a consequence of MSC infusion due to yet unclear modulation of cytokine storm and protection of epithelial and endothelial cells. Studies suggest that the source of the MSCs play a crucial role in attenuating the COVID‐19 infection induced cytokine storm. A better understanding of the implicated bio‐active molecules would not only improve the rational of MSC for COVID‐19 but additionally help in understating the pathophysiology of this still obscure and dismal condition, ultimately decreasing a surprisingly high mortality rate by cell‐based therapeutic.
CONFLICT OF INTEREST
K.S. owns equity in and is a member of the Board of Directors of AMASA Therapeutics, a company developing stem cell‐based therapies for cancer. K.S.'s interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict‐of‐interest policies. D.B. is a consultant at AMASA Therapeutics. R.C. is member of the Advisor Board Takeda Italia on the use of Darvadstrocel in fistulizing Crohn's disease. M.D. declared advisory role for Rigenerand SRL that is developing cell and gene therapy for cancer. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
K.S.: conceived and designed the review, data collection, manuscript writing; N.S., H.W., F.R., D.B., R.C., K.‐S.C., J.K.K., I.M., A.V.S., R.C., M.D.: data collection, manuscript writing.
ACKNOWLEDGMENT
We would like to thank all the members of CSTI for their support and hard work during the conception and writing of this review.
Song N, Wakimoto H, Rossignoli F, et al. Mesenchymal stem cell immunomodulation: In pursuit of controlling COVID‐19 related cytokine storm. Stem Cells. 2021;39:707–722. 10.1002/stem.3354
Funding information Departmental funds
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Moore B, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368:473‐474. [DOI] [PubMed] [Google Scholar]
- 2. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID‐19: implications for the cardiovascular system. Circulation. 2020. [DOI] [PubMed] [Google Scholar]
- 3. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS‐CoV‐2. Nature. 2020;581:221‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20(5):269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ur A, Verma K. Cytokine storm in COVID19: a neural hypothesis. ACS Chem Nerosci. 2020;11(13):1868‐1870. [DOI] [PubMed] [Google Scholar]
- 8. Qin C, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta P, McAuley D, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC‐MSCs) as a therapeutic strategy in managing critically ill COVID‐19 patients: the case for compassionate use. Pain Physician. 2020;23:E71‐E83. [PubMed] [Google Scholar]
- 11. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rizzo P, Vieceli Dalla Sega F, Fortini F, Marracino L, Rapezzi C, Ferrari R. COVID‐19 in the heart and the lungs: could we “notch” the inflammatory storm? Basic Res Cardiol. 2020;115(3):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cilloniz C, et al. Lethal influenza virus infection in macaques is associated with early dysregulation of inflammatory related genes. PLoS Pathog. 2009;5(10):e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SM, Chan RWY, Gardy JL, et al. Systems‐level comparison of host responses induced by pandemic and seasonal influenza A H1N1 viruses in primary human type I‐like alveolar epithelial cells in vitro. Respir Res. 2010;11:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID‐19: present or future. Stem Cell Rev Rep. 2020;427‐433:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Metcalfe SM. Mesenchymal stem cells and management of COVID‐19 pneumonia. Med Drug Discov. 2020;5:100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou G, Chen S, Chen Z. Advances in COVID‐19: the virus, the pathogenesis, and evidence‐based control and therapeutic strategies. Front Med. 2020;14(2):117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liao M, Liu Y, Yuan J, et al. Single‐cell landscape of bronchoalveolar immune cells in patients with COVID‐19. Nat Med. 2020;26(6):842‐844. [DOI] [PubMed] [Google Scholar]
- 19. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tufan A, Avanoglu Guler A, Matucci‐Cerinic M. COVID‐19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI‐1):620‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID‐19: immunology and treatment options. Clin Immunol. 2020;215:108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327‐331. [DOI] [PubMed] [Google Scholar]
- 23. Zbinden‐Foncea H, Francaux M, Deldicque L, Hawley JA. Does high cardiorespiratory fitness confer some protection against proinflammatory responses after infection by SARS‐CoV‐2? Obesity. 2020;28:1378‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shneider A, Kudriavtsev A, Vakhrusheva A. Can melatonin reduce the severity of COVID‐19 pandemic? Int Rev Immunol. 2020;39(4):153‐162. [DOI] [PubMed] [Google Scholar]
- 25. Guo J, et al. Coronavirus disease 2019 (COVID‐19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9(7):e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID‐19? J Transl Med. 2020;18(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection, a hint for COVID‐19 treatment. Engineering. 2020;6:1153‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Russell B, et al. COVID‐19 and treatment with NSAIDs and corticosteroids: should we be limiting their use in the clinical setting? Ecancermedicalscience. 2020;14:1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Temesgen Z, et al. First clinical use of lenzilumab to neutralize GM‐CSF in patients with severe COVID‐19 pneumonia. medRxiv, 2020. 10.1101/2020.06.08.20125369. [DOI]
- 31. Martínez‐Urbistondo D, Costa Segovia R, Suárez del Villar Carrero R, Risco Risco C, Villares Fernández P. Early combination of tocilizumab and corticosteroids: an upgrade in anti‐inflammatory therapy for severe COVID. Clin Infect Dis. 2020;ciaa910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teoh N, Farrell G. Statins as early therapy to mitigate COVID‐19 (SARS‐CoV‐2)‐associated ARDS and cytokine storm syndrome ‐ time is of the essence. J Clin Transl Res. 2020;5(5):227‐229. [PMC free article] [PubMed] [Google Scholar]
- 33. Giudice V, Pagliano P, Vatrella A, et al. Combination of ruxolitinib and eculizumab for treatment of severe SARS‐CoV‐2‐related acute respiratory distress syndrome: a controlled study. Front Pharmacol. 2020;11:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Altay O, et al. Current status of COVID‐19 therapies and drug repositioning applications. iScience. 2020;23(7):101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saad J, Mathew D. Nonsteroidal anti‐inflammatory drugs (NSAID) toxicity. StatPearls. Treasure Island, FL: StatPearls Publishing LLC; 2020. [PubMed] [Google Scholar]
- 36. D'Souza N, et al. Mesenchymal stem/stromal cells as a delivery platform in cell and gene therapies. BMC Med. 2015;13:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salvadori M, Cesari N, Murgia A, Puccini P, Riccardi B, Dominici M. Dissecting the pharmacodynamics and pharmacokinetics of MSCs to overcome limitations in their clinical translation. Mol Ther Methods Clin Dev. 2019;14:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Snelgrove RJ, Goulding J, Didierlaurent AM, et al. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9(9):1074‐1083. [DOI] [PubMed] [Google Scholar]
- 39. Dalberto TP, Nardi NB, Camassola M. Mesenchymal stem cells as a platform for gene therapy protocols. Sci Prog. 2010;93(2):129‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dominici M, le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315‐317. [DOI] [PubMed] [Google Scholar]
- 41. Wang M, Yuan Q, Xie L. Mesenchymal stem cell‐based immunomodulation: properties and clinical application. Stem Cells Int. 2018;2018:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019;3(2):90‐104. [DOI] [PubMed] [Google Scholar]
- 43. Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med. 2019;8(7):1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74(13):2345‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abraham A, Krasnodembskaya A. Mesenchymal stem cell‐derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Translational Medicine. 2020;9(1):28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18(2):128‐134. [DOI] [PubMed] [Google Scholar]
- 47. Wang Q, Yang Q, Wang Z, et al. Comparative analysis of human mesenchymal stem cells from fetal‐bone marrow, adipose tissue, and Warton's jelly as sources of cell immunomodulatory therapy. Hum Vaccin Immunother. 2016;12(1):85‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida‐Porada G, Gonçalves RM. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre‐conditioning. Front Immunol. 2018;9:2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Silva LH, et al. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther. 2018;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu X, et al. Human umbilical cord mesenchymal stem cells inhibit the function of allogeneic activated Vγ9Vδ2 T lymphocytes in vitro. Biomed Res Int. 2015;2015:317801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Malcherek G, Jin N, Hückelhoven AG, et al. Mesenchymal stromal cells inhibit proliferation of virus‐specific CD8(+) T cells. Leukemia. 2014;28(12):2388‐2394. [DOI] [PubMed] [Google Scholar]
- 52. Karlsson H, Samarasinghe S, Ball LM, et al. Mesenchymal stem cells exert differential effects on alloantigen and virus‐specific T‐cell responses. Blood. 2008;112(3):532‐541. [DOI] [PubMed] [Google Scholar]
- 53. Thompson M, et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: an updated systematic review and meta‐analysis. EClinicalMedicine. 2020;19:100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gorman E, Shankar‐Hari M, Hopkins P, et al. Repair of acute respiratory distress syndrome by stromal cell administration in COVID‐19 (REALIST‐COVID‐19): a structured summary of a study protocol for a randomised, controlled trial. Trials. 2020;21(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID‐19. Stem Cells Dev. 2020;29(12):747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tsuchiya A, Takeuchi S, Iwasawa T, et al. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID‐19) cases. Inflamm Regen. 2020;40:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Le Blanc K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890‐896. [DOI] [PubMed] [Google Scholar]
- 58. Sundin M, Ringden O, Sundberg B, Nava S, Gotherstrom C, le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti‐fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92(9):1208‐1215. [DOI] [PubMed] [Google Scholar]
- 59. Reinders ME, et al. Safety of allogeneic bone marrow derived mesenchymal stromal cell therapy in renal transplant recipients: the neptune study. J Transl Med. 2015;13:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Prockop DJ, Brenner M, Fibbe WE, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12(5):576‐578. [DOI] [PubMed] [Google Scholar]
- 62. Orqueda AJ, Gimenez CA, Pereyra‐Bonnet F. iPSCs: a minireview from bench to bed, including organoids and the CRISPR system. Stem Cells Int. 2016;2016:5934782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schafer R, et al. Human mesenchymal stromal cells are resistant to SARS‐CoV‐2 infection under steady‐state, inflammatory conditions and in the presence of SARS‐CoV‐2‐infected cells. Stem Cell Reports. 2020. 10.1016/j.stemcr.2020.09.003 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ji F, Li L, Li Z, Jin Y, Liu W. Mesenchymal stem cells as a potential treatment for critically ill patients with coronavirus disease 2019. Stem Cells Translational Medicine. 2020;9:813‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Johnson CL, Soeder Y, Dahlke MH. Concise review: mesenchymal stromal cell‐based approaches for the treatment of acute respiratory distress and sepsis syndromes. Stem Cells Translational Medicine. 2017;6(4):1141‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone‐marrow‐derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ciccocioppo R, Klersy C, Leffler DA, Rogers R, Bennett D, Corazza GR. Systematic review with meta‐analysis: safety and efficacy of local injections of mesenchymal stem cells in perianal fistulas. JGH Open. 2019;3(3):249‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abati E, Bresolin N, Comi G, Corti S. Advances, challenges, and perspectives in translational stem cell therapy for amyotrophic lateral sclerosis. Mol Neurobiol. 2019;56(10):6703‐6715. [DOI] [PubMed] [Google Scholar]
- 69. de Windt TS, Vonk LA, Slaper‐Cortenbach ICM, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single‐stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35(1):256‐264. [DOI] [PubMed] [Google Scholar]
- 70. Hu Q, Li Y, Huang P. Treatment of critical limb ischemia with localized scleroderma by local injection of umbilical cord mesenchymal stem cells. Med Clin. 2020;S0025‐7753(20)30551‐0. [DOI] [PubMed] [Google Scholar]
- 71. Bustos ML, Huleihel L, Meyer EM, et al. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)‐10 and IL‐1RN levels. Stem Cells Translational Medicine. 2013;2(11):884‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martinez‐Gonzalez I, et al. Human mesenchymal stem cells overexpressing the IL‐33 antagonist soluble IL‐1 receptor‐like‐1 attenuate endotoxin‐induced acute lung injury. Am J Respir Cell Mol Biol. 2013;49(4):552‐562. [DOI] [PubMed] [Google Scholar]
- 73. He H, Liu L, Chen Q, et al. Mesenchymal stem cells overexpressing angiotensin‐converting enzyme 2 rescue lipopolysaccharide‐induced lung injury. Cell Transplant. 2015;24(9):1699‐1715. [DOI] [PubMed] [Google Scholar]
- 74. Bari E, Ferrarotti I, Saracino L, Perteghella S, Torre ML, Corsico AG. Mesenchymal stromal cell secretome for severe COVID‐19 infections: premises for the therapeutic use. Cell. 2020;9(4):924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jayaramayya K, Mahalaxmi I, Subramaniam MD, et al. Immunomodulatory effect of mesenchymal stem cells and mesenchymal stem‐cell‐derived exosomes for COVID‐19 treatment. BMB Rep. 2020;53(8):400‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Muraca M, Pessina A, Pozzobon M, et al. Mesenchymal stromal cells and their secreted extracellular vesicles as therapeutic tools for COVID‐19 pneumonia? J Control Release. 2020;325:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Watanabe T, Tsuchiya A, Takeuchi S, et al. Development of a non‐alcoholic steatohepatitis model with rapid accumulation of fibrosis, and its treatment using mesenchymal stem cells and their small extracellular vesicles. Regen Ther. 2020;14:252‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lopes‐Pacheco M, Robba C, Rocco PRM, Pelosi P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. Cell Biol Toxicol. 2020;36(1):83‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Condor JM, et al. Treatment with human Wharton's jelly‐derived mesenchymal stem cells attenuates sepsis‐induced kidney injury, liver injury, and endothelial dysfunction. Stem Cells Translational Medicine. 2016;5(8):1048‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhou F, Yu T, du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lessons from the past. J Clin Virol. 2020;127:104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. George MJ, Prabhakara K, Toledano‐Furman NE, et al. Clinical cellular therapeutics accelerate clot formation. Stem Cells Translational Medicine. 2018;7(10):731‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moll G, Ignatowicz L, Catar R, et al. Different procoagulant activity of therapeutic mesenchymal stromal cells derived from bone marrow and placental decidua. Stem Cells Dev. 2015;24(19):2269‐2279. [DOI] [PubMed] [Google Scholar]
- 85. Witkowski M, Landmesser U, Rauch U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc Med. 2016;26(4):297‐303. [DOI] [PubMed] [Google Scholar]
- 86. Tatsumi K, Ohashi K, Matsubara Y, et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem Biophys Res Commun. 2013;431(2):203‐209. [DOI] [PubMed] [Google Scholar]
- 87. Wu Z, Zhang S, Zhou L, et al. Thromboembolism induced by umbilical cord mesenchymal stem cell infusion: a report of two cases and literature review. Transplant Proc. 2017;49(7):1656‐1658. [DOI] [PubMed] [Google Scholar]
- 88. Moll G, Hult A, Bahr L, et al. Do ABO blood group antigens hamper the therapeutic efficacy of mesenchymal stromal cells? PLoS One. 2014;9(1):e85040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hashi CK, Zhu Y, Yang GY, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 2007;104(29):11915‐11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Netsch P, Elvers‐Hornung S, Uhlig S, et al. Human mesenchymal stromal cells inhibit platelet activation and aggregation involving CD73‐converted adenosine. Stem Cell Res Ther. 2018;9(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187(7):751‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chan MC, et al. Human mesenchymal stromal cells reduce influenza A H5N1‐associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA. 2016;113(13):3621‐3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li Y, Xu J, Shi W, et al. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus‐induced acute lung injury in mice. Stem Cell Res Ther. 2016;7(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Loy H, Kuok DIT, Hui KPY, et al. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus‐associated acute lung injury. J Infect Dis. 2019;219(2):186‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Klimczak A. Perspectives on mesenchymal stem/progenitor cells and their derivates as potential therapies for lung damage caused by COVID‐19. World J Stem Cells. 2020;12(9):1013‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mesoblast . 83% Survival in COVID‐19 Patients with Moderate/Severe Acute Respiratory Distress Syndrome Treated in New York with Mesoblast's Cell Therapy Remestemcel‐L, ET, Source: Mesoblast Limited; 2020. https://www.globenewswire.com/news‐release/2020/04/24/2021558/0/en/83‐Survival‐in‐COVID‐19‐Patients‐with‐Moderate‐Severe‐Acute‐Respiratory‐Distress‐Syndrome‐Treated‐in‐New‐York‐with‐Mesoblast‐s‐Cell‐Therapy‐Remestemcel‐L.html.
- 97. Mays R, Deans R. Adult adherent cell therapy for ischemic stroke: clinical results and development experience using MultiStem. Transfusion. 2016;56(4):6S‐8S. [DOI] [PubMed] [Google Scholar]
- 98. Ye Q, Wang H, Xia X, et al. Safety and efficacy assessment of allogeneic human dental pulp stem cells to treat patients with severe COVID‐19: structured summary of a study protocol for a randomized controlled trial (phase I/II). Trials. 2020;21(1):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2‐mesenchymal stem cells improves the outcome of patients with COVID‐19 pneumonia. Aging Dis. 2020;11(2):216‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID‐19)‐induced pneumonia. Aging Dis. 2020;11(2):462‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ruppert KA, et al. Human adipose‐derived mesenchymal stem cells for acute and sub‐acute TBI. PLoS One. 2020;15(5):e0233263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID‐19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine. 2020;99(31):e21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lightner AL, García‐Olmo D. Mesenchymal stem cell therapy can transcend perianal Crohn's disease: how colorectal surgeons can help in the COVID‐19 crisis. Dis Colon Rectum. 2020;63:874‐878. [DOI] [PubMed] [Google Scholar]
- 104. Gentile P, Sterodimas A. Adipose‐derived stromal stem cells (ASCs) as a new regenerative immediate therapy combating coronavirus (COVID‐19)‐induced pneumonia. Expert Opin Biol Ther. 2020;20(7):711‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. He HL, Liu L, Chen QH, et al. MSCs modified with ACE2 restore endothelial function following LPS challenge by inhibiting the activation of RAS. J Cell Physiol. 2015;230(3):691‐701. [DOI] [PubMed] [Google Scholar]
- 106. Min F, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin‐converting enzyme 2 gene on bleomycin‐induced lung fibrosis injury. Mol Med Rep. 2015;11(4):2387‐2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lakdawala SS, Menachery VD. The search for a COVID‐19 animal model. Science. 2020;368(6494):942‐943. [DOI] [PubMed] [Google Scholar]
- 108. Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell‐based therapies for respiratory virus infections: applicability to COVID‐19. Eur Respir J. 2020;55:2000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell‐based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196(3):266‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang Q. Hace2 Transgenic Mouse Model For Coronavirus (COVID‐19) Research. 2020. https://www.jax.org/news-and-insights/2020/february/introducing-mouse-model-for-corona-virus.
- 111. Wang Y, et al. Temporal changes of CT findings in 90 patients with COVID‐19 pneumonia: a longitudinal study. Radiology. 2020;296(2):E55‐E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Peteranderl C, Herold S. The impact of the interferon/TNF‐related apoptosis‐inducing ligand signaling axis on disease progression in respiratory viral infection and beyond. Front Immunol. 2017;8:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kirshner JR, Karpova AY, Kops M, Howley PM. Identification of TRAIL as an interferon regulatory factor 3 transcriptional target. J Virol. 2005;79(14):9320‐9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Law HK, Cheung C, Sia S, Chan Y, Peiris JSM, Lau Y. Toll‐like receptors, chemokine receptors and death receptor ligands responses in SARS coronavirus infected human monocyte derived dendritic cells. BMC Immunol. 2009;10(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shoji‐Kawata S, Levine B. Autophagy, antiviral immunity, and viral countermeasures. Biochim Biophys Acta. 2009;1793(9):1478‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kaczorowski A, Hammer K, Liu L, et al. Delivery of improved oncolytic adenoviruses by mesenchymal stromal cells for elimination of tumorigenic pancreatic cancer cells. Oncotarget. 2016;7(8):9046‐9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bagci‐Onder T, et al. Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells. Brain. 2015;138(pt 6):1710‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rossignoli F, Spano C, Grisendi G, et al. MSC‐delivered soluble TRAIL and paclitaxel as novel combinatory treatment for pancreatic adenocarcinoma. Theranostics. 2019;9(2):436‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhu Z, Liang L, Zhang R, et al. Whole blood microRNA markers are associated with acute respiratory distress syndrome. Intensive Care Med Exp. 2017;5(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Monsel A, Zhu YG, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell‐derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192(3):324‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tang XD, Shi L, Monsel A, et al. Mesenchymal stem cell microvesicles attenuate acute lung injury in mice partly mediated by Ang‐1 mRNA. Stem Cells. 2017;35(7):1849‐1859. [DOI] [PubMed] [Google Scholar]
- 123. Li JW, Wei L, Han Z, Chen Z. Mesenchymal stromal cells‐derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti‐apoptotic miR‐21‐5p. Eur J Pharmacol. 2019;852:68‐76. [DOI] [PubMed] [Google Scholar]
- 124. Yi X, et al. Exosomes derived from microRNA‐30b‐3p‐overexpressing mesenchymal stem cells protect against lipopolysaccharide‐induced acute lung injury by inhibiting SAA3. Exp Cell Res. 2019;383(2):111454. [DOI] [PubMed] [Google Scholar]
- 125. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tan M, Liu Y, Zhou R, et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology. 2020;160(3):261‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


