Abstract
Introduction
Evidence about coronavirus disease 2019 (COVID‐19) and pregnancy has rapidly increased since December 2019, making it difficult to make rigorous evidence‐based decisions. The objective of this overview of systematic reviews is to conduct a comprehensive analysis of the current evidence on prognosis of COVID‐19 in pregnant women.
Material and methods
We used the Living OVerview of Evidence (L·OVE) platform for COVID‐19, which continually retrieves studies from 46 data sources (including PubMed/MEDLINE, Embase, other electronic databases, clinical trials registries, and preprint repositories, among other sources relevant to COVID‐19), mapping them into PICO (population, intervention, control, and outcomes) questions. The search covered the period from the inception date of each database to 13 September 2020. We included systematic reviews assessing outcomes of pregnant women with COVID‐19 and/or their newborns. Two authors independently screened the titles and abstracts, assessed full texts to select the studies that met the inclusion criteria, extracted data, and appraised the risk of bias of each included systematic review. We measured the overlap of primary studies included among the selected systematic reviews by building a matrix of evidence, calculating the corrected covered area, and assessing the level of overlap for every pair of systematic reviews.
Results
Our search yielded 1132 references. 52 systematic reviews met inclusion criteria and were included in this overview. Only one review had a low risk of bias, three had an unclear risk of bias, and 48 had a high risk of bias. Most of the included reviews were highly overlapped among each other. In the included reviews, rates of maternal death varied from 0% to 11.1%, admission to intensive care from 2.1% to 28.5%, preterm deliveries before 37 weeks from 14.3% to 61.2%, and cesarean delivery from 48.3% to 100%. Regarding neonatal outcomes, neonatal death varied from 0% to 11.7% and the estimated infection status of the newborn varied between 0% and 11.5%.
Conclusions
Only one of 52 systematic reviews had a low risk of bias. Results were heterogeneous and the overlap of primary studies was frequently very high between pairs of systematic reviews. High‐quality evidence syntheses of comparative studies are needed to guide future clinical decisions.
Keywords: coronavirus infections, coronavirus disease 2019, infant, newborn, overview, pregnant women, systematic reviews as topic
Abbreviations
- CCA
corrected covered area
- SR
systematic review
Key message.
This overview summarizes and critically appraises 52 systematic reviews, of which only one was assessed as having low risk of bias. The overlap of primary studies between pairs of reviews was high, with highly variable results in each systematic review.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is an infection caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 It was first identified in Wuhan, China, on 31 December 2019; 2 10 months later, more than 43 million cases of contagion had been identified across the globe. 3
Pregnant women are a special group of concern during this outbreak. Physiological changes in the immunologic, cardiovascular, and respiratory systems may increase the severity of respiratory diseases, especially during the third trimester. 4 , 5 , 6 The available evidence about the effect of other coronaviruses—causing SARS and Middle Eastern respiratory syndrome—is scarce, but it suggests that coronavirus infection during pregnancy is associated with adverse perinatal outcomes, 7 , 8 , 9 high rates of maternal and perinatal mortality, cesarean section, and preterm birth. 10 , 11
Since the beginning of the pandemic, several studies (observational studies and reviews) have been conducted assessing critical outcomes of COVID‐19 in pregnant women and their newborns. 12 , 13 This continuous and rapid increase of the available evidence may lead to duplication of efforts and overlapping results. 13 Also, if low‐quality studies are produced, they may hinder those making healthcare decisions when producing evidence‐based guidelines or public policies.
This overview of systematic reviews aims to conduct a comprehensive analysis by mapping, summarizing, critically appraising, and assessing bias and overlap of the current evidence on maternal and perinatal outcomes of COVID‐19 and pregnancy.
2. MATERIAL AND METHODS
Considering that no guideline for reporting overviews has been released so far, 14 this manuscript follow the Cochrane's guidance for overviews of systematic reviews 15 and complies with an adapted version of the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines for reporting systematic reviews and meta‐analyses. 16 Our overview is framed within the COVID‐19 L·OVE Working Group's project (https://www.epistemonikos.cl/working‐group/). A protocol describing the shared objectives and methodology of multiple evidence syntheses (systematic reviews and overviews of systematic reviews) to be conducted in parallel for different questions relevant to COVID‐19 was published elsewhere. 17 The protocol of this overview was adapted to the specificities of our methodology design and is available in the Open Science Framework repository (https://osf.io/64qyz/).
2.1. Data sources
The Living Overview of the Evidence (L·OVE) platform retrieves studies from the Epistemonikos database (https://www.epistemonikos.org), a comprehensive database of systematic reviews (SRs) and other types of evidence with more than 300 000 SRs, and over 400 000 studies included in those reviews, yielded through regular searches in 10 electronic databases (https://www.epistemonikos.org/en/about_us/methods). To supplement the information regarding to COVID‐19, the L·OVE platform is continually conducting additional searches in 36 other sources relevant to COVID‐19 (https://app.iloveevidence.com/covid‐19). Thus, our search encompasses: PubMed/MEDLINE, Embase, CINAHL, Cochrane Database of Systematic Reviews, PsycINFO, LILACS, Database of Abstracts of Reviews of Effects, Wanfang Database, The Campbell Collaboration online library, JBI Database of Systematic Reviews and Implementation Reports, EPPI‐center Evidence Library, CBM (Chinese Biomedical Literature Database), CNKI (Chinese National Knowledge Infrastructure), VIP (Chinese Scientific Journal Database), IRIS (WHO Institutional Repository for Information Sharing), IRIS PAHO (PAHO Institutional Repository for Information Sharing), IBECS (Índice Bibliográfico Español en Ciencias de la Salud [Spanish Bibliographic Index on Health Sciences]), Microsoft Academic, medRxiv, bioRxiv, SSRN Preprints, ChinaXiv, SciELO Preprints, Research Square, and 22 clinical trial registries—not as critical for this overview as the aforementioned sources.
The searches covered from the inception date of each database until 13 September 2020. No study design, publication status or language restriction was applied to the searches.
The strategy used in the electronic searches and its terms are shown in the Supplementary material (Appendix S1).
2.2. Eligibility criteria
We included all SRs (defined operationally as any secondary research that included only clinical primary studies, reporting an explicit search strategy in at least two databases) assessing maternal and perinatal outcomes related to COVID‐19 and pregnancy. We included those SRs that were broader in scope but presenting separate and distinguishable data for our population of interest.
We excluded primary studies, clinical practice guidelines, overviews, and other types of study design aimed at synthesizing evidence.
We included studies assessing both maternal and neonatal outcomes, only maternal outcomes, or only neonatal outcomes regarding COVID‐19 and pregnancy. We did not include information about other coronavirus infections.
2.3. Study selection
The results of the electronic searches were automatically incorporated into the L·OVE platform, where they were de‐duplicated by an algorithm that compared unique identifiers (database ID, DOI, trial registry ID), and citation details (ie, author names, journal, year of publication, volume number, pages, article title, and article abstract).
Using the L·OVE platform, two researchers (LVM and SBB) independently screened the titles and abstracts yielded by the searches, against the inclusion criteria. We obtained the full reports of all the titles that appeared to meet the inclusion criteria or required further analysis to decide about their inclusion.
In each search stage, we recorded the reasons for excluding reviews and outlined the study selection process in a PRISMA flow diagram adapted for the purpose of this project.
2.4. Extraction and management of data
Using standardized forms, two independent reviewers extracted data from each included SR in duplicate (LVM, CCP, CC, NM, LO, and SBB). We did not extract data from primary studies.
We recorded the following characteristics of included SRs: publication date, search sources and strategies, number of included studies, number of included studies relevant to our overview, assessment of evidence quality of the included studies, and the elements of the systematic review question (patients, exposure definition, and assessed outcomes).
We also extracted synthesized results from SRs, both narrative and quantitative. The collected data for maternal outcomes were: (1) maternal mortality, (2) admission to intensive care units, (3) mechanical ventilation support, (4) preterm delivery at <37 weeks of gestation, (5) preterm delivery at <34 weeks of gestation, (6) premature rupture of membranes, and (7) cesarean delivery. The collected data for neonatal outcomes were: (1) stillbirth, (2) neonatal mortality, (3) neonatal admission to special care and/or intensive care unit, (4) mechanical ventilation support, (5) APGAR score below 7 at 5 min, and (6) infection status of the newborn (or products of conception) as defined by the authors of the included SRs.
2.5. Overlap assessment
We built a matrix of evidence to visually examine the overlap among the primary studies included in the different SRs. Primary studies were presented in the rows of the matrix and the systematic reviews were given in the columns. We calculated the corrected covered area (CCA), which is a quantitative measure of overlap of primary studies among systematic reviews. 18 We considered overlap as low if CCA was below 5%, moderate if CCA was between 5% and 10%, high if CCA was between 10% and 15%, and very high if CCA was above 15%. In order to identify which specific pairs of reviews were highly overlapped, we followed the previously described methods 19 to assess the overlap degree of every pair of SRs: we calculated the CCA for each possible pair of SRs included in our matrix of evidence.
2.6. Risk of bias assessment
Two authors independently assessed the risk of bias of each included SR using the tool Risk of Bias in Systematic Review (ROBIS). 20 We did not assess the quality of the primary studies included in the SRs or the quality of reporting of each SR.
2.7. Data synthesis
We expressed the results of the included SRs by using the range of the effect measure reported by the different SRs. We did not calculate any pooled estimates. We tabulated the characteristics of each included SR and summarized their results by maternal and perinatal outcomes, as defined above. We graphically presented the overlap of primary studies, and the risk of bias assessment.
3. RESULTS
Our initial search yielded 1132 references. After the initial screening, we assessed the eligibility of 77 full‐text articles; we excluded 25 of them (see Supplementary material, Table S1), which led to the inclusion of 52 SRs. 7 , 9 , 11 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 Figure 1 provides the PRISMA flow diagram. The 52 included SRs referenced a total of 205 primary studies, 142 of which were included in two or more SRs.
FIGURE 1.
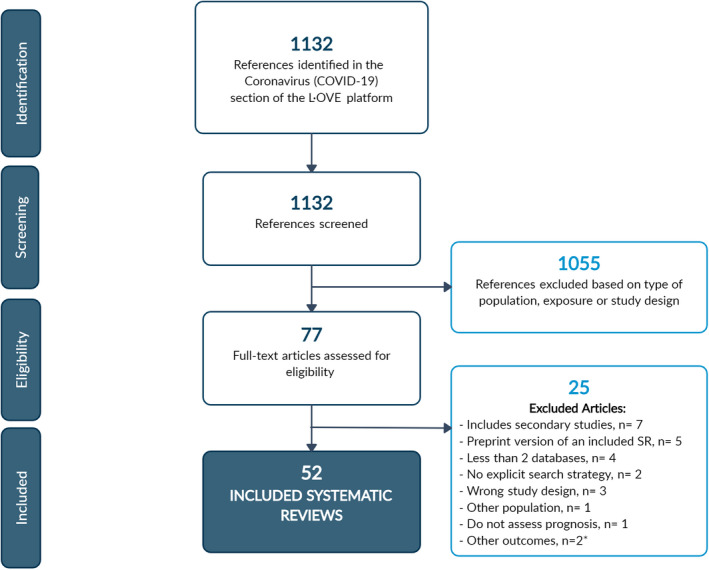
PRISMA flowchart. SR, systematic review. *These two articles correspond to the same review (preprint version and journal article)
Most of the SRs were published as journal articles, 7 , 9 , 11 , 22 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 46 , 47 , 48 , 49 , 51 , 52 , 54 , 55 , 56 , 57 , 58 , 61 , 62 , 63 , 64 , 65 , 66 , 67 while some were available as pre‐print articles. 21 , 24 , 28 , 37 , 45 , 50 , 53 , 59 , 60 , 68 , 69 The SRs were published between 17 March 2020 7 and 4 September 2020. 33 The median number of relevant primary studies included in the SRs was 16 (interquartile range 21). The median number of included pregnant women with COVID‐19 was 145.5 (interquartile range 296.5). Fifty‐one SRs assessed maternal and neonatal outcomes, one SR assessed only maternal outcomes, 42 and none of the included SRs assessed only perinatal outcomes. The most commonly reported maternal outcomes were cesarean delivery, preterm delivery before 37 weeks of pregnancy, and maternal death; and the most commonly mentioned perinatal outcomes were neonatal death, infection status of the newborn, and stillbirth. Table 1 provides the main characteristics of the included studies.
TABLE 1.
Main characteristics of the included systematic reviews
| Review | Status | Databases (as the included SRs reported) | Design of included studies | Included studies, N | Relevant a included studies, N | Pregnant women b , N | Newborns, N |
|---|---|---|---|---|---|---|---|
| AbdelMassih (2020) 21 | Preprint | Embase, MEDLINE, CENTRAL | Case reports, case series, other observational studies | 66 | 66 | 1787 | 1787 |
| Akhtar (2020) 22 | Journal article | MEDLINE, PubMed, Scopus, Google scholar | Case reports, case series, other observational studies | 22 | 22 | 156 | 108 |
| Allotey (2020) 23 | Journal article | MEDLINE, Embase, Cochrane database, WHO COVID‐19 database, CNKI, Wanfang, L·OVE | Non‐comparative cohorts with a minimum of 10 participants | 77 | 72 | 11 432 | N/A |
| Arabi (2020) 24 | Preprint | MEDLINE, Embase, CENTRAL | Case reports, case series, other observational studies | 7 | 7 | 50 | N/A |
| Ashraf (2020) 25 | Journal article | PubMed, Scopus, WoS, Embase, Google Scholar | Case reports, case series | 26 | 20 | 90 | 92 |
| Banaei (2020) 26 | Journal article | MEDLINE, Embase, Scopus, WoS, ProQuest, Google Scholar | Case reports, case series, other observational studies | 16 | 16 | 123 | 124 |
| Capobianco (2020) 27 | Journal article | PubMed, Scopus | Case reports, case series, other observational studies | 13 | 13 | 114 | 108 |
| Chamseddine (2020) 28 | Preprint | PubMed, medRxiv | Case reports, case series, other observational studies | 20 | 16 | 164 | 128 |
| Chang (2020) 29 | Journal article | PubMed, Embase, Google Scholar, Chinese Medical Journal Network | Case reports, case series | 9 | 9 | 18 | 19 |
| Chi (2020) 30 | Journal article | PubMed/MEDLINE, Embase, CINAHL, National Digital Library of Theses and Dissertations in Taiwan database, Art Image Indexing Service on the Internet Database (Chinese database), CENTRAL | Case reports, case series | 14 | 14 | 107 | 105 |
| Della Gatta (2020) 31 | Journal article | PubMed, Scopus, CINAHL | Case reports, case series, other observational studies | 6 | 6 | 51 | 48 |
| Dhir (2020) 32 | Journal article | PubMed, Embase, WoS | Case reports, case series | 86 | 76 | 2035 | 1141 |
| Di Mascio c (2020) 11 | Journal article | MEDLINE, Embase, CINAHL, ClinicalTrials.gov | Case reports, case series, other observational studies | 20 | 6 | 41 | N/A |
| Diriba c (2020) 33 | Journal article | PubMed, WoS, Embase, Google Scholar, Cochrane library, ScienceDirect | Case reports, case series | 39 | 23 | 1271 | N/A |
| Duran (2020) 34 | Journal article | Google Scholar, LILACS, PubMed | Case reports, case series, other observational studies | 20 | 18 | 195 | 222 |
| Elshafeey (2020) 35 | Journal article | LitCovid, EBSCO, MEDLINE, CENTRAL, CINAHL, WoS, Scopus | Case reports, case series, other observational studies | 33 | 33 | 385 | 256 |
| Furlan (2020) 36 | Journal article | SciELO, LILACS, CAPES, PubMed, Google Scholar | Case series, other observational studies | 27 | 22 | 399 | 188 |
| Gajbhiye (2020) 37 | Preprint | PubMed, MEDLINE, Google Scholar, medRxiv, bioRxiv, arXiv | Case reports, case series, other observational studies | 50 | 48 | 441 | 391 |
| Gao (2020) 38 | Journal article | MEDLINE, PubMed, WoS, Embase | Case reports, case series, other observational studies | 14 | 13 | 236 | N/A |
| Gordon (2020) 39 | Journal article | MEDLINE, PubMed, Embase, CINAHL | Case reports, case series, other observational studies | 8 | 5 | N/A | 46 |
| Huntley (2020) 40 | Journal article | MEDLINE, Ovid, ClinicalTrials.gov, medRxiv, Scopus | Case series | 13 | 12 | 538 | 435 |
| Juan (2020) 41 | Journal article | PubMed, Embase, the Cochrane Library, CNKI, Wan Fang Data | Case reports, case series, excluded case reports from China or case series that included fewer than 10 cases from China | 24 | 24 | 324 | 240 |
| Jutzeler d (2020) 42 | Journal article | Embase, PubMed/MEDLINE, Scopus, WoS | Case reports, case series, other observational studies | 148 | 11 | 35 | N/A |
| Kasraeian (2020) 43 | Journal article | PubMed, Google Scholar, medRxiv, and UpToDate search engines | N/A | 9 | 8 | 87 | 86 |
| Khalil (2020) 44 | Journal article | MEDLINE, Embase, ClinicalTrials.gov, CENTRAL | Case reports, case series, other observational studies | 86 | 81 | 2567 | N/A |
| Khan (2020) 45 | Journal article | MEDLINE, WoS, Scopus, CINAHL | Case reports, case series, other observational studies | 9 | 9 | 101 | 56 |
| Kotlyar (2020) 46 | Journal article | PubMed, Embase, medRxiv, bioRxiv | Case reports, case series, other observational studies | 65 | 64 | 1566 | 979 |
| Lopes de Sousa (2020) 47 | Journal article | PubMed, Scopus, Embase, ScienceDirect, WoS, Google Scholar, bioRxiv, medRxiv | Case reports, case series, other observational studies | 49 | 49 | 755 | 598 |
| Matar (2020) 48 | Journal article | Ovid MEDLINE and Epub Ahead of Print, In‐Process and Other Nonindexed Citations; Ovid Embase; Ovid Cochrane Central Register of Controlled Trials; Scopus | Case reports, case series, other observational studies | 24 | 24 | 136 | 94 |
| Melo (2020) 49 | Journal article | PubMed, Scopus, LILACS, WoS, bioRxiv, medRxiv, Preprints | Case reports, case series, other observational studies e | 38 | 38 | 60 | 432 |
| Mirbeyk (2020) 50 | Preprint | PubMed, WoS, Google Scholar, Scopus, WHO COVID‐19 database | Case reports, case series | 37 | 36 | 386 | 302 |
| Muhidin (2020) 51 | Journal article | PubMed, Scopus, Embase, ProQuest, ScienceDirect | Case reports, case series, other observational studies | 9 | 9 | 89 | 89 |
| Mullins c (2020) 7 | Journal article | PubMed, medRxiv | Case reports, case series | 21 | 5 | 32 | 30 |
| Parazzini (2020) 52 | Journal article | PubMed, Embase | Case reports, case series | 14 | 13 | 71 | 65 |
| Paulino Vigil‐De Gracia (2020) 53 | Preprint | PubMed, Google Scholar | Case series, other observational studies | 13 | 13 | 83 | 84 |
| Pettirosso (2020) 54 | Journal article | Embase, MEDLINE, WHO COVID‐19 database, the Cochrane Library | Case reports, case series, other observational studies | 60 | 56 | 1287 | N/A |
| Rodríguez‐Blanco c (2020) 55 | Journal article | MEDLINE, SciELO, CUIDEN | Case reports, case series, other observational studies | 20 | 10 | 102 | 74 |
| Sepúlveda‐ Martinez (2020) 68 | Preprint | MEDLINE, LILACS | Case series, other observational studies | 14 | 14 | 292 | 252 |
| Sharps (2020) 56 | Journal article | MEDLINE, Google Scholar, medRxiv | Case reports, case series, other observational studies | 50 | 39 | 325 | N/A |
| Simões (2020) 57 | Journal article | PubMed, Embase, LILACS, Cochrane, Scopus, SciELO | Case reports, case series | 12 | 10 | 51 | 67 |
| Smith (2020) 58 | Journal article | PubMed, MEDLINE, Embase | Case reports, case series | 9 | 9 | 92 | 60 |
| Soheili (2020) 59 | Preprint | PubMed, MEDLINE, Embase, Scopus, WoS, CENTRAL, Ovid, CINAHL | Case reports, case series, other observational studies | 11 | 7 | 177 | N/A |
| Sun c (2020) 60 | Journal article | PubMed, Embase, WoS, CNKI, CENTRAL | Case reports, case series, other observational studies | 17 | 7 | 41 | N/A |
| Teles Abrao (2020) 61 | Journal article | Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Scopus, ClinicalTrials.Gov | Case reports, case series | 16 | 16 | 155 | 118 |
| Trippella (2020) 62 | Journal article | MEDLINE, Embase, Google Scholar, medRxiv | Case reports, case series | 37 | 29 | 275 | 248 |
| Trocado (2020) 63 | Journal article | PubMed, Scopus database, and WHO database | Case reports, case series, other observational studies | 8 | 8 | 95 | 51 |
| Turan (2020) 64 | Journal article | PubMed, Ovid MEDLINE, WoS, China Academic Literature Database | Case reports, case series | 63 | 62 | 637 | 479 |
| Yang (2020a) 9 | Journal article | PubMed, Google Scholar, CNKI, Wanfang Data, VIP, CBMdisc | Case reports, case series, other observational studies | 18 | 17 | 114 | N/A |
| Yang (2020b) 65 | Journal article | PubMed, CNKI, CBMdisc, Wanfang Data | Case reports, case series, other observational studies | 22 | 22 | N/A | 83 |
| Yee (2020) 69 | Preprint | PubMed, Embase, WoS | Case series | 9 | 9 | 93 | 103 |
| Yoon (2020) 66 | Journal article | PubMed/MEDLINE, Embase | Case reports, case series | 28 | 28 | 223 | 201 |
| Zaigham (2020) 67 | Journal article | MEDLINE, Embase, Google Scholar | Case reports, case series | 18 | 18 | 108 | 87 |
Abbreviations: CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature; CNKI, China National Knowledge Infrastructure; N/A, not available; WHO, World Health Organization; WoS, Web of Science.
All the primary studies providing information about any of the outcomes of interest were considered as relevant. This number may be different from the number of included studies in the review for several reasons: the review may have a broader scope, a primary study did not assess any of our outcomes of interest, or primary studies were not well referenced in the review and the authors did not answer our emails.
Pregnant women infected with SARS‐CoV‐2.
This systematic review also included pregnant women infected with severe acute respiratory syndrome coronavirus or Middle Eastern respiratory syndrome coronavirus.
This systematic review also included non‐pregnant adults and children but described separately outcomes in pregnant women.
For outcomes “preterm delivery” and “birthweight£ it included case‐control or cohort study with pregnant women without COVID‐19 as a control group. For outcome “vertical transmission” it included cross‐sectional studies, case‐control, cohort, case report, or case series.
Overall, 48 SRs had a high risk of bias. 7 , 11 , 21 , 22 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 67 , 68 , 69 One SR had a low risk of bias 23 and three SRs had an unclear risk of bias. 9 , 53 , 66 Figure 2 provides the overall assessment, and Table 2 provides the detailed assessments with the ROBIS tool. Regarding the overlap assessment, the overall CCA was 9.93%, with 64.7% of all possible pairs of SRs showing a very high overlap. Figure 3 provides a detailed assessment of the CCA and the Table S2 provides a matrix of evidence with the included SRs in the columns and their respective primary studies in the rows.
FIGURE 2.
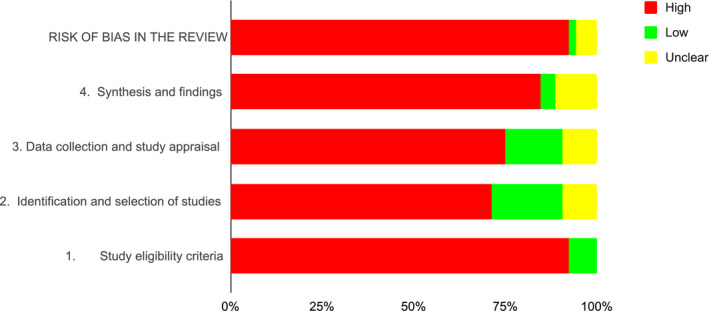
Overall risk of bias of the included systematic reviews
TABLE 2.
Risk of bias of each included systematic review using ROBIS
| Review | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|
| 1. Study eligibility criteria | 2. Identification and selection of studies | 3. Data collection and study appraisal | 4. Synthesis and findings | Risk of bias in the review | |
| AbdelMassih (2020) 21 |

|

|

|

|

|
| Akhtar (2020) 22 |

|

|

|

|

|
| Allotey (2020) 23 |

|

|

|

|

|
| Arabi (2020) 24 |

|

|

|

|

|
| Ashraf (2020) 25 |

|

|
? | ? |

|
| Banaei (2020) 26 |

|

|
? |

|

|
| Capobianco (2020) 27 |

|

|
? |

|

|
| Chamseddine (2020) 28 |

|

|

|

|

|
| Chang (2020) 29 |

|

|

|

|

|
| Chi (2020) 30 |

|

|

|

|

|
| Della Gatta (2020) 31 |

|

|

|

|

|
| Dhir (2020) 32 |

|

|

|

|

|
| Di Mascio (2020) 11 |

|

|

|

|

|
| Diriba (2020) 33 |

|

|

|

|

|
| Duran (2020) 34 |

|

|

|

|

|
| Elshafeey (2020) 35 |

|

|

|

|

|
| Furlan (2020) 36 |

|

|

|

|

|
| Gajbhiye (2020) 37 |

|
? |

|

|

|
| Gao (2020) 38 |

|

|

|

|

|
| Gordon (2020) 39 |

|

|

|

|

|
| Huntley (2020) 40 |

|

|

|

|

|
| Juan (2020) 41 |

|

|

|
? |

|
| Jutzeler (2020) 42 |

|

|

|

|

|
| Kasraeian (2020) 43 |

|

|

|

|

|
| Khalil (2020) 44 |

|

|
? |

|

|
| Khan (2020) 45 |

|

|

|

|

|
| Kotlyar (2020) 46 |

|

|

|

|

|
| Lopes de Sousa (2020) 47 |

|

|

|

|

|
| Matar (2020) 48 |

|

|

|

|

|
| Melo (2020) 49 |

|

|

|

|

|
| Mirbeyk (2020) 50 |

|

|

|

|

|
| Muhidin (2020) 51 |

|

|

|
? |

|
| Mullins (2020) 7 |

|

|

|

|

|
| Parazzini (2020) 52 |

|

|

|

|

|
| Paulino Vigil‐De Gracia (2020) 53 |

|
? |

|
? | ? |
| Pettirosso (2020) 54 |

|

|

|

|

|
| Rodríguez‐Blanco (2020) 55 |

|

|

|

|

|
| Sepúlveda‐ Martinez (2020) 68 |

|

|

|

|

|
| Sharps (2020) 56 |

|

|
? |

|

|
| Simões (2020) 57 |

|
? |

|

|

|
| Smith (2020) 58 |

|

|

|

|

|
| Soheili (2020) 59 |

|

|

|

|

|
| Sun (2020)‡, 60 |

|

|

|

|

|
| Teles Abrao (2020) 61 |

|

|

|
? |

|
| Trippella (2020) 62 |

|

|

|

|

|
| Trocado (2020) 63 |

|

|

|

|

|
| Turan (2020) 64 |

|

|

|

|

|
| Yang (2020) A 9 |

|

|

|
? | ? |
| Yang (2020) B 65 |

|

|

|

|

|
| Yee (2020) 69 |

|

|

|

|

|
| Yoon (2020) 66 |

|
? |

|

|
? |
| Zaigham (2020) 67 |

|
? |

|

|

|
Abbreviations:  , high risk;
, high risk;  , low risk;
, low risk;  , unclear risk.
, unclear risk.
FIGURE 3.
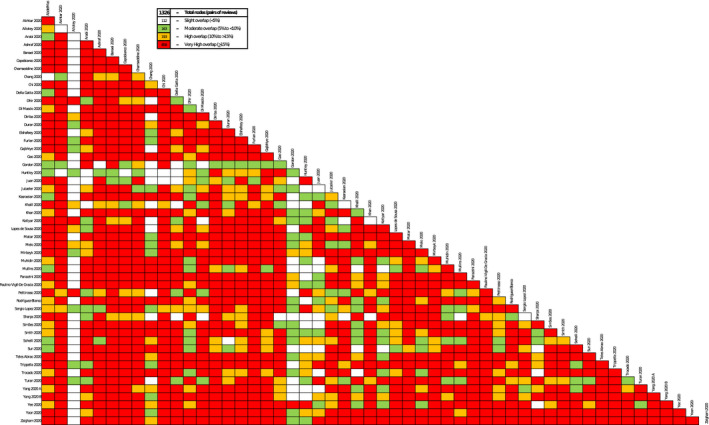
Detailed assessment of corrected covered area. Our overview includes several systematic reviews (SRs), and each SR includes primary studies. It is expected that some primary studies are included in two or more SRs, which is known as “overlap of primary studies”. To assess this overlap, there is a formula known as corrected covered area (CCA), 18 where values below 5% are considered low overlap; between 5% and 10% are considered moderate; between 10% and 15% are considered high; and above 15% are considered very high. Usually overlap is presented as an overall assessment for the whole body of evidence, but this approach sometimes fails to identify which specific SRs are contributing to double‐counting of the same primary studies. In this figure, we present not an overall CCA, but a CCA for each pair of SRs. White boxes represent low overlap (CCA <5%), green boxes represent moderate overlap (CCA between >5% and <10%), yellow boxes represent high overlap (CCA between >10% and <15%), and red boxes represent very high overlap (CCA ≥ 15%). The interpretation of each one of these boxes or “nodes” involves two SRs: a white node means that there are none or a minimum proportion of primary studies shared by the two SRs assessed, whereas a red node means that there is a considerable amount of primary studies shared by the pair of SRs assessed
3.1. Maternal outcomes
Maternal death was reported in 32 SRs, 7 , 11 , 22 , 35 , 37 , 40 , 41 , 42 , 43 , 44 , 45 , 47 , 48 , 50 , 51 , 53 , 54 , 55 , 57 , 58 , 59 , 60 , 62 , 64 , 67 , 68 and varied from 0% to 11.1% among the included reviews. 33 SRs 11 , 22 , 23 , 35 , 36 , 37 , 40 , 41 , 42 , 43 , 44 , 47 , 48 , 50 , 51 , 52 , 53 , 55 , 58 , 59 , 60 , 61 , 62 , 64 , 66 , 67 , 68 assessed the requirement of admission to intensive care or mechanical ventilation support, with overall rates varying from 2.1% to 28.5% and from 1.6% to 11%, respectively. Forty‐two SRs estimated preterm deliveries for <37 weeks of gestation, 7 , 9 , 11 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 35 , 36 , 37 , 38 , 39 , 40 , 43 , 44 , 45 , 48 , 49 , 50 , 51 , 52 , 53 , 56 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 66 , 67 , 69 with rates varying between 14.3% and 61.2%. Another eight SRs 11 , 21 , 33 , 36 , 39 , 44 , 48 , 64 estimated preterm deliveries for <34 weeks of gestation, with rates varying between 3.3% and 40.3%. Premature rupture of membranes varied between 2.5% and 26.5% in 23 SRs, 11 , 22 , 43 , 48 , 51 , 53 , 55 , 56 , 59 , 60 , 61 , 62 , 63 , 64 , 66 , 69 and cesarean delivery varied between 48.3% and 100% in 47 SRs. 7 , 9 , 11 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 55 , 56 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 Table 3 provides details of the results for each maternal outcome.
TABLE 3.
Maternal outcomes
| Review | No. of pregnant women a | No. delivered a | Maternal death, n/N (%) b | Admission to ICU, n/N (%) b | Mechanical ventilation required, n/N (%) b | Delivery <37 weeks, n/N (%) b | Delivery <34 weeks, n/N (%) b | Preterm rupture of membranes, n/N (%) b | Cesarean delivery, n/N (%) b |
|---|---|---|---|---|---|---|---|---|---|
| AbdelMassih (2020) 21 | 1787 | 1787 | N/A | N/A | N/A | 3 | 1 | N/A | N/A |
| Akhtar (2020) 22 | 156 | N/A | 8 | N/A | 11 | 27 | N/A | 12 | 66 |
| Allotey c (2020) 23 | 11 432 | N/A | 73/11 580 (0%; 95% CI 0%–1%) | 323/10 901 (4%; 95% CI 2%–7%) | 155/10 713 (3%; 95% CI 1%–5%) | 386/1872 (17%; 95% CI 13%–21%) | N/A | 28/436 (5%; 95% CI 3%–8%) | 1060/1933 (65%; 95% CI 57%–73%) |
| Arabi c (2020) 24 | 50 | N/A | N/A | N/A | N/A | (20%; 95% CI 4%–4.1%) | N/A | N/A | 47 (100%; 95% CI 95%–100%) |
| Ashraf (2020) 25 | 90 | N/A | 1 | 3 | 3 | 29 | N/A | 16 | 81 |
| Banaei (2020) 26 | 123 | N/A | 0 | 1 | 1 | 30 | N/A | N/A | 99 |
| Capobianco c (2020) 27 | 114 | N/A | 0 | 3 (13%; 95% CI 4%−25%) | N/A | 22 (23%; 95% CI 11%−39%) | N/A | 5 | 95 (88%; 95% CI 82%−94%) |
| Chamseddine (2020) 28 | 164 | 110 | 1/163 (0.6%) | N/A | N/A | 26/128 (20%) | N/A | 4/110 (3.6%) | 93/110 (84.5%) |
| Chang (2020) 29 | 18 | 18 | N/A | N/A | N/A | 10/18 (56%) | N/A | N/A | 16/18 (89%) |
| Chi (2020) 30 | 107 | N/A | N/A | 2/107 | N/A | 25/105 (23.8%) | N/A | N/A | 92/105 (87.6%) |
| Della Gatta (2020) 31 | 51 | 48 | N/A | 2 | 1 | 15 | N/A | 9/34 (26.5%) | 46/48 (95.8%) |
| Dhir (2020) 32 | 2035 | 1184 | N/A | N/A | N/A | 297/1168 (25%) | N/A | N/A | 730/1168 (65%) |
| Di Mascio c (2020) 11 | 41 | 41 | 0/41 (0%) | 2/32 (6.3%) | 1/31 (3.2%) | 14/32 (43.8%) | 4/32 (12.5%) | 5/31 (16.1%) | 38/41 (92.7%) |
| Diriba c (2020) 33 | 1271 | N/A | 8/523 (1.5%; 95% CI 1.2%–9.6%) | 53/186 (28.5%; 95% CI 23.1%–54.4%) | N/A | 86/602 (14.3%; 95% CI 9.2%–33.2%) | 61/682 (8.9%; 95% CI 6.1%–19.3%) | 16/179 (8.9%; 95% CI 5.5%–14.6%) | 426/747 (57%; 95% CI 48.9%–78.7%) |
| Duran (2020) 34 | 195 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 48 |
| Elshafeey (2020) 35 | 385 | 252 | 1 | 17/385 (4.4%) | 6/385 (1.6%) | 39/256 (15.2%) | N/A | N/A | 175/252 (69.4%) |
| Furlan (2020) 36 | 284 | N/A | N/A | 6/284 (2.1%) | N/A | 60 | 3 | N/A | 149 |
| Gajbhiye (2020) 37 | 441 | 387 | 9/441 (2%) | (11%) | (11%) | /380 (26%) | N/A | /344 (9%) | (80%) |
| Gao (2020) 38 | 236 | N/A | N/A | N/A | N/A | 27/116 (23.3%) | N/A | N/A | 129/187 (69%) |
| Gordon (2020) 39 | N/A | N/A | N/A | N/A | N/A | 3/9 (33%) | 2/9 (22%) | N/A | 6/7 (86%) |
| Huntley (2020) 40 | 538 | 435 | 0/348 (0%) | 8/263 (3%) | N/A | 57/284 (20%) | N/A | N/A | 332/392 (85%) |
| Juan d (2020) 41 | 295 | 219 | 7/295 (2.4%) | 12/253 (4.7%) | 3/170 (1.8%) | N/A | N/A | N/A | 171/219 (78.1%) |
| Jutzeler (2020) 42 | N/A | N/A | 0/9 (0%) | 1/1 | N/A | N/A | N/A | N/A | N/A |
| Kasraeian c (2020) 43 | 87 | N/A | 0/87 (0%; 95% CI 0%–7%) | /32 (2.7%) | N/A | /41 (61.2%) | N/A | 4/31 (13.9%) | /69 (92.2%) |
| Khalil c (2020) 44 | 2567 | 746 | 43/2468 (0.9%; 95% CI 0.4%–2.3%) | 159/1591 (7.0%; 95% CI 4.4%–10.9%) | 92/1680 (3.4%; 95% CI 1.5%–7.7%) | 183/746 (21.8%; 95% CI 14.6%−31.3%) | 13/147 (3.3%; 95% CI 0.2%–31.9%) | N/A | 390/746 (48.3%; 95% CI 34.1%–62.7%) |
| Khan (2020) 45 | 101 | 56 | 1/101 (1%) | N/A | N/A | 17/56 (30%) | N/A | N/A | 47/56 (84%) |
| Kotlyar (2020) 46 | 1566 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
659/901 (73%) 32/44 (73%) e |
| Lopes de Sousa (2020) 47 | 755 | 587 | 8/755 (1%) | 100/598 f (16.7%) | N/A | N/A | N/A | N/A | 380/587 f (64.7%) |
| Matar c (2020) 48 | 136 | N/A | 1 (11.1%; 95% CI 6.3%–18.7%) | N/A | 2 | 31/94 (37.7%; 95% CI 26.9%–50.0%) | 5 | 8 | (76.3%; 95% CI 65.8%–84.2%) |
| Melo (2020) 49 | 60 f | 60 | N/A | N/A | N/A | 10/60 | N/A | N/A | 31 |
| Mirbeyk (2020) 50 | 386 | 299 | 2/386 (0.5%) | N/A | 10 (2.8%) | 65/276 (23.6%) | N/A | N/A | 257/299 (86.0%) |
| Muhidin (2020) 51 | 89 | 86 | 0 | 2 | 2 | 30 | N/A | 6 | 79/86 (91.9%) |
| Mullins (2020) 7 | 32 | 29 | 0/32 (0%) | N/A | N/A | 15/32 (46.9%) | N/A | N/A | 27/29 (93%) |
| Parazzini c (2020) 52 | 71 | 64 | N/A | 2/31 (6.5%; 95% CI 0.8%–2.4%) | N/A | 19/48 (39.6%; 95% CI 25.8%–54.7%) | N/A | N/A | 58 |
| Paulino Vigil‐De Gracia (2020) 53 | 83 | N/A | 0/83 (0%) | N/A | 3/83 (3.6%) | 4 | N/A | (9.6%) | (89%) |
| Pettirosso (2020) 54 | 1287 | 1002 | 8 | N/A | N/A | N/A | N/A | N/A | N/A |
| Rodríguez‐Blanco (2020) 55 | 79 | N/A | 0/79 (0%) | 3/70 (4.3%) | 3/70 (4.3%) | N/A | N/A | 9/74 (12.2%) | 65/73 (89.0%) |
| Sepúlveda‐Martinez c (2020) 68 | 292 | 251 | 0/292 (0%) | N/A | 3/292 (2%; 95% CI 1%–4%) | N/A | N/A | N/A | 176/220 (79%; 95% CI 69%–88%) |
| Sharps (2020) 56 | 325 | N/A | N/A | N/A | N/A | 58/225 (25.8%) | N/A | 9 (2.5%) | 83 (58%) |
| Simões (2020) 57 | N/A | N/A | 0 | N/A | N/A | N/A | N/A | N/A | N/A |
| Smith (2020) 58 | 92 | N/A | 0 | 1/23 (4.3%) | 1/23 (4.3%) | 6/13 (46%) | N/A | N/A | 40/50 (80%) |
| Soheili c (2020) 59 | 177 | N/A | 0 | 2 | 1 | 43/151 (28%; 95% CI 12%–44%) g | N/A | 11 | /94 (86%; 95% CI 75%–95%) |
| Sun c (2020) 60 | 41 | N/A | 0/41 (0%) | 2/41 (4.9%) | 2/41 (4.9%) | 17/41 (46%; 95% CI 30%–60%) | N/A | 3 (14%; 95% CI 3%–26%) | 33 (91.7%) |
| Teles Abrao (2020) 61 | 155 | 116 | N/A | 5/155 (3.2%) | N/A | 20/118 (17%) h | N/A | 10/116 (8.6%) | 107/116 (92.2%) |
| Trippella (2020) 62 | 275 | 239 | 1/275 (0.4%) | 10/275 (3.6%) | 5/275 (2%) | 48/208 (23%) | N/A | 24/275 (8.7%) | 179/239 (74.9%) |
| Trocado (2020) 63 | 95 | 50 | N/A | N/A | N/A | 18/51 (35.3%) | N/A | 5 (5%) | 47/50 (94%) |
| Turan (2020) 64 | 637 | 485 | 10/637 (1.6%) | 61/637 (9.6%) | 51/637 (8.0%) | 161/479 (33.6%) | 48/119 (40.3%) | 8 | 403/485 (83%) |
| Yang (2020) A 9 | 114 | 98 | N/A | N/A | N/A | (21.3%) | N/A | N/A | 89/98 (90.8%) |
| Yang (2020) B 65 | N/A | 83 | N/A | N/A | N/A | N/A | N/A | N/A | 73/83 (88%) |
| Yee c (2020) 69 | 93 | N/A | N/A | N/A | N/A | 17/68 (29.4%; 95% CI 9.6%–53.6%) | N/A | 9/71 (11.7%; 95% CI 4.5%–21.1%) | N/A |
| Yoon (2020) 66 | 223 | 201 | N/A | 5/223 (2.2%) | 5/223 (2.2%) | 48/185 (25.9%) g | N/A | 16/126 (12.7%) | 163/185 (88.1%) |
| Zaigham (2020) 67 | 108 | 86 | 0/108 (0%) | 3/108 (3%) | N/A | 20/48 (42%) | N/A | N/A | 79/86 (92%) |
Abbreviation: N/A, not available.
Pregnant women infected with SARS‐CoV‐2.
n, n/N (%) or (%; 95% CI) from meta‐analyses (fixed or random effect), according to the availability of data in the included systematic reviews.
Some outcomes were estimated from meta‐analyses using fixed or random effects.
Data from consecutive case series are presented, as the author of the review used these data to combine results from primary studies.
Data from case reports are presented separately for this outcome.
Inconsistency between tables and text, or within the manuscript for this outcome.
Weeks were not specified.
Nineteen women delivered between 32 and 36 weeks, and one before 32 weeks.
3.2. Neonatal outcomes
Stillbirth and neonatal death were assessed in 35 SRs 7 , 9 , 11 , 30 , 31 , 35 , 36 , 37 , 38 , 43 , 44 , 45 , 47 , 48 , 50 , 51 , 53 , 54 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 64 , 66 , 67 , 69 and 45 SRs, 7 , 9 , 11 , 22 , 43 , 44 , 45 , 47 , 48 , 50 , 51 , 52 , 53 , 54 , 55 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 66 , 67 , 68 , 69 respectively, with rates varying from 0% to 8% for stillbirth, and from 0% to 11.7% for neonatal death. Estimates of admission to special or intensive care units among neonates born to pregnant women infected with SARS‐CoV‐2 varied between 2.1% and 76.9% in 16 SRs, 11 , 41 , 48 , 52 , 58 , 61 , 62 , 64 and the requirement for mechanical ventilation varied between 0.4% and 1.2% in four SRs. 21 , 35 , 62 , 66 One SR 32 estimated the rates of admission to special or intensive care units (38%), and requirement for mechanical ventilation only among newborns who were infected with SARS‐CoV‐2 (17.2%). Fifteen SRs 11 , 58 , 60 , 62 , 63 , 64 , 68 estimated the rate of APGAR score below 7 at 5 min among neonates born to mothers with COVID‐19 between 0% and 4.4%, and 45 SRs 9 , 11 , 21 , 22 , 40 , 41 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 58 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 estimated the rates of infection status of the newborn between 0% and 11.5%. Table 4 provides details of the results for each neonatal outcome.
TABLE 4.
Neonatal outcomes
| Review | No. of newborns a | No. of tested newborns | Stillbirth, n/N (%) b | Neonatal death, n/N (%) b | Neonatal admission to special care and/or NICU, n/N (%) b | Mechanical ventilation required, n/N (%) b | APGAR score <7 at 5 min, n/N (%) b | Infection status of the newborn, n/N (%) b |
|---|---|---|---|---|---|---|---|---|
| AbdelMassih 2020 21 | 1787 | N/A | N/A | N/A | N/A | 2 | N/A | 45 |
| Akhtar 2020 22 | 108 | N/A | 9 | 3 | N/A | N/A | N/A | 7 |
| Allotey c 2020 23 | N/A | N/A | 18/2837 (0%; 95% CI 0%–0%) | 6/1728 (0%; 95% CI 0%–0%) | 368/1348 (25%; 95% CI 14%–37%) | N/A | 11/500 (1%; 95% CI 0%–2%) | N/A |
| Arabi c 2020 24 | N/A | N/A | N/A | 0 (0%; 95% CI 0%–2%) | N/A | N/A | N/A | N/A |
| Ashraf 2020 25 | 92 | N/A | 1 | 1 | N/A | N/A | N/A | 4 |
| Banaei 2020 26 | 124 | N/A | 1 | 1 | 4 | N/A | N/A | 5 |
| Capobianco c 2020 27 | 108 | N/A | 1 | 2 | N/A | N/A | N/A | 4 (6%; 95% CI 2%−12%) |
| Chamseddine 2020 28 | 128 | 44 | 3/163 (1.8%) | 1/128 (0.8%) | N/A | N/A | 3/68 (4.4%) | 3/44 (6.8%) |
| Chang 2020 29 | 19 | 19 | N/A | 0 | N/A | N/A | N/A | 0/19 (0%) |
| Chi 2020 30 | 105 | 91 | 1/107 (0.9%) | 1/105 (1.0%) | N/A | N/A | N/A | 8/91 (8.8%) |
| Della Gatta 2020 31 | 48 | 48 | 1/48 (2.1%) | 1/48 (2.1%) | 1/48 (2.1%) | N/A | N/A | 1/48 (2.1%) |
| Dhir 2020 32 | 1184 | 1048 | N/A | 1/1184 (0.1%) | 22/58 (38%) d | 10/58 (17.2%) d | 2/25 e | 58/1048 (5.5%) |
| Di Mascio c 2020 11 | 42 | 42 | 1/41 (2.4%) | 1/41 (2.4%) | 1/10 (10%) | N/A | 1/41 (2.4%) | 0/42 (0%) |
| Diriba c 2020 33 | N/A | N/A | N/A | 5/430 (1.2%; 95% CI 1%–8.7%) | 8/69 (11.6%; 95% CI 5.4%–22.6%) | N/A | 1/72 (1.4%; 95% CI 0%–8.9%) | 0/1271 (0%; 95% CI 0%–1.5%) f |
| Duran 2020 34 | 222 | N/A | N/A | 1 | 111 | N/A | N/A | 13 |
| Elshafeey 2020 35 | 256 | N/A | 2/385 (0.5%) | 1/256 (0.4%) | 8/256 (3.1%) | 3/256 (1.2%) | N/A | 4/256 (1.6%) |
| Furlan 2020 36 | 188 | N/A | 1/188 | 1/188 | N/A | N/A | N/A | 4 |
| Gajbhiye 2020 37 | 391 | 313 | 6/344 (1.7%) | 4/369 (1.1%) | (8%) | N/A | N/A | 24/313 (7.7%) |
| Gao 2020 38 | N/A | N/A | 1/13 (7.7%) | 1/9 (11.1%) | N/A | N/A | N/A | 3/167 (1.8%) |
| Gordon g 2020 39 | 46 | 10 | N/A | 0/10 (0%) | N/A | N/A | 0/3 (0%) | 7/8 (87.5%) |
| Huntley 2020 40 | 435 | 310 | N/A | 1/313 (0.3%) | 137/211 (64.9%) | N/A | 1/203 (0.5%) | 0/310 (0%) |
| Juan 2020 41 | 221 | 160 | N/A | 1/221 (0.5%) | 49/173 (28.3%) | N/A | N/A | 3/160 (1.9%) |
| Jutzeler 2020 42 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Kasraeian c 2020 43 | 86 | 50 | 0.2% | 0.2% | N/A | N/A | N/A | 0/50 (0%) |
| Khalil c 2020 44 | N/A | N/A | 12/1362 (0.9%; 95% CI 0.5%–1.5%) | 4/688 (0.6%; 95% CI 0.2%–1.5%) | N/A | N/A | N/A | 19/751 (1.4%; 95% CI 0.4%–4.7%) |
| Khan 2020 45 | 56 | 43 | 0/56 (0%) | 1/56 (1.8%) | N/A | N/A | N/A | 1/43 (2.3%) |
| Kotlyar c 2020 46 | N/A | 979 | N/A | N/A | N/A | N/A | N/A | 27/936 (3.2%; 95% CI 2.2%–4.3%) |
| Lopes de Sousa 2020 47 | 598 | 493 | 2/755 (0.3%) h | 10/598 (1.7%) | N/A | N/A | 16/595 (2.7%) | 9/493 (1.8%) |
| Matar c 2020 48 | 94 | N/A | 2 | 3 (11.7%, 95% CI 6.8%–19.2%) | (63.7%; 95% CI 37.8%–83.5) | N/A | N/A | 2 (11.5%; 95% CI 6.7%–19.2%) |
| Melo 2020 49 | 432 h | 432 h | N/A | N/A | N/A | N/A | 1/10 (10%) d | 10/432 (2.3%) i |
| Mirbeyk 2020 50 | 302 | 219 | 1/386 (0.3%) | 3/302 (1.0%) | N/A | N/A | N/A | 11/219 (5%) |
| Muhidin 2020 51 | 89 | N/A | 1 | 2/89 (2.2%) | N/A | N/A | N/A | 0 |
| Mullins 2020 7 | 29 | 15 | 1/32 (3.1%) | 1/29 (3.4%) | N/A | N/A | N/A | N/A |
| Parazzini 2020 52 | 65 | 45 | N/A | 1/65 (1.5%) | 3 | N/A | 0/54 (0%) | 2/45 (4.4%) |
| Paulino Vigil‐De Gracia 2020 53 | 84 | N/A | 1 | 1 | N/A | N/A | N/A | 4 |
| Pettirosso 2020 54 | N/A | 655 | 7 | 6 | N/A | N/A | 6 | 19/655 (2.9%) |
| Rodríguez‐Blanco c 2020 55 | 74 | 66 | N/A | 1/74 (1.4%) | N/A | N/A | 0/57 (0%) | 0/66 (0%) |
| Sepúlveda‐ Martinez c 2020 68 | 252 | 223 | N/A | 2/252 (1%; 95% CI 0%–3%) | N/A | N/A | 1/198 (0.5%) | 5/223 (1%; 95% CI, 2%–19%) |
| Sharps 2020 56 | N/A | 307 | 11 | N/A | N/A | N/A | N/A | 7/307 (2.3%) |
| Simões 2020 57 | N/A | N/A | 0 | 1 | N/A | N/A | N/A | N/A |
| Smith 2020 58 | 60 | 18 | 1/37 (2.7%) | 1/37 (2.7%) | 11/13 (76.9%) | N/A | 0/32 (0%) | 1/21 (4.8%) |
| Soheili c 2020 59 | N/A | N/A | 2/65 (2%; 95% CI 1%–6%) | 2/65 (4%; 95% CI 1%–9%) | N/A | N/A | N/A | N/A |
| Sun c 2020 60 | N/A | 29 | 1/41 (8%; 95% CI −0.07% to 23%) | 1 | N/A | N/A | 0 | 0/29 (0%) |
| Teles Abrao 2020 61 | 118 | 95 | 1/118 (0.8%) | 1/118 (0.8%) | 24/118 (20.3%) | N/A | N/A | 1/95 (1.1%) |
| Trippella 2020 62 | 248 | 191 | 2/248 (0.8%) | 1/248 (0.4%) | 4/16 (25%) | 1/248 (0.4%) | 5/190 (2.6%) | 16/191 (8.4%) |
| Trocado 2020 63 | 51 | 48 | N/A | 1/51 (2.0%) | N/A | N/A | 0 | 1/48 (2.1%) |
| Turan 2020 64 | 479 | 405 | 7/479 (1.4%) | 5/479 (1.0%) | 54/479 (11.3%) | N/A | 6/361 (1.7%) | 8/405 (2%) |
| Yang 2020 A 9 | 84 | N/A | 1/98 (1.0%) | 1/84 (1.2%) | N/A | N/A | N/A | 7/84 (8.3%) |
| Yang 2020 B 65 | 83 | 83 | N/A | N/A | N/A | N/A | N/A | 9/83 (10.9%) |
| Yee c 2020 69 | 103 | 68 | 2/56 (1.7%; 95% CI 0.0%–8.8%) | 0/70 (0%; 95% CI 0.0%–2.5%) | N/A | N/A | N/A | 4/68 (2.2%; 95% CI 0.0%–9.3%) |
| Yoon 2020 66 | 201 | 167 | 2/201 (1.0%) | 1/177 (0.6%) | N/A | 1 | N/A | 4/167 (2.4%) |
| Zaigham 2020 67 | 87 | 75 | 1/87 (1.1%) | 1/87 (1.1%) | N/A | N/A | N/A | 1/75 (1.3%) |
Abbreviation: N/A, not available.
Born to women infected with SARS‐COV‐2.
n, n/N (%) or (%; 95% CI) from meta‐analyses (fixed or random effect), according to the availability of data in the included systematic reviews.
Some outcomes were estimated from meta‐analyses using fixed or random effects.
Data from newborns with confirmed SARS‐CoV‐2 infection.
Only reported for case reports included in the review.
The review estimated this outcome using the number of pregnant women as the denominator (N).
Only newborns infected with SARS‐CoV‐2 are included in this review.
Inconsistency between tables and text or within the manuscript for this outcome.
Sixteen newborns had a positive RT‐PCR in nasopharyngeal swab but authors of the systematic review only considered ten as possible vertical transmission.
4. DISCUSSION
This overview of SRs summarizes and critically appraises findings regarding the prognosis of pregnant women with COVID‐19 and their newborns. We retrieved a total of 52 SRs assessing maternal and perinatal outcomes in COVID‐19. However, only one of them (2%) of them was at low risk of bias; this SR 23 was qualified at low risk of bias by satisfactorily fulfilling all steps of the ROBIS. There was a moderate overall overlap of primary studies (CCA = 9.93%), with 858 pairs of SRs presenting a very high overlap, which indicates redundant efforts. Despite this overlap, the included SRs reported very heterogeneous results for maternal and perinatal outcomes related to COVID‐19 in pregnancy, and considering the confidence intervals reported by the reviews, the heterogeneity among the results was even higher.
During this pandemic, healthcare decision‐makers urgently required information to produce evidence‐based guidelines: this requirement probably explains the high number of retrieved SRs. However, and probably in response to the rush when elaborating the SRs, more than 95% of the SRs included in this overview were at high risk of bias, resulting in useless information for the above‐mentioned purpose. Multiple factors may be involved in the variability of the reported results among the reviews. First, the number of included primary studies that were relevant in the included SRs ranged from 5 7 , 39 to 81, 44 and the number of pregnant women included ranged from 18 29 to 11 432 23 among the reviews. For this reason, certain reported results might falsely alarm clinicians, for example: one SR 43 reports that 61% of the deliveries were preterm (before 37 weeks of gestation) using a sample of only 41 pregnancies, and another SR 31 reports 26.5% premature rupture of membranes estimation from a sample size of 34 patients. In both examples, patients were only from case reports and series of cases, which further reduces reliability. Another important factor is that the inclusion criteria for the pregnant women varied among different primary studies and SRs, resulting in inclusion of patients with diverse severity of disease. Outcomes from primary studies would depend on the testing strategies that were used: if a population‐based study includes all pregnant women who tested positive for SARS‐CoV‐2 regardless of the severity of their disease, it would surely report better outcomes than a series of independent cases. Because of this variability in the reported results and the high risk of bias of more than 95% of the reviews, we cannot safely draw conclusions about maternal and perinatal outcomes.
Despite the above, the SR by Allotey et al 23 is at low risk of bias, so some of its results should be highlighted. The authors report a 17% (95% CI 13%‐21%) rate of preterm births among live births, which is slightly higher than the global report of 11% in non‐COVID‐19 pregnancies. 70 Interestingly, when they analyzed the preterm births in pregnant women with COVID‐19, the rates of premature rupture of membranes and spontaneous labor among those women reached only 5% and 6%, respectively, 23 allowing us to hypothesize that the preterm deliveries reported in the other included SRs were mostly iatrogenic. On the other hand, the rate of cesarean section reported by Allotey et al seems alarming: 65% (95% CI 57%‐73%). This is higher than the global report published in The Lancet, showing cesarean sections rates of 28.8% in East Asia and Pacific, 32% in North America, and 26.9% in western Europe, 71 and is surely conflicts with WHO’s statement, which declares that cesarean section frequencies higher than 15% are not associated with reductions in maternal and newborn mortality rates. 72
Allotey et al reported high rates of intensive care admission of neonates born to women with COVID‐19 (25%), but the authors did not assess the neonatal requirement for mechanical ventilation. Other SRs, 35 , 62 , 73 at a high risk of bias, reported a 0.4%‐1.2% neonatal requirement for mechanical ventilation. Although no SR describes the criteria for neonatal intensive care admission, some SRs 48 , 61 , 62 , 65 , 67 showed that an important proportion of mothers and newborns were isolated for 14 days, which leads us to hypothesize that this isolation may have increased the rate of neonatal intensive care requirement.
The SR at low risk of bias did not assess the infection status of the newborn, but Khalil et al 44 —in an SR at high risk of bias including 2567 pregnant women—reported a rate of 1.4% neonatal SARS‐CoV‐2 positivity, which is certainly infrequent, but leads us to ponder that in utero and intrapartum vertical transmission might be possible. The presence of IgG antibodies but not IgM antibodies against SARS‐CoV‐2 in newborns of mothers with positive antibodies suggests transplacental passage of antibodies more than in utero vertical transmission of SARS‐CoV‐2. 73 Besides, the presence of SARS‐CoV‐2 has been described in such different tissues as placenta, umbilical cord, and amniotic fluid, and in neonatal swabs, such as rectal and nasopharyngeal. 46 If we consider that transplacental passage of pathogens increases with the advance of gestational age and that positive viremia occurs in only 1% of adult patients with COVID‐19, the transplacental passage of SARS‐CoV‐2 seems to be unlikely. 74 Regarding intrapartum vertical transmission, it is important to note that the available literature has shown no cases of vaginal samples testing positive for SARS‐CoV‐2. 75 , 76 Finally, the clinical implementation of a correct classification system and a case definition of SARS‐CoV‐2 in pregnant women, fetuses, and neonates is required to guide good clinical practice and future investigations. 77
Our overview has some limitations. We did not undertake a pooled analysis of the results for each outcome because of the expected variability of methods and study designs among the primary and secondary studies retrieved. Also, we did not assess the risk of bias of the primary studies included in each SR, which makes it impossible for us to prudently conclude about clinical outcomes reported in the reviews. Our overview has several strengths. We comprehensively appraised the risk of bias of the included SRs and the overlap of the primary studies among SRs. We performed an exhaustive search and selection of studies, we considered all clinically relevant maternal and perinatal outcomes, and we comprehensively described the characteristics and the results of each included SR.
The available information regarding COVID‐19 has grown rapidly since WHO declared the outbreak a pandemic. 12 In the case of maternal and perinatal outcomes related to SARS‐CoV‐2 infection, the 52 included SRs have already searched the research field. The primary data summarized by these SRs derive mainly from case reports and case series, which are the first studies to become available to researchers aiming to provide information on an emerging clinical phenomenon. More recent SRs have included more representative observational studies, 23 but they are still insufficient to guide clinical recommendations with the required certainty of the evidence.
In addition to the lack of major observational studies, most SRs at high risk of bias did not report any concern about the risk of duplicating patients included among the primary studies they summarized, Allotey et al 23 being the most rigorous exception. Duplicate reporting of the same patients—especially when conducting meta‐analyses—is a major methodological error that may distance the findings from a reliable estimation, either under‐ or over‐estimating them. This overview highlights the existence of redundant efforts and provides a starting point for researchers who aim to investigate the prognosis of COVID‐19 in pregnant women and their newborns.
5. CONCLUSION
Only one of the 52 systematic reviews included in this overview were assessed as having low risk of bias and after assessing all possible pairs of included systematic reviews, 64.7% showed a very high overlap of primary studies. The high risk of bias and the overlap among the included reviews highlights the importance of avoiding unnecessary duplication of work and the need to conduct new, high‐quality evidence syntheses of comparative studies to guide clinical decisions.
CONFLICT OF INTEREST
The authors have stated explicitly that there is no conflict of interest in connection with this article.
Supporting information
Appendix S1
Table S1
Table S2
ACKNOWLEDGMENTS
The members of the COVID‐19 L·OVE Working Group and Epistemonikos Foundation have made it possible to build the systems and compile the information needed by this project. Franco Pesce and Gabriel Rada helped to revise the final draft of the manuscript.
REFERENCES
- 1. Eurosurveillance Editorial Team . Note from the editors: World Health Organization declares novel coronavirus (2019‐nCoV) sixth public health emergency of international concern. Euro Surveill. 2020;25:200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui DS, I Azhar E, Madani TA, et al. Novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2019;2020(91):264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20:533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12:1638‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lapinsky SE. Acute respiratory failure in pregnancy. Obstet Med. 2015;8:126‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz DA. An analysis of 38 pregnant women with COVID‐19, their newborn infants, and maternal‐fetal transmission of SARS‐CoV‐2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144(7):799‐805. [DOI] [PubMed] [Google Scholar]
- 7. Mullins E, Evans D, Viner RM, et al. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55:586‐592. [DOI] [PubMed] [Google Scholar]
- 8. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Z, Wang M, Zhu Z, et al. Coronavirus disease 2019 (COVID‐19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2020;30:1‐4. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz DA, Graham AL. Potential maternal and infant outcomes from coronavirus 2019‐nCoV (SARS‐CoV‐2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1–19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM. 2020;2:100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma M, Scarr S, Kelland K. Speed Science: The risks of swiftly spreading coronavirus research. 2020. https://graphics.reuters.com/CHINA‐HEALTH‐RESEARCH/0100B5ES3MG/index.html.
- 13. Glasziou PP, Sanders S, Hoffmann T. Waste in covid‐19 research. BMJ. 2020;369:m1847. [DOI] [PubMed] [Google Scholar]
- 14. Pollock M, Fernandes RM, Pieper D, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pollock M, Fernandes RM, Becker LA, et al. Chapter V: Overviews of Reviews. Cochrane Handbook for Systematic Reviews of Interventions. 2020. https://training.cochrane.org/handbook/current/chapter‐v.
- 16. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rada G, Verdugo‐Paiva F, Ávila C, et al. Evidence synthesis relevant to COVID‐19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020;20:e7868. [DOI] [PubMed] [Google Scholar]
- 18. Pieper D, Antoine S‐L, Mathes T, et al. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67:368‐375. [DOI] [PubMed] [Google Scholar]
- 19. Pérez‐Bracchiglione J, Niño de Guzmán E, Roqué Figuls M, et al. Graphical representation of overlap degree of primary studies in systematic reviews included in overviews. In: Abstracts of the 26th Cochrane Colloquium. Cochrane Database Syst Rev. 2020;1:151‐152. [Google Scholar]
- 20. Whiting P, Savović J, Higgins JPT, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. AbdelMassih A, Fouda R, Essam R, et al. COVID‐19 during pregnancy should we really worry from vertical transmission or rather from fetal hypoxia and placental insufficiency? A systematic review and meta‐analysis. Research Square. Posted 8 Sept 2020. 10.21203/rs.3.rs-71847/v1. [DOI]
- 22. Akhtar H, Patel C, Abuelgasim E, et al. COVID‐19 (SARS‐CoV‐2) infection in pregnancy: a systematic review. Gynecol Obstet Invest. 2020;85(4):295‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arabi S, Vaseghi G, Heidari Z, et al. Clinical characteristics of COVID‐19 infection in pregnant women: a systematic review and meta‐analysis. medRxiv. Posted April 07, 2020. https://www.medrxiv.org/content/10.1101/2020.04.05.20053983v1. [Google Scholar]
- 25. Ashraf MA, Keshavarz P, Hosseinpour P, et al. Coronavirus disease 2019 (COVID‐19): a systematic review of pregnancy and the possibility of vertical transmission. J Reprod Infertil. 2020;21:157‐168. [PMC free article] [PubMed] [Google Scholar]
- 26. Banaei M, Ghasemi V, Saei ghare naz M, et al. Obstetrics and neonatal outcomes in pregnant women with COVID‐19: a systematic review. Iran J Public Health. 2020;49:38‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Capobianco G, Saderi L, Aliberti S, et al. COVID‐19 in pregnant women: a systematic review and meta‐analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:543‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chamseddine RS, Wahbeh F, Chervenak F, Salomon LJ, Ahmed B, Rafii A. Pregnancy and neonatal outcomes in SARS‐CoV‐2 infection: a systematic review. J Pregnancy. 2020;2020:4592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang T‐H, Wu J‐L, Chang L‐Y. Clinical characteristics and diagnostic challenges of pediatric COVID‐19: a systematic review and meta‐analysis. J Formos Med Assoc. 2020;119:982‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chi H, Chiu N‐C, Tai Y‐L, et al. Clinical features of neonates born to mothers with coronavirus disease‐2019: a systematic review of 105 neonates. J Microbiol Immunol Infect. 2020:S1684–1182(20)30182–1. 10.1016/j.jmii.2020.07.024. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Della Gatta AN, Rizzo R, Pilu G, et al. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. 2020;223:36‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dhir SK, Kumar J, Meena J, Kumar P. Clinical features and outcome of SARS‐CoV‐2 infection in neonates: a systematic review. J Trop Pediatr. 2020:fmaa059. 10.1093/tropej/fmaa059. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS‐CoV‐2, MERS‐CoV, and SARS‐CoV) during pregnancy and the possibility of vertical maternal‐fetal transmission: a systematic review and meta‐analysis. Eur J Med Res. 2020;25:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duran P, Berman S, Niermeyer S, et al. COVID‐19 and newborn health: systematic review. Rev Panam Salud Publica. 2020;44:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elshafeey F, Magdi R, Hindi N, et al. A systematic scoping review of COVID‐19 during pregnancy and childbirth. Int J Gynecol Obstet. 2020;150:47‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furlan MCR, Jurado SR, Uliana CH, et al. A systematic review of pregnancy and coronavirus infection: maternal, fetal and neonatal outcomes. Rev Cuid. 2020;11(2):e1211‐e1311. [Google Scholar]
- 37. Gajbhiye R, Modi D, Mahale S. Pregnancy outcomes, Newborn complications and Maternal‐Fetal Transmission of SARS‐CoV‐2 in women with COVID‐19: a systematic review of 441 cases. Posted May 05, 2020. medRxiv 2020.04.11.20062356. 2020. 10.1101/2020.04.11.20062356. [DOI] [Google Scholar]
- 38. Gao Y‐J, Ye L, Zhang J‐S, et al. Clinical features and outcomes of pregnant women with COVID‐19: a systematic review and meta‐analysis. BMC Infect Dis. 2020;20:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gordon M, Kagalwala T, Rezk K, et al. Rapid systematic review of neonatal COVID‐19 including a case of presumed vertical transmission. BMJ Paediatr Open. 2020;4:e000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huntley BJF, Huntley ES, Di Mascio D, et al. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS‐Co‐V‐2) infection: a systematic review. Obstet Gynecol. 2020;136:303‐312. [DOI] [PubMed] [Google Scholar]
- 41. Juan J, Gil MM, Rong Z, et al. Effect of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jutzeler CR, Bourguignon L, Weis CV, et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;37:101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kasraeian M, Zare M, Vafaei H, et al. COVID‐19 pneumonia and pregnancy; a systematic review and meta‐analysis. J Matern Fetal Neonatal Med. 2020;9:1‐8. 10.1080/14767058.2020.1763952. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 44. Khalil A, Kalafat E, Benlioglu C, et al. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25:100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Khan MMA, Khan MN, Mustagir MG, et al. COVID‐19 infection during pregnancy: A systematic review to summarize possible symptoms, treatments, and pregnancy outcomes. Posted April 10, 2020. medRxiv 2020.03.31.20049304. 2020. 10.1101/2020.03.31.20049304. [DOI] [Google Scholar]
- 46. Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta‐analysis. Am J Obstet Gynecol. 2021;224(35–53):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lopes de Sousa ÁF, Carvalho HEFd, Oliveira LBD, et al. Effects of COVID‐19 infection during pregnancy and neonatal prognosis: what is the evidence? Int J Environ Res Public Health. 2020;17(11):4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matar R, Alrahmani L, Monzer N, et al. Clinical presentation and outcomes of pregnant women with COVID‐19: a systematic review and meta‐analysis. Clin Infect Dis. 2020:ciaa828. 10.1093/cid/ciaa828. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melo GCD, Araújo KCGMd. COVID‐19 infection in pregnant women, preterm delivery, birth weight, and vertical transmission: a systematic review and meta‐analysis. Cad Saude Publica. 2020;36:e00087320. [DOI] [PubMed] [Google Scholar]
- 50. Mirbeyk M, Rezaei N. The impact of COVID‐19 on pregnancy and neonatal health: a systematic review. Posted 01 May, 2020. PREPRINT (Version 1) available at Research Square. 10.21203/rs.3.rs-25861/v1. [DOI]
- 51. Muhidin S, Behboodi Moghadam Z, Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019‐nCoV; a systematic review. Arch Acad Emerg Med. 2020;8:e49. [PMC free article] [PubMed] [Google Scholar]
- 52. Parazzini F, Bortolus R, Mauri PA, et al. Delivery in pregnant women infected with SARS‐CoV‐2: a fast review. Int J Gynaecol Obstet. 2020;150:41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Paulino Vigil‐De G, Carlos L. Coronavirus infection (SARS‐CoV‐2) in pregnant women: systematic review. April 28, 2020. https://www.authorea.com/users/303207/articles/439306‐coronavirus‐infection‐sars‐cov‐2‐in‐pregnant‐women‐systematic‐review.
- 54. Pettirosso E, Giles M, Cole S, Rees M. COVID‐19 and pregnancy: a review of clinical characteristics, obstetric outcomes and vertical transmission. Aust N Z J Obstet Gynaecol. 2020;60:640‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rodríguez‐Blanco N, Vegara‐Lopez I, Aleo‐Giner L, et al. Revisión exploratoria sobre series de casos de coronavirus (SARS‐CoV, MERS‐CoV y SARS‐CoV‐2) y sus resultados obstétricos y neonatales. [Scoping review of coronavirus case series (SARS‐CoV, MERS‐CoV and SARS‐CoV‐2) and their obstetric and neonatal results]. In Spanish. Rev Esp Quimioter. 2020;33:313‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharps MC, Hayes DJL, Lee S, et al. A structured review of placental morphology and histopathological lesions associated with SARS‐CoV‐2 infection. Placenta. 2020;101:13‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simões E, Silva AC, Leal CRV. Is SARS‐CoV‐2 vertically transmitted? Front Pediatr. 2020;8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smith V, Seo D, Warty R, et al. Maternal and neonatal outcomes associated with COVID‐19 infection: a systematic review. PLoS One. 2020;15:e0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Soheili M, Moradi G, Baradaran HR, et al. Clinical Manifestation and Maternal Complications and Neonatal outcomes in Pregnant Women with COVID 19: An Update a Systematic Review and Meta‐analysis. Posted 12 May, 2020 PREPRINT (Version 1) available at Research Square. 10.21203/rs.3.rs-27383/v1. [DOI] [PubMed]
- 60. Sun P, Gao H, Huang X, et al. Comparison of perinatal outcomes of pregnant women with SARS, MERS and COVID‐19: a systematic review and meta‐analysis. Posted: 1 Jun 2020 Available at SSRN: https://ssrn.com/abstract=3582809.
- 61. Teles Abrao Trad A, Ibirogba ER, Elrefaei A, et al. Complications and outcomes of SARS‐CoV‐2 in pregnancy: where and what is the evidence? Hypertens Pregnancy. 2020;39:361‐369. [DOI] [PubMed] [Google Scholar]
- 62. Trippella G, Ciarcià M, Ferrari M, et al. COVID‐19 in pregnant women and neonates: a systematic review of the literature with quality assessment of the studies. Pathogens. 2020;9:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Trocado V, Silvestre‐Machado J, Azevedo L, et al. Pregnancy and COVID‐19: a systematic review of maternal, obstetric and neonatal outcomes. J Matern Fetal Neonatal Med. 2020;9:1‐13. 10.1080/14767058.2020.1781809. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64. Turan O, Hakim A, Dashraath P, et al. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS‐CoV‐2 infection among hospitalized pregnant women: a systematic review. Int J Gynaecol Obstet. 2020;151:7‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang Z, Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a systematic review. Am J Perinatol. 2020;37:1055‐1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yoon SH, Kang JM, Ahn JG. Clinical outcomes of 201 neonates born to mothers with COVID‐19: a systematic review. Eur Rev Med Pharmacol Sci. 2020;24:7804‐7815. [DOI] [PubMed] [Google Scholar]
- 67. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99:823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sepulveda‐Martinez AS, Lopez S, Riquelme N, Silva MC, Muñoz H, Parra‐Cordero M. Perinatal outcomes and vertical transmission by SARS‐CoV2 infection (COVID‐19) during pregnancy: systematic review and meta‐analysis. Authorea. May 22, 2020. 10.22541/au.159015267.77344658. [DOI]
- 69. Yee J, Kim W, Han JM, et al. Clinical manifestations and perinatal outcomes of pregnant women with COVID‐19: a systematic review and meta‐analysis. Sci Rep. 2020;10:18126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162‐2172. [DOI] [PubMed] [Google Scholar]
- 71. Boerma T, Ronsmans C, Melesse DY, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet. 2018;392:1341‐1348. [DOI] [PubMed] [Google Scholar]
- 72. World Health Organization Human Reproduction Programme, 10 April 2015 . WHO Statement on caesarean section rates. Reprod Health Matters. 2015;23:149‐150. [DOI] [PubMed] [Google Scholar]
- 73. Egerup P, Fich Olsen L, Christiansen A‐M, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibodies at delivery in women, partners, and newborns. Obstet Gynecol. 2021;137:49‐55. [DOI] [PubMed] [Google Scholar]
- 74. Lamouroux A, Attie‐Bitach T, Martinovic J, et al. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus 2. Am J Obstet Gynecol. 2020;223:91.e1‐91.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wu Y, Liu C, Dong L, et al. Coronavirus disease 2019 among pregnant Chinese women: case series data on the safety of vaginal birth and breastfeeding. BJOG. 2020;127:1109‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qiu L, Liu X, Xiao M, et al. SARS‐CoV‐2 is not detectable in the vaginal fluid of women with severe COVID‐19 infection. Clin Infect Dis. 2020;71:813‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shah PS, Diambomba Y, Acharya G, et al. Classification system and case definition for SARS‐CoV‐2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99:565‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1
Table S2


