Dear Editors,
The coronavirus disease 2019 (COVID‐19) emerged in December 2019 and spread worldwide very quickly. The clinical course of the disease varies from complete recovery to an acute respiratory distress syndrome. Patients with COVID‐19 usually show a coagulopathy characterized by a marked increase in D‐dimer, but with a low incidence of both thrombocytopenia and hypofibrinogenaemia. Also, a tendency to develop thrombosis has been described. 1 , 2 These features are different from the coagulopathy observed in other infections and from common disseminated intravascular coagulation (DIC). 3 A considerably marked increase in inflammation markers has been observed in patients with COVID‐19 disease, 4 , 5 , 6 and this pro‐inflammatory state can cause endothelial dysfunction, an increase in tissue factor and von Willebrand factor (VWF) release and an activation of the coagulation cascade, leading altogether to a prothrombotic situation. A disintegrin and metalloprotease with thrombospondin motif 13 (ADAMTS13) regulates the size of VWF multimers and is known to be a marker of endothelium dysfunction in sepsis, and changes in ADAMTS13 and VWF have been reported in the context of an endothelial activation. 7 , 8 , 9 Furthermore, ultrastructural features of endothelial cell destruction and SARS‐CoV‐2 visible within the cell have been described in patients who died from COVID‐19. 10
We aimed to assess the possible involvement of ADAMTS13 in the coagulopathy associated with COVID‐19 and its relevance in the severity and prognosis of the infection.
We carried out a retrospective study in 100 consecutive PCR‐proven COVID‐19 hospitalized patients between 15 March and 1 April 2020. Demographic and clinical information, and laboratory data were obtained from the hospital electronic medical records at the time of admission. Patients were classified into two groups according to the severity of COVID‐19 disease: nonsevere disease and severe disease. Patients were classified as severe if at least one of the following criteria was met: hypoxaemia in need for invasive mechanical ventilation, D‐dimer plasma concentration >3000 ng/mL and at least three of the next: CRP > 15 mg/dL, ferritin > 1000 ng/mL, D‐dimer > 1,500 ng/mL, lymphopenia < 800 x 109/L and/or IL‐6 > 40. 50 patients were classified as severe COVID‐19 disease cases and 50 as nonsevere form of COVID‐19 disease. Blood samples were collected at patient's admission. Laboratory results for prothrombin activity (PA; RecombiPlasTin 2G), active partial thromboplastin (aPTT; SynthAsIL), derived fibrinogen and D‐dimer (D‐Dimer HS 500) were measured using an ACL‐TOP 550 coagulation analyser (Werfen Diagnostics). VWF antigen and ADAMTS13 activity were performed using chemiluminescent assays in ACL AcuStar analyser (Werfen). Platelet and lymphocyte counts were performed in DxH900 analyser (Beckman Coulter). LDH, CRP and ferritin were performed in a Cobas 8000 analyser (Roche Diagnostics). IL‐6 was measured by cytometric bead array (Becton). Continuous variables are presented as median and interquartile range, and discrete variables as numbers and percentage. Normality distribution was tested in our data; the Mann‐Whitney test was used to analyse differences in continuous variables. Spearman's r was used to explore the relationship between ADAMTS13 and VWF, D‐dimer, ferritin and LDH. Fisher's exact was used to explore the association between ADAMTS13 activity below median value and increased D‐dimer plasma concentration. The Kaplan‐Meier analysis was performed to assess whether ADAMTS13 activity can predict mortality, and patients were tracked for 60 days. Univariate and multivariate analyses were performed to assess the influence of laboratory values on the risk of death. Statistical significance was set at P values <.05. Statistical analyses were performed using ibm spss 23.0 (IBM Inc).
A total of 100 hospitalized patients with confirmed COVID‐19 were included in the study. Of the 100 patients, 50 were considered as severe COVID‐19 disease cases and 50 as nonsevere form of COVID‐19 disease. Median age was 60.5 years. Regarding age, there were no significant differences between both groups, and 55% of patients were older than 60 years. There were 70% males and 30% females. 4 cases developed venous thromboembolism during admission and 1 case presented an arterial thrombotic event, all of them within the severe disease group. All patients received low molecular weight heparin (LMWH) as thromboprophylaxis. Overall, there were 19 deaths (19%), all of them in the severe disease group. Analytical data of the whole sample and of both groups are shown in Table 1.
TABLE 1.
Analytical parameters
| Parameter | Whole sample (100) | Nonsevere COVID‐19 (50) | Severe COVID‐19 (50) |
Significance P (nonsevere vs severe) |
|---|---|---|---|---|
| PA (%), median (IQR) |
85% (77‐92) |
85% (75.6‐92.3) |
85.5% (78.8‐92) |
.55 |
| aPTT, median (IQR) |
29.6 s (27.4‐31.9) |
29.7 s (27.6‐31.5) |
29.4 s (27.3‐32.2) |
.78 |
| Fibrinogen, median (IQR) |
700 mg/dL (564‐884) |
673 mg/dL (568‐827) |
706 mg/dL (559‐924) |
.43 |
| D‐Dimer, median (IQR) |
1089 ng/mL (550‐2512) |
639 ng/mL (413‐1070) |
2079 ng/mL (1112‐5411) |
<.0001 |
| Platelets, median (IQR) |
326x109/L (215‐438) |
332 x109/L (221‐456) |
324 x109/L (212‐418) |
.41 |
| Lymphocyte, median (IQR) |
1000x109/L (700‐1375) |
1000x109/L (675‐1325) |
1000x109/L (700‐1400) |
.9 |
| IL‐6, median (IQR) |
34 pg/mL (9.5‐516) |
23 pg/mL (7‐510) |
34.5 pg/mL (15.8‐606) |
.49 |
| CRP, median (IQR) |
5.89 mg/dL (1.91‐14.7) |
4.5 mg/dL (1.1‐13.8) |
7.72 mg/dL (2.9‐23.8) |
.06 |
| Ferritin, median (IQR) |
1163 ng/mL (453‐2226) |
674 ng/mL (420‐1190) |
1751 ng/mL (850‐3472) |
<.0001 |
| LDH, median (IQR) |
380 U/L (289‐483) |
341 U/L (264‐428) |
427 U/L (358‐549) |
<.0001 |
Abbreviations: aPTT, active partial thromboplastin time; CRP, C‐reactive protein; IL‐6, interleukin‐6; IQR, interquartile range; LDH, lactate dehydrogenase; PA, prothrombin activity.
D‐dimer, ferritin and lactate dehydrogenase were significantly increased in the severe COVID‐19 disease cases. Severe cases had significantly lower ADAMTS13 activity and higher plasma concentration of VWF (Table 2). ADAMTS13 activity was negatively correlated with VWF‐Ag (Spearman's r: −.52, P < .0001), D‐dimer plasma concentration (Spearman's r: −.41, P < .0001), ferritin (Spearman's r: −.44, P < .0001) and LDH (Spearman's r: −.33, P = .001). In multivariate analysis, D‐dimer > 1079 ng/mL (odds ratio: 2.83, CI: 1.07‐7.5, P = .03) and ferritin > 1163 ng/mL (odds ratio: 3.82, CI: 1.2‐12.1, P = .02) were significantly associated with increased risk of ADAMTS13 activity lower than the median value (61%).
TABLE 2.
ADAMTS13 activity and VWF Ag plasma concentration. ADAMTS13 activity and VWF Ag plasma concentration in survivors and nonsurvivors. ADAMTS13 activity and VWF Ag plasma concentration in nonsurvivors and severe disease survivors
| Parameter | Whole sample (100) | Nonsevere COVID‐19 (50) | Severe COVID‐19 (50) |
Significance P (nonsevere vs severe) |
|---|---|---|---|---|
| ADAMTS13, median (IQR) |
61% (47.2‐74.6) |
69% (54.2‐84.9) |
53.2% (38.8‐65.3) |
<.0001 |
| VWF‐Ag, median (IQR) |
289% (228‐382) |
261.4% (213‐326) |
355% (267‐400) |
<.0001 |
| Parameter |
Survivors (81) |
Nonsurvivors (19) |
Significance P |
|
|---|---|---|---|---|
| ADAMTS13, median (IQR) |
62.8% (52.3‐80.1) |
42.4% (33.8‐57.3) |
<.0001 | |
| VWF‐Ag, median (IQR) |
270% (218‐353) |
395% (294‐400) |
.002 |
| Parameter | Severe disease survivors (31) | Nonsurvivors (19) |
Significance P |
|
|---|---|---|---|---|
| ADAMTS13, median (IQR) |
59.1% (46.2‐69.8) |
42.4% (33.8‐57.3) |
.016 | |
| VWF‐Ag, median (IQR) |
315% (236‐400) |
395% (294‐400) |
.18 |
Abbreviations: ADAMTS13, A disintegrin‐like and metalloprotease with thrombospondin type 1 motif no. 13; IQR, interquartile range; VWF Ag, von Willebrand factor antigen.
The 19 nonsurvivors had significantly lower ADAMTS13 activity and higher plasma concentration of VWF (Table 2). 15 out of 19 nonsurvivors had ADAMTS13 activity lower than 61%. Cases with ADAMTS13 activity lower than 61% showed a significant association with in‐hospital death (P = .011). The Kaplan‐Meier analysis confirmed a significantly lower survival in those cases with ADAMTS13 levels below 61% (Figure 1). Univariate logistic regression analysis was performed, showing that ADAMTS13 activity below 61% (odds ratio: 4.46, 95% CI: 1.36‐14.6, P < .014), VWF higher than 289% (odds ratio: 4.93, CI: 1.5‐16.2, P = .008), D‐dimer higher than 1089 ng/mL (odds ratio: 3.5, CI: 1.15‐10.6, P = .027) and LDH higher than 380 U/L (odds ratio: 6.91, CI: 1.85‐25.8, P = .004) were significantly associated with increased risk of in‐hospital death. In multivariate analysis, only ADAMTS13 activity below 61% (odds ratio: 3.64, CI: 1.05‐12.6, P = .04) and LDH higher than 380 U/L (odds ratio: 6.26, CI: 1.64‐23.8, P = .007) remained significantly associated with increased risk of in‐hospital death. Despite receiving antithrombotic prophylaxis, 5 patients had thrombotic events, all of them in the severe disease group. 4 out of 5 presented ADAMTS13 activity below 61% and VWF higher than 289%.
FIGURE 1.
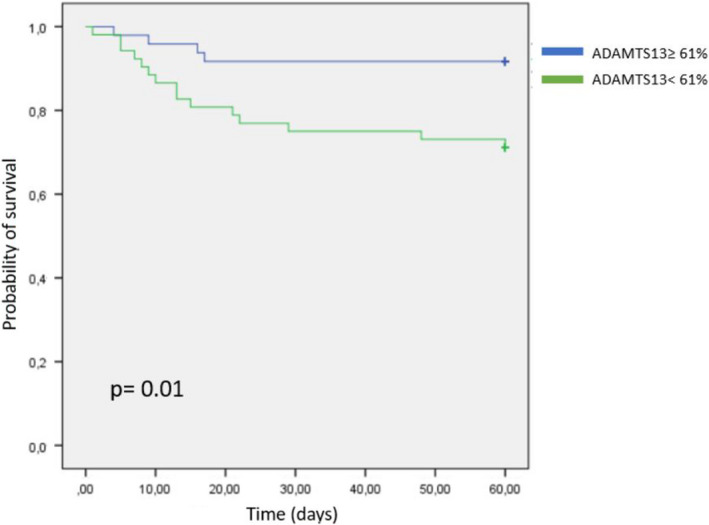
The Kaplan‐Meier survival analysis according to A disintegrin‐like and metalloprotease with thrombospondin type 1 motif no. 13 activity levels: 15 deaths were observed in the group with values <61%
Overall, in our study we observed higher levels of VWF and lower ADAMTS13 in severe disease patients, probably due to the strong inflammation and to the endothelial dysfunction, both derived from the SARS‐CoV‐2 infection. Besides, ADAMTS13 activity lower than the median value (61%) was associated with higher risk of in‐hospital death. The Kaplan‐Meier analysis confirmed a significantly lower survival in those cases with ADAMTS13 below 61%. Finally, in our cohort, there were no cases with hypofibrinogenaemia, thrombocytopenia or prolongation of both PT and aPTT. This might suggest that the coagulopathy caused by COVID‐19 would not be a conventional form of DIC.
In conclusion, the strong inflammation and the endothelial dysfunction caused by the SARS‐CoV‐2 infection might play a fundamental role in the coagulopathy, the levels of VWF and ADAMTS13 activity, the severity of the disease and the increased thrombotic risk. These data are consistent with previous findings 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 and might suggest that ADAMTS13 activity could be a prognosis marker in COVID‐19 patients.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Mario Rodríguez Rodríguez, Nerea Castro Quismondo, Denis Zafra Torres and Daniel Gil Alos collected data. Mario Rodríguez wrote the manuscript. Mario Rodríguez, Rosa Ayala and Joaquín Martinez‐Lopez processed statistical data.
1.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Klok FA, Kruip MJHA, van der Meer NJM, et al.Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. 191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(04):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Lancet Haematology . COVID‐19 coagulopathy: an evolving story. Lancet Haematol. 2020;7(6):e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Labò N, Ohnuki H, Tosato G. Vasculopathy and coagulopathy associated with SARS‐CoV‐2 infection. Cells. 2020;9(7):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giamarellos‐Bourbolis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(06):992‐1003.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levi M, Scully M, Singer M. The role of ADAMTS‐13 in the coagulopathy of sepsis. J Thromb Haemost. 2018;16(4):646‐651. [DOI] [PubMed] [Google Scholar]
- 8. Vasileadis I, Politou M, Dimopoulos S, et al. Variation of endothelium‐related hemostatic factors during sepsis. Microcirculation. 2018;25(08):e12500. [DOI] [PubMed] [Google Scholar]
- 9. Chen J, Chung DW. Inflammation, von Willebrand factor, and ADAMTS13. Blood. 2018;132(02):141‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid‐19. N Engl J Med. 2020;383(2):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D. Los ADAMTS 13 plasma levels are predictors of mortality in COVID‐19 patients. Intern Emerg Med. 2020;15:861‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tiscia GL, Favuzzi G, De Laurenzo A, et al. Reduction of ADAMTS13 levels predicts mortality in SARS‐CoV‐2 patients. TH Open. 2020;4:e203‐e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huisman A, Beun R, Sikma M, Westerink J, Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS‐CoV‐2. Int J Lab Hematol. 2020;42:e211‐e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adam EH, Zacharowski K, Miesbach W. A comprehensive assessment of the coagulation profile in critically ill COVID‐19 patients. Thromb Res. 2020;194:42‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinelli N, Montagnana M, Pizzolo F, et al. A relative ADAMTS13 deficiency supports the presence of secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Escher R, Breakey N, Lammle B. ADAMTS13 activity, von Willebrand factor, factor VIII and D‐dimers in COVID‐19 inpatients. Thromb Res. 2020;192:174‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mancini I, Baronciani L, Artoni A, et al. The ADAMTS13‐von Willebrand factor axis in COVID‐19 patients. J Thromb Haemost. 2020:1–9. 10.1111/jth.15191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Von Meijenfeldt FA, Havervall S, Adelmeijer J, et al. Prothrombotic changes in patients with COVID‐19 are associated with disease severity and mortality. Front Physiol. 2020;10(11):587013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


