Abstract
Background
The coronavirus disease 2019 (COVID‐19) pandemic has resulted in millions of deaths and overburdened healthcare systems worldwide. Systemic low‐dose corticosteroids have proven clinical benefit in patients with severe COVID‐19. Higher doses of corticosteroids are used in other inflammatory lung diseases and may offer additional clinical benefits in COVID‐19. At present, the balance between benefits and harms of higher vs. lower doses of corticosteroids for patients with COVID‐19 is unclear.
Methods
The COVID STEROID 2 trial is an investigator‐initiated, international, parallel‐grouped, blinded, centrally randomised and stratified clinical trial assessing higher (12 mg) vs. lower (6 mg) doses of dexamethasone for adults with COVID‐19 and severe hypoxia. We plan to enrol 1,000 patients in Denmark, Sweden, Switzerland and India. The primary outcome is days alive without life support (invasive mechanical ventilation, circulatory support or renal replacement therapy) at day 28. Secondary outcomes include serious adverse reactions at day 28; all‐cause mortality at day 28, 90 and 180; days alive without life support at day 90; days alive and out of hospital at day 90; and health‐related quality of life at day 180. The primary outcome will be analysed using the Kryger Jensen and Lange test adjusted for stratification variables and reported as adjusted mean differences and median differences. The full statistical analysis plan is outlined in this protocol.
Discussion
The COVID STEROID 2 trial will provide evidence on the optimal dosing of systemic corticosteroids for COVID‐19 patients with severe hypoxia with important implications for patients, their relatives and society.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has resulted in thousands of deaths and overburdened healthcare systems worldwide. 1 SARS‐CoV‐2 can cause systemic hyperinflammation and acute respiratory distress syndrome (ARDS), 2 a condition occurring in up to 40% of hospitalised patients with COVID‐19. 3 , 4 , 5 , 6 , 7
Until recently, the care of COVID‐19 patients was primarily supportive, including respiratory support. On 16 June 2020, the preliminary results of the Randomised Evaluation of COVid‐19 thERapY (RECOVERY) trial were publicly announced. 8 In this randomised clinical trial (RCT), 6,425 hospitalised patients with suspected or confirmed COVID‐19 were randomised to open‐label dexamethasone 6 mg daily for up to 10 days or usual care. 8 The results demonstrated a 17% relative risk reduction (95% confidence interval (CI) 0.74 to 0.92%) in 28‐day mortality with dexamethasone with a possible larger clinical benefit in patients receiving invasive mechanical ventilation. 8
The results from all critically ill patients included in the RECOVERY trial and 6 other ongoing or recently completed RCTs were summarised in a prospective meta‐analysis that demonstrated lower 28‐day mortality among patients who received corticosteroids as compared with usual care or placebo (odds ratio (OR) 0.66, 95% CI 0.53 to 0.82). 9 Following these results, updated guidelines from the World Health Organization (WHO), the National Institutes of Health and the Infectious Diseases Society of America now recommend systemic low‐dose corticosteroids for 7‐10 days in patients with COVID‐19 requiring supplemental oxygen. 10 , 11 , 12
In July 2020, we surveyed doctors at potential COVID STEROID 2 trial sites to assess clinician's preferences for systemic corticosteroid use in patients with severe COVID‐19 after the preprint publication of the RECOVERY trial (unpublished data). Among 240 anonymous responders, 56% would use 6 mg of dexamethasone or equivalent, and 36% would use a dose above 6 mg (Figure 1). As for preferences for an upcoming trial, most would enrol patients with severe COVID‐19 into a trial of different doses of corticosteroids, and into one comparing 12 mg vs 6 mg of dexamethasone (55% Figure 2).
FIGURE 1.
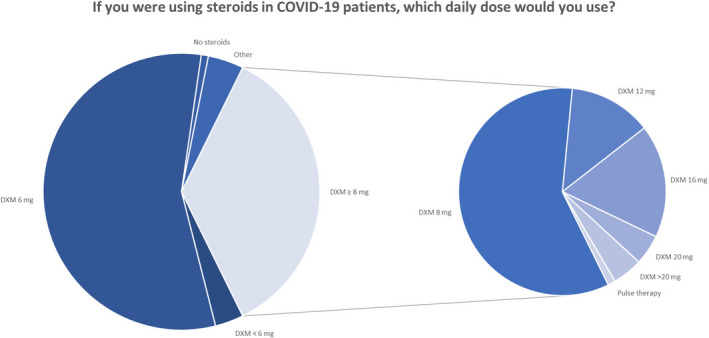
Dose preferences in corticosteroid use for COVID‐19 patients among 240 survey responders from Denmark, Sweden, Switzerland and India. In the survey, all dose options were stated as equivalents of dexamethasone, betamethasone, hydrocortisone, methylprednisolone and prednisone. COVID‐19, coronavirus disease 19; DXM, dexamethasone; mg, milligrams
FIGURE 2.
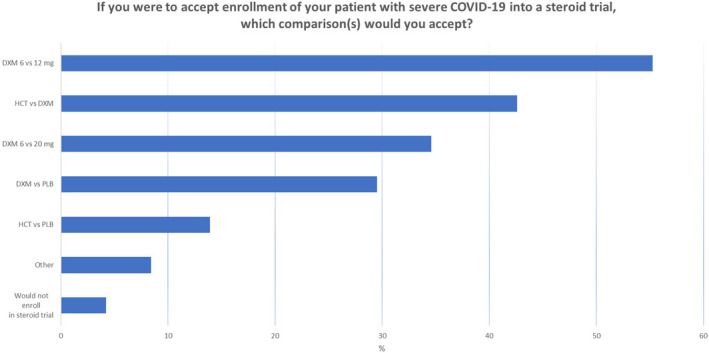
Preferences in comparisons for future trial in COVID‐19 patients among 237 responders from Denmark, Sweden, Switzerland and India. Survey responders were able to tick one or more comparisons. COVID‐19, coronavirus disease 19, DXM, dexamethasone, mg, milligrams, HCT, hydrocortisone; PLB, placebo.
With short‐term use in healthy volunteers, a dose‐dependent activation of the corticosteroid receptor has been observed for increasing doses up to 60 mg of prednisone (equivalent to 12 mg of dexamethasone), suggesting that doses up to 12 mg of dexamethasone may offer additional anti‐inflammatory effects, while adverse effects seemed independent of the dosing. 13 Higher doses of dexamethasone have also been used in a RCT in non‐COVID‐19 patients with ARDS suggesting benefit without an increased risk of serious adverse events. 14 Several ongoing or completed trials have assessed corticosteroids for severe COVID‐19 using different types (ie dexamethasone, methylprednisolone, hydrocortisone or prednisone) and doses (median dose in dexamethasone equivalents 15 mg, interquartile range (IQR) 10 to 16 mg) of corticosteroids. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 Most of these trials have not yet been published, 16 , 17 , 18 , 28 , 29 and none of them directly compare different dosing regimens, leaving the optimal dosing for severe COVID‐19 uncertain.
The aim of the COVID STEROID 2 trial is to compare the effects of 12 mg vs 6 mg of intravenous (IV) dexamethasone for up to 10 days on the number of days alive without life support and other patient‐centred outcomes in adult patients with COVID‐19 and severe hypoxia. We hypothesise that dexamethasone 12 mg will increase the number of days alive without life support as compared to dexamethasone 6 mg in patients with COVID‐19 and severe hypoxia.
2. METHODS
2.1. Trial design
The COVID STEROID 2 trial is an investigator‐initiated, international, parallel group, blinded, centrally randomised and stratified clinical trial. We plan to enrol 1,000 hospitalised adult patients with COVID‐19 and severe hypoxia from sites in Denmark, Sweden, Switzerland and India. All trial results will be reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement. 30
2.2. Trial conduct
We have prepared the COVID STEROID 2 protocol in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Guidelines (Supporting Information S1). 31 The trial will be conducted in accordance with the trial protocol, the Helsinki Declaration (latest version), 32 the International Conference on Harmonization (ICH) Good Clinical Practice (GCP) guidelines (latest version) 33 and all applicable laws in the participating countries.
2.3. Randomisation
Participants will be randomised in a 1:1 ratio to 12 mg vs 6 mg of dexamethasone using a central web‐based randomisation system administered by the Copenhagen Trial Unit (CTU). The randomisation will be performed using computer‐generated allocation sequence lists stratified by trial site, invasive mechanical ventilation (yes/no) and age below 70 years (yes/no) with varying block sizes.
2.4. Allocation concealment
The allocation sequence will be unknown to the trial staff preparing the trial medication, the clinicians, the investigators and the statistician conducting the analyses. The group allocations will remain masked (coded as 0 and 1) until two versions of the abstract for the trial report have been written.
2.5. Blinding
The allocation will be masked for all participants, clinical staff, trial staff reporting outcome data, the COVID STEROID 2 Management Committee and the trial statistician.
Unblinded trial staff will prepare the trial medication and register protocol adherence and any violations (ie trial medication not administered as per protocol and use of open‐label corticosteroids, respectively) during the intervention period. The unblinded staff will not be involved in the care of trial participants, the entry of outcome data or the statistical analysis. They will be instructed not to reveal the allocation unless the participant is subject to emergency unblinding.
2.6. Inclusion criteria
We will screen for enrolment of adults with COVID‐19 and severe hypoxia fulfilling the following three inclusion criteria:
-
‐
Aged 18 years or above AND
-
‐
Confirmed SARS‐CoV‐2 requiring hospitalisation AND
-
‐
Use of one of the following:
-
•
Invasive mechanical ventilation OR
-
•
Non‐invasive ventilation or continuous use of continuous positive airway pressure (CPAP) for hypoxia OR
-
•
Oxygen supplementation with an oxygen flow of at least 10 L/min independent of delivery system.
A detailed description of the inclusion criteria is provided in the Supporting Information S2.
2.7. Exclusion criteria
Any patient fulfilling one or more of the following exclusion criteria at the time of screening will be excluded from the trial:
-
‐
Use of systemic corticosteroids for other indications than COVID‐19 in doses higher than 6 mg dexamethasone equivalents.
-
‐
Use of systemic corticosteroids for COVID‐19 for 5 consecutive days or more.
-
‐
Invasive fungal infection.
-
‐
Active tuberculosis.
-
‐
Fertile woman (<60 years of age) with positive urine human gonadotropin (hCG) or plasma‐hCG.
-
‐
Known hypersensitivity to dexamethasone.
-
‐
Previously randomised in the COVID STEROID 2 trial.
-
‐
Informed consent not obtainable.
We will allow co‐enrolment with other clinical trials unless the interventions or protocols of the trials collide. A detailed description of the exclusion criteria is provided in the Supporting Information S3.
2.8. Trial interventions
We will use shelf‐medications from the hospital departments’ pharmacies for the trial medication. For each participant, the trial medication will be prepared once daily by the unblinded trial staff and administered as a bolus injection (5 ml) by the clinical staff. All other interventions will be given at the discretion of the treating clinicians.
A list of trade names and a detailed description of the preparation of trial medication used in the COVID STEROID 2 trial is provided in the Supporting Information S4 and S5.
2.8.1. Higher dose of dexamethasone
For patients randomised to a higher dose of dexamethasone, 12 mg of IV dexamethasone will be given daily as bolus injection (5 ml) for up to 10 days. We will allow the use of betamethasone 12 mg at sites where dexamethasone is not available as these are diastereomers and likely equipotent. 34
2.8.2. Lower dose of dexamethasone
For patients randomised to a lower dose of dexamethasone, 6 mg of IV dexamethasone will be given daily as bolus injection (5 ml) for up to 10 days. We will allow the use of betamethasone 6 mg at sites where dexamethasone is not available as these are diastereomers and likely equipotent. 34
2.8.3. Intervention period
The intervention period is up to 10 days from randomisation or until hospital discharge or death, whichever is earlier. For each participant, the intervention period will depend on the number of consecutive days with corticosteroid use for COVID‐19 before randomisation (ie the number of consecutive days with corticosteroid use will be subtracted from the 10‐day intervention period). The intervention period will range from 6 to 10 days as all patients who have received corticosteroids for COVID‐19 for 5 consecutive days or more will be excluded from the trial.
2.9. Outcomes
Detailed definitions of all outcome measures are provided in the Supporting Information S6.
2.9.1. Primary outcome
Days alive without life support (ie invasive mechanical ventilation, circulatory support or renal replacement therapy (including days in between intermittent renal replacement therapy)) from randomisation to day 28.
2.9.2. Secondary outcomes
-
‐
Number of participants with one or more serious adverse reactions (SARs) to dexamethasone from randomisation to day 28 defined as new episodes of septic shock, invasive fungal infection, clinically important gastrointestinal (GI) bleeding or anaphylactic reaction to IV dexamethasone.
-
‐
All‐cause mortality at day 28.
-
‐
All‐cause mortality at day 90.
-
‐
Days alive without life support at day 90.
-
‐
Days alive and out of hospital at day 90.
-
‐
All‐cause mortality at day 180.
-
‐
HRQoL at day 180 using EQ‐5D‐5L and EQ‐VAS. 35
2.10. Registered variables
Detailed definitions of the registered variables are provided in Supporting Information S7. Data will be entered in an online electronic case report form (OpenClinica).
2.10.1. Baseline variables
Sex.
Age at enrolment (date of birth).
Date of admission to hospital.
Number of days with symptoms of COVID‐19 before hospital admission.
Type of department at which the participant was included (ie emergency department, hospital ward, intermediate care unit, intensive care unit).
-
Use of respiratory support at randomisation:
-
‐
Closed system: invasive mechanical ventilation or non‐invasive ventilation or continuous use of CPAP (including latest fraction of inspired oxygen (FiO2) and number of days on closed system ventilation prior to randomisation).
-
‐
Open system ventilation with an oxygen flow ≥ 10 L/min (including maximum supplemental oxygen flow at randomisation ± 1 hour).
-
‐
Limitations of care (ie limitations for invasive mechanical ventilation, circulatory support, renal replacement therapy, or cardio‐pulmonary resuscitation) at the time of randomisation.
Chronic use of systemic corticosteroids for other indications than COVID‐19.
-
Treatment for COVID‐19 during current hospital admission prior to randomisation:
-
‐
Agents with potential anti‐viral action (ie remdesivir, convalescent plasma, other).
-
‐
Agents with potential anti‐inflammatory action (ie Janus kinase inhibitor, interleukin‐6 inhibitors, other).
-
‐
Treatment with systemic antibacterial agents in the 24 hours prior to randomisation.
-
Chronic co‐morbidities:
-
‐
History of ischaemic heart disease or heart failure.
-
‐
Treatment at the time of hospital admission with any anti‐diabetic drug indicating diabetes mellitus.
-
‐
Treatment at the time of hospital admission with any drug indicating chronic pulmonary disease.
-
‐
Use of immunosuppressive therapy within the last 3‐months.
-
‐
-
Laboratory values, interventions and vital parameters:
-
‐
Participant weight (kilograms).
-
‐
Arterial partial pressure of oxygen (PaO2).
-
‐
Saturation of oxygen (SaO2) from arterial blood gas sample (preferred) or pulse oximeter.
-
‐
Use of circulatory support within the last 24 hours prior to randomisation.
-
‐
Use of any form of renal replacement therapy within the last 72 hours prior to randomisation.
-
‐
Highest plasma lactate within the last 24 hours prior to randomisation.
-
‐
2.10.2. Variables registered daily during admission for the first 14 days after randomisation (day forms)
Use of invasive mechanical ventilation.
Use of circulatory support (continuous infusion of vasopressor/inotrope for a minimum of 1 hour).
Use of any form of renal replacement therapy (including days between intermittent renal replacement therapy).
-
SAR(s)
-
‐
New episodes of septic shock.
-
‐
Invasive fungal infection.
-
‐
Clinically important GI bleeding.
-
‐
Anaphylactic reaction to IV dexamethasone (only recorded during the intervention period)
-
‐
2.10.3. Protocol violations during the intervention period
Protocol violations will be recorded during the intervention period (for up to 10 days).
Use of open‐label systemic corticosteroids.
Trial medication not administered as per protocol (1 bolus injection of either 12 mg or 6 mg dexamethasone according to the allocation on each day during the intervention period).
2.10.4. Follow‐up 28 days after randomisation
Vital status (if dead, date of death).
Number of days on invasive mechanical ventilation from day 15‐28.
Number of days with circulatory support (continuous infusion of vasopressor/inotrope for a minimum of 1 hour) from day 15‐28.
Number of days on renal replacement therapy (including days between intermittent renal replacement therapy) from day 15‐28.
The occurrence of SAR(s) (section 2.10.2) from day 15‐28 (if yes, apply date(s)).
Use of extracorporeal membrane oxygenation from randomisation to day 28 (y/n)
Discharged against medical advice to home/other hospital/other facility, including degree of life support (ie mechanical ventilation, circulatory support, renal replacement therapy) or supplementary oxygen at the time of discharge.
We will not register the occurrence of suspected unexpected serious adverse reactions (SUSARs) in dayforms or at 28‐day follow‐up as these rarely occur. Instead, SUSARs will be reported directly and without delay by the site investigator to the sponsor.
2.10.5. Follow‐up 90 days after randomisation
Vital status (if dead, date of death).
Number of days on invasive mechanical ventilation from day 29‐90.
Number of days with circulatory support (continuous infusion of vasopressor/inotrope for a minimum of 1 hour) from day 29‐90.
Number of days on renal replacement therapy (including days between intermittent renal replacement therapy) from day 29‐90.
Date of discharge from hospital.
Additional hospital admissions (date(s) of re‐admission(s) and discharge(s)).
2.10.6. Follow‐up 180 days after randomisation
2.11. General analytic principles
We will conduct the primary analyses of both primary and secondary outcomes in the intention‐to‐treat (ITT) population (ie all randomised participants for whom consent has been given to use data). For the primary outcome, this analysis will be supplemented with a sensitivity analysis in the per‐protocol (PP) population (ie the ITT population except those having one or more major protocol violations as defined in section 2.10.3). All statistical tests will be two‐tailed and reported with confidence intervals (CIs).
Significance level is set to 5% including the interim analysis. In practice, this means that a P‐value below 0.0492 will be considered significant if the trial is not stopped at the interim analysis and accordingly 95.08% confidence intervals will be employed. For all secondary outcomes, we will employ 99% CIs for (P‐value threshold for significance 0.01) due to the multiplicity of these. If not stopped early, the mortality outcomes will be tested in a hierarchical procedure along with the primary outcome (first primary outcome, then 28 days mortality and finally 90 days mortality) reusing the alpha if the previous test was significant. If the primary outcome is insignificant at trial conclusion, 0.01 significance thresholds will be employed for all additional outcomes, but the results interpreted with caution.
2.12. Missing data
We will perform complete case analyses if less than 5% of patients have missing data for variables included in the primary or secondary outcome analyses. If 5% or more of patients have missing data for the outcome/covariates in any analysis, we will use multiple imputation with chained equations for that analysis. If multiple imputation is used, we will use the predictive mean matching and logistic regression methods for numerical and categorical variables, respectively, with 25 datasets imputed separately in each treatment group. 36 , 37 We will include all stratification variables, all variables used in the applicable analyses, important baseline prognostic variables (age, all co‐morbidities listed above, use of all 3 life support measures at baseline), and all outcomes available at the time of analysis in the imputation models. If multiple imputation is used, these results will be reported as the primary and supplemented with complete case analyses and best‐worst/worst‐best analyses (as previously described 38 ).
2.13. Statistical analyses
2.13.1. Primary outcome
Primary analysis of the primary outcome
1. Kryger Jensen and Lange test adjusted for stratification variables (site, invasive mechanical ventilation, and age below 70 years) in the ITT population. 39
Sensitivity analysis of the primary outcome
2. Kryger Jensen and Lange test adjusted for stratification variables (site, invasive mechanical ventilation and age below 70 years) and additional important prognostic baseline risk factors, that is all co‐morbidities listed above, and use of circulatory support or renal replacement therapy in the ITT population. 39
3. Kryger Jensen and Lange test adjusted for stratification variables (site, invasive mechanical ventilation and age below 70 years) in the PP population. 39
4. If 5% or more of patients have missing data and multiple imputation is used: best‐worst/worst‐best case analyses and complete case analysis. 37
Subgroup analyses of the primary outcome
5. Test of interaction between the intervention and the pre‐planned subgroups (section 2.13.4) by Kryger Jensen and Lange test adjusted for stratification variables (site, invasive mechanical ventilation and age below 70 years) in the ITT population.
The Kryger Jensen and Lange test is a joint test for no treatment effect on an outcome which can have probability point mass in a single value (ie zero days alive without life support within 28 days). 39 The test builds on combining two regressions; we can therefore adjust as per usual analysis despite the expectation that the outcome will be highly skewed. 39
Results from all analyses of the primary outcome will be reported as adjusted mean differences and median differences with confidence intervals (see preceding section for details on significance level). Secondarily, we will report the unadjusted (crude) mean differences and median differences.
As the primary outcome is composite, we will report results from the analysis of each component in a supplement to the main report.
2.13.2. Secondary outcomes
Binary outcomes
We will conduct the following analyses for all binary outcomes (ie number of participants with one or more SARs at day 28; all‐cause mortality at day 28, 90 and 180):
Primary analysis
1. Generalised linear models with log links and binomial error distributions adjusted for the stratification variables in the ITT population. 40
Secondary analysis
2. Fisher's exact test in the ITT population.
3. Kaplan‐Meier survival curve for the crude data on all‐cause mortality at day 28, 90 and 180.
Results will be reported as adjusted relative risks and secondarily adjusted risk differences with corresponding confidence intervals (see section 2.11 for details on significance level). Secondarily, we will report the unadjusted (crude) relative risks and absolute risk differences.
Continuous outcomes
We will conduct the following analyses for all continuous outcomes (ie days alive without life support at day 90; days alive and out of hospital at day 90; HRQoL at day 180 using EQ‐5D‐5L and EQ‐VAS):
Kryger Jensen and Lange test adjusted for the stratification variables in the ITT population. 39
The results will be reported as adjusted mean differences and median differences with confidence intervals (see preceding section for details on significance level) and unadjusted (crude) mean differences and median differences.
For composite outcomes (ie number of participants with one or more SARs at day 28; days alive without life support at day 90), we will report results from the analysis of each component in a supplement to the main report.
2.13.3. Power estimations
Sample size and power estimations for the primary outcome
At maximum, we will randomise 1000 participants. A blinded statistician will conduct an interim analysis after the first 500 participants have been followed for 28 days. The alpha values for the interim analysis and the final analysis are 0.0054 and 0.0492, respectively as by the O'Brien‐Fleming bounds, which preserves type I error at the usual 5%. The trial has 85% power to detect a 15% relative reduction in 28‐day mortality combined with a 10% reduction in time on life support among the survivors.
Power estimations for the secondary outcomes
We have 80% statistical power to detect the following effects for the secondary outcomes:
-
‐
A 21% relative risk reduction for the mortality at day 28 (control event rate 30%)
-
‐
A 18% relative risk reduction for the mortality at day 90 (control event rate 40%)
-
‐
A 32% relative risk reduction for the number of participants with one or more SARs (control event rate 15%)
-
‐
A 15% relative risk reduction for the mortality at day 180 (control event rate 50%)
The estimates of control event rates for mortality at day 28 originate in data of previous COVID‐19 studies; 4 , 41 the estimates of the control event rates for mortality at day 90 and the number of participants with SARs are based on our best clinical estimate. We expect the outcomes “days alive out of hospital at day 90” and “HRQoL at 180 days” to be highly skewed (non‐normally distributed). The power estimations for these outcomes would be uncertain, and we therefore refrain from making these estimates.
2.13.4. Pre‐planned subgroup analyses
We will assess the heterogeneity of the intervention effects on the primary outcome in the following subgroups based on baseline characteristics:
-
‐
Patients ≥ 70 years compared to < 70 years of age: hypothesised larger beneficial effect of higher dose dexamethasone in patients < 70 years of age.
-
‐
Patients who receive invasive mechanical ventilation compared to oxygen by other delivery systems: hypothesised larger beneficial effect of higher dose dexamethasone in patients who receive oxygen by other delivery systems.
-
‐
Patients who received corticosteroids for COVID‐19 for 0 to 2 days compared to 3 to 4 days before enrolment: hypothesised larger beneficial effect of higher dose dexamethasone in patients with short duration (≤2 days) of corticosteroid use before enrolment.
-
‐
Patients who receive IL‐6 inhibitors at baseline compared to patients who do not receive IL‐6 inhibitors at baseline: hypothesised larger beneficial effect of higher dose dexamethasone in patients who receive IL‐6 inhibitors at baseline.
-
‐
Patients with limitations of care (ie not for invasive mechanical ventilation, circulatory support, renal replacement therapy, cardio‐pulmonary resuscitation) compared to patients without limitations of care: hypothesised larger beneficial effect of higher dose dexamethasone in patients without limitations of care.
-
‐
Patients with compared to without chronic use of systemic corticosteroids: hypothesised larger beneficial effect of higher dose dexamethasone in patients without chronic use of systemic corticosteroids.
-
‐
Patients enrolled in India compared to patients enrolled in Denmark, Sweden and Switzerland: hypothesised different effect of higher dose dexamethasone in patients enrolled in India as compared to patients enrolled in Denmark, Sweden and Switzerland.
Detailed definitions of the subgroups are available in Supporting Information S8.
2.14. Independent data monitoring and safety committee
We have formed an Independent Data Monitoring and Safety Committee (IDMSC) consisting of a multidisciplinary group of a clinician, a trialist and a biostatistician that, collectively, have experience in the conduct, monitoring and analysis of randomised clinical trials. The charter for the IDMSC is provided in Supporting Information S9.
2.15. Interim analysis
The independent statistician of the IDMSC will conduct one blinded interim analysis after 500 participants (50%) have been followed for 28 days. The alpha value for the interim analysis is 0.0054 as by the O'Brien‐Fleming bounds, which preserves type I error at the usual 5%. 42 The trial will be stopped early if the alpha cut‐off is crossed at the interim analysis.
The IDMSC will be provided with the following outcome data with the two groups masked (eg interventions coded as 0 and 1):
-
‐
Days alive without life support (ie invasive mechanical ventilation, circulatory support or renal replacement therapy (including days in between intermittent renal replacement therapy)) from randomisation to day 28.
-
‐
Number of participants with one or more SARs or SUSARs from randomisation to day 28.
The data set will also include data on the stratification (ie site, invasive mechanical ventilation, and age below 70 years) and baseline variables.
2.15.1. Statistical analyses conducted by the IDMSC
Days alive without life support at day 28: Kryger Jensen and Lange test adjusted for stratification variables (site, invasive mechanical ventilation, and age below 70 years) in the ITT population. 39
Number of participants with one or more SARs or SUSARs from randomisation to day 28: generalised linear model with log links and binomial error distributions adjusted for the stratification variables (site, invasive mechanical ventilation, and age below 70 years) in the ITT population. 40
The IDMSC can request additional data from the coordinating centre or unblinding of the intervention groups during the whole course of the trial. The IDMSC will submit their recommendations to the trial Management Committee, which makes the final decision to continue, pause or stop the trial.
2.16. Monitoring during the study
The trial will be externally monitored according to the GCP Directive. A monitoring and data verification plan has been developed together with the GCP unit at Rigshospitalet, University of Copenhagen.
The trial will also be centrally monitored by the Sponsor or his delegates through the electronic case report form (eCRF), including monitoring of protocol adherence.
2.17. Close out
We will ensure that a plan for long‐term storage of data and source documentation has been made at each site upon completion of the trial.
3. DISCUSSION
Low‐dose systemic corticosteroids are strongly recommended by the WHO for COVID‐19 patients with hypoxia. 8 Yet, the optimal dose is still not clear. Higher doses of corticosteroids may offer additional anti‐inflammatory effects, 43 but maybe also a be associated with a higher risk of serious adverse events. The COVID STEROID 2 trial intends to provide important evidence on the optimal dosing of corticosteroids for COVID‐19 patients with severe hypoxia.
3.1. Strengths
The COVID STEROID 2 trial has all the strengths of a large international, collaborative trial and will exclusively assess patient‐centred outcomes, including days alive without life support, SARs, mortality and HRQoL. In addition, i) we publish this protocol and statistical analysis plan reported according to the SPIRIT guidelines, 31 ii) adhere to the CONSORT statement, 30 iii) external monitoring according to the GCP directive 33 and central monitoring to ensure high quality of the collected data. We will allow co‐enrolment with other clinical trials unless the interventions or protocols collide to ensure that the participants are offered all potential beneficial interventions.
We will conduct an interim analysis after 50% of the participants have been followed for 28 days in order to early identify benefits and harms of higher vs. lower doses of dexamethasone. We will supplement the primary reports of the trial with a pre‐planned secondary Bayesian analysis of all outcomes collected up to 90 days after randomisation to help interpretation of the trial results. Details on this Bayesian re‐analysis will be specified in another protocol before database lockdown.
3.2. Limitations
The number of COVID‐19 patients may decline during the conduct of the trial, hindering the timely recruitment of trial participants.
We expect protocol violations (ie use of open‐label corticosteroids and/or failure to administer trial interventions during the intervention period) to occur during the trial. To account for this, we will challenge the primary results of the trial from the ITT population with a sensitivity analysis of the primary outcome in PP population (ie trial participants without protocol violations). Protocol violations will only be recorded during the intervention period for each trial participant, and some participants may receive open‐label corticosteroids after the intervention period. This may affect the results of the trial but will not be challenged in a sensitivity analysis.
Days alive without life support were chosen as the primary outcome of the trial as this is likely important to both patients, relatives and society. A reduction in days with life support will for most patients also mean a reduction in the time spent in the ICU. During the COVID‐19 pandemic, numerous ICUs worldwide are experiencing strained capacity. 44 The results of the COVID STEROID 2 trial may not only benefit patients and relatives, but also society by lowering the pressure on the healthcare systems. Mortality may, however, have been a more important outcome to patients and relatives.
3.3. Perspectives
There are other ongoing trials assessing higher vs. lower doses of corticosteroids for COVID‐19 patients with hypoxia. Together, results from all these trials may accrue enough evidence to show benefit or harm from a higher dose of corticosteroids as compared with the standard lower dose (6 mg of dexamethasone or equivalent) in COVID‐19 patients with hypoxia. As of 21 August 2020, only a few trials with a similar scope have been registered on ClinicalTrials.gov, 15 , 45 , 46 highlighting the need for the COVID STEROID 2 trial.
4. CONCLUSION
The COVID STEROID 2 trial is an investigator‐initiated, international, parallel‐grouped, blinded, centrally randomised and stratified clinical trial assessing the effects of higher vs. lower doses of dexamethasone in COVID‐19 patients with severe hypoxia. The trial protocol and statistical analysis plan is outlined in this manuscript. The COVID STEROID 2 trial will provide evidence on the optimal dosing of corticosteroids for COVID‐19 patients with severe hypoxia with important implications for both patients, relatives, healthcare systems and society in general.
4.1. Ethical considerations
The trial was registered at the European Union Drug Regulation Authorities Clinical Trials Database (EudraCT, 2020‐003363‐25) and at ClinicalTrials.gov (NCT04509973) before commencement. The trial was also registered at the Swiss National Clinical Trials Portal (SNCTP000004116) before commencing enrolment in Switzerland and at the Clinical Trials Registry—India (CTRI/2020/10/028731) before commencing enrolment in India.
In Denmark, the trial is approved by the Danish Medicines Agency (2020‐07‐16), the Committees on Health Research Ethics in the Capital Region of Denmark (H‐20051056), and The Capital Region Knowledge Centre for Data Compliance (P‐2020‐842). In Sweden, the trial is approved by the Swedish Ethical Review Authority (Dnr 2020‐02582 and Dnr 2020‐04403) and the Swedish Medical Product Agency with the additional requirement that the patient must have GCS ≥ 14 and give consent before inclusion (EudraCT number 2020‐003363‐25). In Switzerland, the trial was approved by the local ethics committee (BASEC 2020‐02165). In India, the trial was approved by the Health Ministry's Screening Committee of the Indian Council of Medical Research (HMSC, proposal ID 2020‐9746). All applicable additional approvals will be obtained before start of enrolment in other participating countries than Denmark. The protocol, including the procedure for obtaining informed consent, will be approved by all required authorities in the participating countries before commencing inclusion in the country concerned. Trial information to participants and consent forms used in the COVID STEROID trial are available from www.cric.nu/covid‐steroid‐2.
4.2. Data sharing statement
We will make the final de‐identified data set available for sharing in accordance with the recent International Committee of Medical Journal Editors (ICMJE) recommendations 47 and data sharing agreements adhering to the laws of the participating countries. All trial‐related documents are available from www.cric.nu/covid‐steroid‐2.
4.3. Dissemination
We plan to publish the trial results in an international peer‐reviewed medical journal irrespective of the findings. The reports will adhere to the CONSORT statement. 30 Upon trial completion, the trial results will also be available from www.cric.nu/covid‐steroid‐2.
4.4. Status
The trial was initiated on 27 August 2020 and is expected to be completed in 2021. As of January 25 2021, 492/1,000 participants (49.2%) have been enrolled at 27 trial sites in 22 hospitals in Denmark, India, Sweden and Switzerland. The recruitment is reported daily on www.cric.nu/covid‐steroid‐2.
5. TRIAL SPONSOR
Anders Perner, Department of Intensive Care, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark. Email: anders.perner@regionh.dk.
6. APPLICABLE PROTOCOL REGISTRATION NUMBERS
ClinicalTrials.gov identifier NCT04509973; Ethics committee number H‐20051056; EudraCT number 2020‐003363‐25; Danish Medicines Agency number 2020‐07‐16; Swiss National Clinical Trials Portal: SNCTP000004116; CTRI number: 2020/10/028731.
CONFLICTS OF INTEREST
Anders Perner and the Department of Intensive Care at Rigshospitalet, University of Copenhagen, has received grants for other research projects from the Novo Nordisk Foundation and Pfizer. The Department of Intensive Care Medicine, Bern University Hospital (Inselspital), has or has had research & development/consulting contracts with Edwards Lifesciences Services GmbH, Phagenesis Limited and Nestlé. The money was paid into a departmental fund, and none of the authors received any financial gain. The Department of Intensive Care Medicine, Bern University Hospital (Inselspital), has received unrestricted educational grants from the following organisations for organising bi‐annual postgraduate courses in the fields of critical care ultrasound, management of extracorporeal membrane oxygenation and mechanical ventilation: Pierre Fabre Pharma AG (formerly known as RobaPharm), Pfizer AG, Bard Medica SA, Abbott AG, Anandic Medical Systems, PanGas AG Healthcare, Orion Pharma, Bracco, Edwards Lifesciences AG, Hamilton Medical AG, Fresenius Kabi (Switzerland) AG, Getinge Group Maquet AG, Dräger Schweiz AG, and Teleflex Medical GmbH. Balasubramanian Venkatesh has received institutional research support from Baxter. Thomas Benfield reports grants from Pfizer, grants from the Novo Nordisk Foundation, grants from Lundbeck Foundation, grants from Simonsen Foundation, grants and personal fees from GSK, grants and personal fees from Pfizer, personal fees from Boehringer Ingelheim, grants and personal fees from Gilead, personal fees from MSD, grants from Lundbeck Foundation, and grants from Kai Hansen Foundation outside the submitted work. Tine Sylvest Meyhoff is a co‐author of a Cochrane systematic review of supplemental perioperative corticosteroids for surgical patients with adrenal insufficiency (protocol stage). Charlotte Suppli Ulrik has received personal fees, grants and/or been on advisory boards for AstraZeneca, GSK, TEVA, Orion Pharma, Actelion, Boehringer Ingelheim, ALK‐Abello, Novartis and Mundipharma outside the present work. The remaining authors have no conflicts of interest to declare.
AUTHORS’ CONTRIBUTIONS
MWM drafted the first version of this protocol, which was critically revised by all authors. All authors contributed to the design or conduct of the COVID STEROID 2 trial.
Supporting information
Table S1
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all patients and relatives agreeing to participate in the COVID STEROID 2 trial; all clinical and research staff at the participating departments for conducting the trial; the regulatory authorities in the participating countries for rapid handling and approvals; and the funding sources.
We would also like to thank all clinical and research staff involved in the design and conduction of the “Low‐dose hydrocortisone in patients with COVID‐19 and severe hypoxia (COVID STEROID) trial” which preceded the COVID STEROID 2 trial.
Balasubramanian Venkatesh have received financial support from the Medical Research Future Fund Australia.
Munch MW, Granholm A, Myatra SN, et al. Higher vs lower doses of dexamethasone in patients with COVID‐19 and severe hypoxia (COVID STEROID 2) trial: Protocol and statistical analysis plan. Acta Anaesthesiol Scand. 2021;65:834–845. 10.1111/aas.13795
FUNDING INFORMATION
Rigshospitalet's Research Council, Grant/Award Number: E‐22703‐06; Novo Nordisk Fonden, Grant/Award Number: 0062998. The funding sources were not involved in the design or conduct of the COVID STEROID 2 trial.
References
- 1. Center for Systems Science and Engineering at Johns Hopkins University . Coronavirus COVID‐19 Global Cases. Available from: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed on March 18 2020.
- 2. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Recovery Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report [Epub ahead of print]. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO Rapid Evidence Appraisal for COVID‐19 Therapies Working Group . Sterne JAC, Murthy S, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID‐19: A Meta‐analysis. JAMA 2020.324(13):1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. National Institutes of Health. Available at. https://www.covid19treatmentguidelines.nih.gov/. Accessed 01 June 2020. [PubMed]
- 11. Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID‐19. Available from: https://www.idsociety.org/globalassets/idsa/practice‐guidelines/covid‐19/treatment/idsa‐covid‐19‐gl‐tx‐and‐mgmt‐v2.1.0.pdf. Accessed on July 10, 2020. 2020. [DOI] [PMC free article] [PubMed]
- 12. World Health Organization . Corticosteroids for COVID‐19. Living guidance. 2 September 2020.
- 13. Fleishaker DL, Mukherjee A, Whaley FS, Daniel S, Zeiher BG. Safety and pharmacodynamic dose response of short‐term prednisone in healthy adult subjects: a dose ranging, randomized, placebo‐controlled, crossover study. BMC Musculoskelet Disord. 2016;17:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Villar J, Ferrando C, Martinez D, et al. Dexamethasone in An. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 2020;8:267‐276. [DOI] [PubMed] [Google Scholar]
- 15. Randomized Embedded . Multifactorial Adaptive Platform Trial for Community‐Acquired Pneumonia (REMAP‐CAP) (NCT02735707). Available from: https://clinicaltrials.gov/ct2/show/NCT02735707?term=remap+cap&draw=2&rank=1. [DOI] [PMC free article] [PubMed]
- 16. Targeted Steroids for ARDS Due to COVID‐19 Pneumonia: A Pilot Randomized Clinical Trial (NCT04360876). Available form: https://clinicaltrials.gov/ct2/show/NCT04360876?term=COVID+STEROID&draw=2&rank=3.
- 17. Steroid Dosing by bioMARker Guided Titration in Critically Ill Patients With Pneumonia (SMART) (NCT03852537). Available from: https://clinicaltrials.gov/ct2/show/NCT03852537?term=COVID+STEROID&draw=2&rank=4.
- 18. Glucocorticoid Therapy for COVID‐19 Critically Ill Patients With Severe Acute Respiratory Failure (NCT04244591). Available from: https://clinicaltrials.gov/ct2/show/NCT04244591?term=COVID+STEROID&draw=2&rank=5.
- 19. Methylprednisolone in the Treatment of Patients With Signs of Severe Acute Respiratory Syndrome in Covid‐19 (MetCOVID) (NCT04343729). Available from: https://clinicaltrials.gov/ct2/show/NCT04343729?term=COVID+methylprednisolone&draw=2&rank=3.
- 20. Efficacy of Dexamethasone Treatment for Patients With ARDS Caused by COVID‐19 (DEXA‐COVID19) (NCT04325061). Available from: https://clinicaltrials.gov/ct2/show/NCT04325061?term=COVID+and+dexa&draw=2&rank=1.
- 21. Community‐Acquired Pneumonia: Evaluation of Corticosteroids (CAPE_COD) (NCT02517489). Available from: https://clinicaltrials.gov/ct2/show/NCT02517489?term=COVID+hydrocortisone&draw=2&rank=5.
- 22. Efficacy and Safety of Corticosteroids in COVID‐19 (NCT04273321). Available from: https://clinicaltrials.gov/ct2/show/NCT04273321.
- 23. Dexamethasone Treatment for Severe Acute Respiratory Distress Syndrome Induced by COVID‐19 (DHYSCO) (NCT04347980). Available from: https://clinicaltrials.gov/ct2/show/NCT04347980.
- 24. Dexamethasone for COVID‐19 Related ARDS: a Multicenter, Randomized Clinical Trial (NCT04395105). Available from: https://clinicaltrials.gov/ct2/show/NCT04395105.
- 25. Dexamethasone and Oxygen Support Strategies in ICU Patients With Covid‐19 Pneumonia (COVIDICUS) (NCT04344730). Available from: https://clinicaltrials.gov/ct2/show/NCT04344730.
- 26. Low‐dose Hydrocortisone for Patients with COVID‐19 and Severe Hypoxia (NCT04348305). Available from: https://clinicaltrials.gov/ct2/show/NCT04348305?term=COVID+STEROID&draw=2&rank=1.
- 27. COVID‐19‐associated ARDS Treated With Dexamethasone: Alliance Covid‐19 Brasil III (CoDEX) (NCT04327401). Available from: https://clinicaltrials.gov/ct2/show/NCT04327401.
- 28. Efficacy and Safety of Corticosteroids in Oxygen‐dependent Patients With COVID‐19 Pneumonia (CORTICOVIDHUGO) (NCT04359511). Available from: https://clinicaltrials.gov/ct2/show/NCT04359511.
- 29. Corticosteroids During Covid‐19 Viral Pneumonia Related to SARS‐Cov‐2 Infection (CORTI‐Covid) (NCT04344288). Available from: https://clinicaltrials.gov/ct2/show/NCT04344288.
- 30. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1:100‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Medical Association . Declaration of Helsinki ‐ Ethical Principles for Medical Research involving Human Subjects. Version 2008. Available from https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/20132013/. Accessed on Feb 26, 2020.
- 33. The Internation Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Expert Working Group. Integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). 2016. Available from https://www.ich.org/page/efficacy‐guidelines
- 34. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury. 2012. [PubMed]
- 35. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727‐1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Buuren S, Groothuis‐Oudshoorn K. Mice. Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45(3).1–67. [Google Scholar]
- 37. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials ‐ a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krag M, Perner A, Wetterslev J, et al. Stress ulcer prophylaxis in the intensive care unit trial: detailed statistical analysis plan. Acta Anaesthesiol Scand. 2017;61:859‐868. [DOI] [PubMed] [Google Scholar]
- 39. Kryger Jensen A, Lange T. A novel high‐power test for continuous outcomes truncated by death. arXiv:1910.12267 [stat.ME]. 2019.
- 40. Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med. 2012;31:328‐340. [DOI] [PubMed] [Google Scholar]
- 41. Cao B, Wang Y, Wen D, et al. A trial of Lopinavir‐ritonavir in adults hospitalized with severe covid‐19. N Engl J Med. 2020.382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549‐556. [PubMed] [Google Scholar]
- 43. Fleischhack G, Hartmann C, Simon A, et al. Meropenem versus ceftazidime as empirical monotherapy in febrile neutropenia of paediatric patients with cancer. The Journal of antimicrobial chemotherapy. 2001;47:841‐853. [DOI] [PubMed] [Google Scholar]
- 44. European Data Portal . COVID‐19: ICU beds use and capacity. Available from: https://european‐data‐portal_interactive.gitlab.io/covid‐19/ic_beds_visualisation.html.
- 45. Dexamethasone for COVID‐19 Related ARDS: a Multicenter, Randomized Clinical Trial (NCT04395105). Available from: https://clinicaltrials.gov/ct2/show/NCT04395105?term=NCT04395105&draw=2&rank=1.
- 46. The Efficacy of Different Hormone Doses in 2019‐nCoV Severe Pneumonia (NCT04263402). Available from: https://clinicaltrials.gov/ct2/show/NCT04263402?term=NCT04263402&draw=2&rank=1.
- 47. International Comittee of Medical Journal Editors (ICMJE) . Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals. Available from: http://www.icmje.org/recommendations/.Accessed on May 5, 2020. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Supplementary Material


