Abstract
Background
Among hospitalized patients with coronavirus disease 2019 (COVID‐19), up to 12% may require intensive care unit (ICU) management. The aim of this prospective cohort study is to assess nutrition status and outcome in patients with COVID‐19 following ICU discharge.
Methods
Patients requiring a minimum of 14 days’ stay in the ICU with mechanical ventilation were included. Nutrition status was assessed at inclusion (ICU discharge) and follow‐up (after 15, 30, and 60 days). All patients had standardized medical nutrition therapy with defined targets regarding energy (30 kcal/kg/d) and protein intake (1.5 g/kg/d).
Results
Fifteen patients were included (67% males); the median age was 60 (33–75) years old. Body mass index at ICU admission was 25.7 (IQR, 24–31) kg/m². After a median ICU stay of 33 (IQR, 26–39) days, malnutrition was present in all patients (11.3% median weight loss and/or low muscle mass based on handgrip strength measurement). Because of postintubation dysphagia in 60% of patients, enteral nutrition was administered (57% nasogastric tube; 43% percutaneous endoscopic gastrostomy). After 2 months, a significant improvement in muscle strength was observed (median handgrip strength, 64.7% [IQR, 51%–73%] of the predicted values for age vs 19% [IQR, 4.8%–28.4%] at ICU discharge [P < 0.0005]), as well as weight gain of 4.3 kg (IQR, 2.7–6.7 kg) (P < 0.0002).
Conclusions
Critically ill patients with COVID‐19 requiring ICU admission and mechanical ventilation have malnutrition and low muscle mass at ICU discharge. Nutrition parameters improve during rehabilitation with standardized medical nutrition therapy.
Keywords: malnutrition, medical nutrition therapy, nutrition support, SARS‐CoV2
CLINICAL RELEVANCY STATEMENT
All patients with COVID‐19 are at nutrition risk, and one‐third of them are malnourished. This study assessed the nutrition status and outcome of the subgroup who may require intensive care management (12%). Malnutrition and low muscle mass are present in the majority of patients after prolonged ICU stay with mechanical ventilation. Standardized medical nutrition therapy allows improvement of nutrition parameters. Nutrition support with adequate protein intake (>1.5 g/kg/day) remains crucial even after hospital discharge during rehabilitation.
INTRODUCTION
In December 2019, Wuhan City, the capital of Hubei Province in China, became the center of an outbreak of viral pneumonia, attributed to a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]). The disease related to the viral infection was designated coronavirus disease 2019 (COVID‐19). 1
Among hospitalized patients with COVID‐19, up to 12% may require intensive care unit (ICU) management with invasive mechanical ventilation, requiring endotracheal intubation and medical nutrition therapy. 1 It is already been shown that in patients with multiple organ dysfunction syndrome due to other causes, muscle wasting occurred early and rapidly during the first week of critical illness according to the decreased of rectus femoris cross‐sectional area from days 1 to 7 (–12.5% [95% confidence interval (CI), –15.8% to –9.1%]). 2 Furthermore, prolonged bed rest leads to sarcopenia, characterized by loss of skeletal muscle mass and function. 3 It is noteworthy that even in young, otherwise healthy adults, strength of the knee extensors may decrease up to 6% per week during bed rest. 4 Specifically in patients with SARS‐CoV‐2 infection, comorbidities such as diabetes, arterial hypertension and cardiovascular disease can coexist and further aggravate nutrition status. 1 , 5 Malnutrition is responsible for high morbidity at discharge from the ICU 6 and, conversely, optimal nutrition status has been shown to be associated with fewer complications, shorter stays in the ICU, and lower mortality in 2 recent large retrospective studies including ICU patients. 7 , 8 Moreover, according to a recent Italian cohort of 268 patients, it has been shown that all patients with COVID‐19 are at nutrition risk, and one‐third of them are malnourished. 9 Recent recommendations regarding nutrition management in patients with COVID‐19 have been issued by the European Society on Enteral and Parenteral Nutrition (ESPEN) and the American Society for Parenteral and Enteral Nutrition (ASPEN), but specific cohort studies in this population are lacking. 10 , 11 Furthermore, optimal management during the early recovery phase following ICU discharge (rehabilitation period) is equally challenging, as many patients present severe low muscle mass, as well as postextubation swallowing disorders limiting oral food intake. 12 , 13 It is therefore recommended to provide sufficient nutrition support to allow physical rehabilitation in post‐ICU patients by achieving an energy intake of 30 kcal/kg/d and 1.5–2 g/kg of protein per day. 14 Up till now, there are no published data regarding nutrition management in COVID‐19 patients during the rehabilitation period after ICU stay.
The objective of the present prospective study is to (1) assess the nutrition status of critical COVID‐19 patients when discharged from the ICU, (2) evaluate if and how the nutrition recommendations (30 kcal/kg/d and 1.5 g/kg/d protein) are met during the rehabilitation period, and (3) measure the evolution of nutrition parameters such as weight, body mass index (BMI), and muscular strength (handgrip and mid‐arm circumference).
MATERIAL AND METHODS
Study design
This is a prospective, observational, monocentric study including consecutive COVID‐19 patients discharged from the ICU and admitted in the post‐ICU ward of the Erasme University Hospital from April 15, 2020, to June 1, 2020. The inclusion criteria were as follows: patients infected with SARS‐CoV‐2 according to the positive result on a reverse transcriptase polymerase chain reaction assay of a specimen collected on a nasopharyngeal swab or bronchoalveolar lavage, age over 18 years, prolonged stay (>2 weeks) in ICU with mechanical ventilation requiring endotracheal intubation, and minimum stay of 7 days in the post‐ICU ward. Patients were followed up over a period of 2 months, either as in‐ or out‐patients. All patients received medical nutrition therapy based on current ESPEN recommendations. 10
Data collection
Demographic and clinical characteristics of the patients
Data collected include demographics (age, sex), weight, height, and BMI, as well as preexisting comorbidities, assessed by the Charlson Comorbidity Index. 15 ICU severity was evaluated with the Sequential Organ Failure Assessment Score 16 and Simplified Acute Physiology Score 3, both predicting mortality risk. 17 Additional recorded parameters included duration of mechanical ventilation, use of extracorporeal membrane oxygenation (ECMO), presence of a tracheostomy, postintubation swallowing disorders, type of nutrition support (enteral nutrition [EN], parenteral nutrition [PN], or oral nutrition supplements [ONS]), the type of enteral access used (nasogastric tube or percutaneous endoscopic gastrostomy [PEG]), and nutrition intake (energy and protein respectively expressed in kcal/kg/d and g/kg/d) and percent related to individual target before ICU reported by the patient, estimated with ideal BMI of 25 kg/m2 for obese patients (>30 kg/m2). Preexisting, post‐ICU and after rehabilitation ward low muscle mass were quantified using thoracic muscle areas on chest computerized tomography (CT) that had been performed on admission in all patients to confirm pneumonia due to SARS‐CoV‐2 and during the follow‐up 18 , 19 (see Supplementary Methods).
Nutrition status data
The diagnosis of malnutrition was made based on the percent weight loss after discharge from ICU (compared with weight at the time of admission) and the presence of low muscle mass. Muscle mass was assessed indirectly by anthropometric measures such as mid‐arm circumference measured in centimeters and handgrip strength using Jamar Hydraulic Hand Dynamometer. 20 , 21
Additional measurements conducted after ICU discharge included the Simplified Nutritional Appetite Questionnaire (SNAQ) 22 and the Simple Evaluation of Food Intake (SEFI) 23 (see Supplementary Methods). Postextubation swallowing disorders were assessed by ear, nose, and throat specialists. 12
Medical nutrition therapy protocol
A standardized medical nutrition therapy protocol based on the ESPEN recommendations was applied 10 (see Supplementary Methods).
Follow‐up
Patients were followed over a period of 2 months after ICU discharge (day 0 [baseline], 7, 14, 21, 30, and 60) Monitoring include weight, BMI, SNAQ score, food intake and protein‐energy nutrition intake assessment, type of medical nutrition therapy administered, and handgrip and mid‐arm circumference measurement. Post‐ICU complications were also listed. Energy and protein intakes were calculated based on dietitian evaluation including oral, EN, and PN on days 0, 7, 14, 21, 20, and 60.
Our inpatient rehabilitation program consisted of a daily 90‐minute session of exercise training. Additional altering 30‐minute sessions of patient education, occupational therapy, nutrition counseling, and psychosocial support are performed daily. The 2‐minute walking test was evaluated at the beginning and end of the rehabilitation ward.
Ethics
This study was approved by the Ethics Committee (CCB B4062020000075) in accordance with the Declaration of Helsinki, and written informed consent was obtained from the patients. All authors had access to the study data and reviewed and approved the final manuscript.
Statistical analysis
Descriptive statistics were used, and results are reported as medians and interquartile ranges (IQRs). Categorical variables were summarized as counts and percentages. The Wilcoxon signed rank test was used to compare matched groups with the baseline values after ICU discharged to determine the evolution of nutrition parameters. Nonparametric Spearman correlation was used. The statistically significant threshold value was fixed at 5%. Analysis was performed with Prism 6 software.
RESULTS
Clinical characteristics of COVID‐19 patients discharged from ICU
From April 15, 2020, to June 1, 2020, 41 COVID‐19 patients were discharged alive from the 82 patients admitted to the ICU. From the above, 20 patients were eligible based on the aforementioned inclusion criteria and 15 were finally enrolled (Figure 1). Median follow‐up was 61 (IQR, 60–63) days and 14 out of 15 (93%) of patients were fully assessed at the end of follow‐up. One patient was not fully assessed because of discharge to an external rehabilitation ward. All demographics and ICU data are shown in Table 1. The median of age of study participants was 60 years (IQR, 55–67) and 67% were men. The median weight before ICU admission was 87 kg (IQR, 71–94) and the median BMI was 25.7 kg/m2 (IQR, 24–31). Four patients (27%) and 6 patients (40%) were respectively obese with a BMI >30 kg/m2 and overweight with a BMI between 25 and 30 kg/m2. The median length of stay in ICU was 33 days (IQR, 26–39). Preexisting low muscle mass based on chest CT according to the height normalized index of dorsal muscle area (DMI) was present on 4 men (44%) and 2 women (40%). The median duration of mechanical ventilation was 24 days (IQR, 19–25 days) and 7 patients (46.6%) required a temporary tracheostomy. In 4 patients (26.6%) ECMO was provided.
Figure 1.
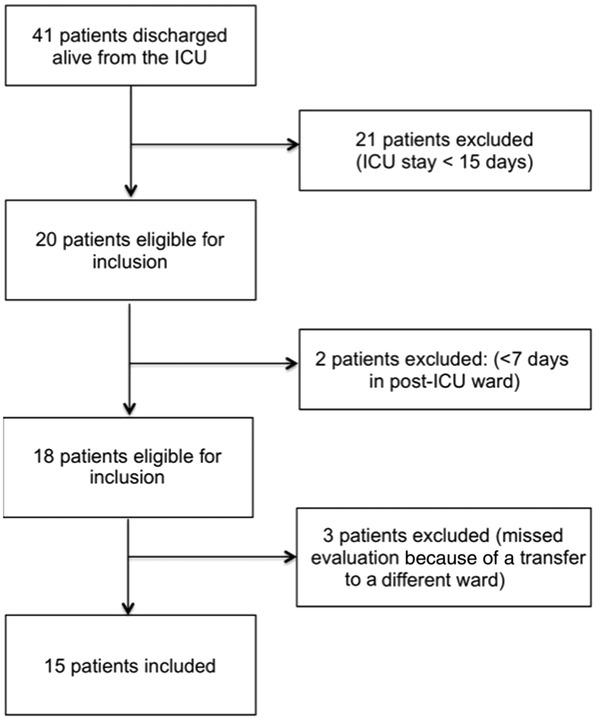
Flow‐chart illustrating enrolment of coronavirus disease 2019 patients discharged from the intensive care unit (ICU)
Table 1.
Clinical characteristics of patients discharged from the ICU
| Characteristics | Patients (n = 15) |
|---|---|
| Age, median (IQR), years | 60 (55–67) |
| Gender, n (%) | |
| Male | 10 (66.6) |
| Female | 5 (33.3) |
| Weight, median, kg (IQR) | 87 (71–94) |
| Body mass index, median (IQR) | 25.7 (24–31) |
| DMI, cm2/m2, median (IQR) a | |
| Male | 11.8 (11.7–15.9) |
| <10.9 cm2/m2, n (%) | 4 (44) |
| Female | 9.5 (7.3–10.9) |
| <7.8 cm2/m2, n (%) | 2 (40) |
| Coexisting comorbidity, n (%) b | |
| Cardiovascular disease | 5 (33.3) |
| Diabetes mellitus | 0 |
| Asthma | 3 (20) |
| Chronic obstructive pulmonary disease | 2 (13.3) |
| Other pulmonary disease | 1 (6.6) |
| Cancer | 3 (20) |
| Chronic kidney disease | 0 |
| Chronic liver disease | 0 |
| Chronic inflammatory disease | 2 (13.3) |
| Hemorrhagic or ischemic stroke | 0 |
| Current or former tobacco smoker | 3 (20) |
| Charlson index, median (IQR) | 1 (0–2) |
| Length of stay in ICU, median (IQR), days | 33 (26–39) |
| Duration of mechanical ventilation, median (IQR), days | 24 (19–25) |
| Tracheostomy, n (%) | 7 (46.6) |
| ECMO, n (%) | 4 (26.6) |
| ICU prognostic scores, median (IQR) | |
| SOFA | 7 (4–9) |
| SAPS3 | 55 (45–58) |
| Complications, n (%) | |
| EP | 3 (20) |
| VAP | 15 (100) |
| Opportunistic infection | 1 (6.6) |
| Dialysis | 2 (13.3) |
Abbreviations: DMI, height normalized index of dorsal muscle area; ECMO, extracorporeal membrane oxygenation; EP, embolism pulmonary; ICU, intensive care unit; IQR, interquartile range; SAPS3, Simplified Acute Physiology Score 3; SOFA, Sequential Organ Failure Assessment Score; VAP, ventilator associated pulmonary.
Data are available for 14 patients.
Preexisting disorder was defined on the basis of the documented clinical diagnostic categories: cardiac, respiratory, renal, liver, inflammatory disease, and immunocompromised host; history of cancer with hematologic cancer within the previous 5 years or active in ICU admission; and diabetes.
Nutrition assessment at time of ICU discharge
At the time of ICU discharge, median BMI was 22.9 kg/m2 (IQR, 20.4–31 kg/m2). One patient had a BMI < 20 kg/m2 (19.1 kg/m2). Median weight was 75.8 kg (IQR, 62–90 kg). Median weight loss compared with former weight was 11.3% (IQR, 7.8%–15.5%). Six patients (40%) lost <10% of their weight, 8 patients (54%) lost >10% of their weight, and 1 patient lost >20% of their weight. Regarding evaluation of low muscle mass, handgrip strength of the dominant arm was performed with a median value of 8 kg (IQR, 2–12 kg). The dominant handgrip strength was 8 kg (IQR, 8–13) and 0 kg (0–11 kg) for, respectively, men and women. Relative handgrip value reported according to age references values 24 was 19% (IQR, 4.8%–28.4%). Median mid‐arm circumference was 28.5 cm (IQR, 26–30 cm). Postextubation swallowing disorders were observed in 9 patients (60%). Medical nutrition therapy was administered to all 15 patients. Enteral access with nasogastric tube was used in 8 patients (57%), and 6 (43%) patients had a PEG (Table 2). Median values of energy intake in ICU during the phases of critical illness were 7.5 kcal/kg/d (IQR, 1.8–11.9 kcal/kg/d) at the first day and gradually increased to reach 18.4 kcal/kg/d (IQR, 6.9–21.95 kcal/kg/d) at the fifth day. On the 15th and 20th days, median energy intake were respectively 21.6 kcal/kg/d (IQR, 20–25.6 kcal/kg/d) and 23.4 kcal/kg/d (IQR, 20.8–26.7 kcal/kg/d) (Figure 2).
Table 2.
Nutrition assessment of coronavirus disease 2019 patients at intensive care unit discharge
| Characteristics | Patients (n =15) |
|---|---|
| Body mass index, n, median (IQR) | 22.9 (20.4–31) |
| Weight loss, median, (IQR), % | 11.3 (7.8–15.5) |
| ≤10%, no. (%) | 6 (40) |
| >10%, no. (%) | 8 (53) |
| >20%, no. (%) | 1 (7) |
| Dominant handgrip strength, median (IQR), kg | 8 (2–12) |
| Male, median (IQR) | 8 (8–13) |
| Female, median (IQR) | 0 (0–11) |
| Median of age reference values, median (IQR), % | 19.4 (6.5–28) |
| Mid arm circumference, median (IQR), cm | 28.5 (26–30) |
| Postintubation swallowing disorders, n (%) | 9 (60) |
| Enteral nutrition, n (%) | 14 (93) |
| Parenteral nutrition, n (%) | 1 (7) |
| Enteral access, n (%) | |
| Nasogastric tube | 8 (57) |
| Percutaneous endoscopic gastrostomy | 6 (43) |
Abbreviations: IQR, interquartile range; no., number of patients.
Figure 2.
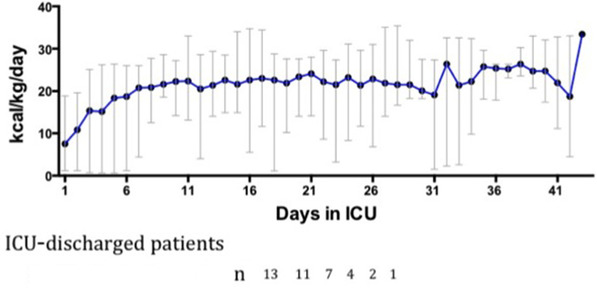
Nutrition intake in intensive care unit (ICU) during the phases of critical illness. Representative median of energy intake according to the kilocalories per kilos per day (kcal/kg/d) (the weight measured upon admission to the ICU) followed until discharge from ICU. Each median of kcal/kg/d is calculated each day with the number of patients remaining in ICU. Each median was the energy data of 13 patients. Two patients were transferred from another hospital and their nutrition data were missing
Medical nutrition therapy and nutrition intake during follow‐up
Following ICU discharge, all of the patients had ongoing medical nutrition therapy (Figure 3A). EN was exclusive for 14 patients with a median duration of 7 days (IQR, 4–10 days). One patient had persistent postextubation swallowing disorders and was kept on exclusive EN for 53 days. EN was combined with oral intake in 13 patients for a median duration of 14 days (IQR, 6–23.5 days). The median duration of oral intake with ONS and oral intake alone were respectively 22 days (IQR, 11–35 days) and 33 days (IQR, 23–42 days). One patient had exclusive PN for 3 days and associated with oral and ONS intake for 8 days. Median values of protein‐energy nutrition intake related to individual targets are represented in Figure 3B. During the follow‐up, energy intake ranged from 28 to 33.5 kcal/kg/d, and at the end of follow‐up, the median value of energy target covered was 83.3%. Similarly, protein intake varied from 1 to 1.6 g/kg/d, and at the end of follow‐up, the median value of protein target covered was 63.3%. Oral intake alone without any additional medical nutrition therapy was observed in 53.3% of the patients at the end of follow‐up.
Figure 3.
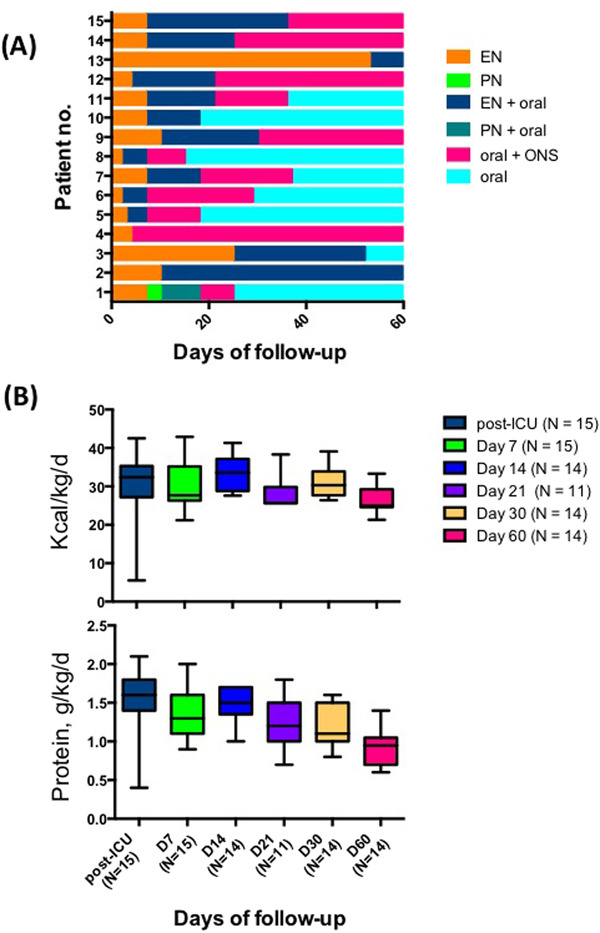
Nutrition support for individual patients and nutrition intake (energy and protein) during the 2 months of follow‐up. (A) Representative nutrition support of the 15 patients and their duration (in days). Each support is represented with a different shade of gray. One patient was lost to follow‐up at day 30. (B) Each plot represented the median of kcal/kg/d and g/kg/d protein at the time to the follow‐up. D, day; ICU, intensive care unit; no., number
Nutrition status and muscle mass assessment during follow‐up
Median weight significantly increased during the follow‐up to reach a weight gain of 4.3 kg (IQR, 2.7–6.7 kg) (P < 0.0002) at 60 days compared with post‐ICU discharge weight (Table 3). Median mid‐arm circumference does not increase significantly during the follow‐up, but the measures carried out at admission are significantly correlated with the BMI (r = 0.738, P = 0.002). Median dominant handgrip significantly increased up to 64.7% (IQR, 51%–73%) of reference values based on patient age at day 60 (Figure 4A and 4B). Low muscle mass criteria quantified on chest CT were compared between ICU admission, post‐ICU ward, and after rehabilitation ward. In the post‐ICU ward, 8 patients had a chest CT and 75% had low muscle mass criteria but with no significant difference compared with their admission data. After rehabilitation ward, 11 patients had a chest CT and 27% had low muscle mass criteria with significant difference compared with their post‐ICU data (P = 0.02) (Table 3). SNAQ evaluation did not increase significantly, and the median score was >14 at the end of the follow‐up.
Table 3.
Nutrition status and muscle mass assessment during follow‐up
| ICU admission | Post‐ICU | Day 7 | Day 14 | Day 21 | Day 30 | Day 60 | |
|---|---|---|---|---|---|---|---|
| Weight, median (IQR), kg | 87 (71– 94) | 75.8(62–90) | 76 (62–90.5) | 76 (68–91) | 74.3 (65–92) | 78.4 (68–89) | 76.5 (69–88) |
| Weight loss, % (IQR) | 11.3 (7.8–15.5) | 10.9 (7–15) | 10 (5–14.5) | 12.5 (5.6–16) | 9.1 (4–12.5) | 6.5 (3–12) | |
| P‐value | .002 | .0002 | .0001 | .0001 | .0002 | ||
| n = 15 | n = 15 | n = 13 | n = 11 | n = 13 | n = 14 | ||
| Dominant HG strength, median (IQR), kg | NA | 8 (2–12) | 15 (6.5‐23) | 18 (7.5–23.5) | 20 (12–28) | 22.5 (10–31.5) | 26 (18–35.5) |
| P‐value | .001 | .0005 | .002 | .0002 | .0002 | ||
| Matched age reference values, median (IQR), % | 19.4 (6.5–28) | 36 (17–55) | 39 (26.5–55) | 45.5 (29–58) | 50.5 (37–75) | 64.7 (51–73) | |
| n = 15 | n = 14 | n = 13 | n = 11 | n = 14 | n = 13 | ||
| Mid‐arm circumference, median (IQR), cm | NA | 28.5 (26–30) | 27.5 (24–31.5) | 29 (25–30) | 27.5 (25–32.5) | 29 (27–31.5) | 29 (26.5–31) |
| P‐value | .337 | .312 | .750 | .554 | .110 | ||
| n = 15 | n = 14 | n = 13 | n = 11 | n = 14 | n = 13 | ||
| SNAQ score, median (IQR) | NA | NA | 16 (14–17) | 15 (14–16) | 16 (14–18) | 16 (15–18) | 17 (16.5–18.5) |
| (n = 6) | (n = 10) | (n = 8) | (n = 12) | (n = 13) | |||
| DMI, cm2/m2 | |||||||
| Male <10.9 and female <7.8, n (%) | 6 (42) | 6 (75) | NA | NA | NA | NA | 3 (27) |
| P‐value | .22 | .02 a | |||||
| n = 14 | n = 8 | n = 11 |
Abbreviations: DMI, height normalized index of dorsal muscle area; HG, handgrip; ICU, intensive care unit; IQR, interquartile range; NA, evaluation not performed; SNAQ, Simplified Nutritional Appetite Questionnaire.
Significant difference according to the post‐ICU data.
Figure 4.
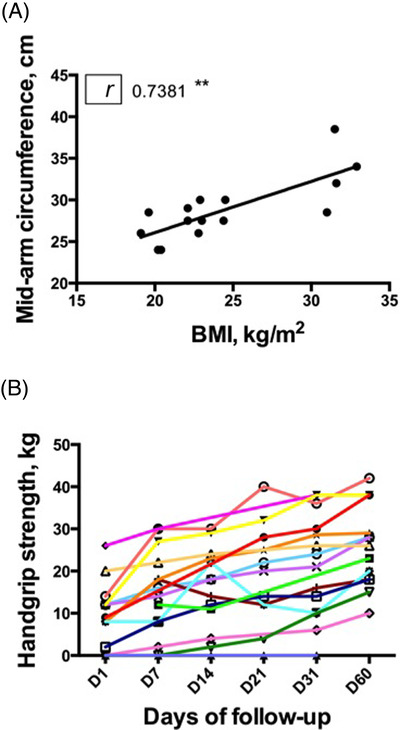
(A) Correlation between body mass index (BMI) and mid‐arm circumference. (B) Individual dominant handgrip strength at the time of intensive care unit discharge and at day (D) 7, 14, 30, and 60 for 13 patients. Each patient is represented with a shade of gray
Outcomes
The median length of stay in the post‐ICU ward was 19 days (IQR, 12–23 days) and 38 days (IQR, 26–51 days) in an in‐hospital or external rehabilitation ward. The median duration of tracheostomy was 25 days (IQR, 17–26 days), and the PEG stayed in place for a median duration of 51 days (IQR, 46–64 days). None of patients had swallowing disorders at the end of the follow‐up. One patient, with preexisting pulmonary fibrosis, still requires nasal oxygenotherapy. Among the complications encountered during the follow‐up, 8 patients (53%) had infections, 2 patients (13%) had haemorrhagic shock (duodenal ulcer and spontaneous psoas hematoma), 7 patients (47%) had pressure sores, and 7 patients (47%) had peripheric nerve injury. Among the 7 patients in our rehabilitation ward, the median of the 2‐minute walk distance was 60 m (IQR, 40–81 m), 31% of predicted distance on admission, and 129 m (IQR, 115–150 m), 70.5% of predicted distance at discharge (P = 0.03). At the end of the follow‐up, 13 patients had been discharged from the hospital, and 2 patients were still in the in‐hospital or external rehabilitation ward at the end of the follow‐up. (see Table S1).
DISCUSSION
The present work prospectively evaluates the nutrition status and outcome in patients with critical COVID‐19 following discharge from the ICU and during the post‐ICU rehabilitation period. Patients infected with SARS‐COV‐2 often present with respiratory symptoms but may deteriorate because of multiorgan failure and may even lead to death. 1 In case of critical COVID‐19, prolonged ICU stay with mechanical ventilation is required, which constitutes a major cause of morbidity and mortality, especially in elderly and polymorbid patients. 25 In our cohort, the majority of patients had a severe malnutrition at the moment of ICU discharge according to the weight loss (9 patients lost >10% of their weight) and/or low muscle mass assessed by handgrip strength (the median of the dominant handgrip strength was 8 kg), 10 even if they received the recommended energy intake during ICU stay, (20–25 kcal/kg/d, data not shown). 14 Low muscle mass criteria quantified on chest CT were already present in 44% of men and 40% of women at the time of ICU admission. At ICU discharge, 60% of patients presented with >10% of weight loss compared with their weight before hospital admission, but nevertheless, the median BMI was normal at 22.9 kg/m2. This can be explained by the fact that 40% of patients were overweight and 27% of patients were obese before ICU admission. Indeed, the prevalence of obesity was higher in patients with critical COVID‐19 than ICU patients without COVID‐19 in a French study. 26 Moreover, a recent meta‐analysis study showed that patients with a BMI > 25kg/m2 are at a high risk of mortality from COVID‐19 infection. 27 Therefore, overweight patients are at increased risk for COVID‐19–related morbidity and mortality, and although they may present a BMI in the reference range at ICU discharge because of ICU‐related weight loss, malnutrition is still a major concern.
Secondary sarcopenia and muscle weakness acquired in the ICU is common and associated with long‐standing consequences that dramatically affect recovery. 28 , 29 Sepsis, persistent systemic inflammation, female gender, prolonged duration of mechanical ventilation, use of neuromuscular blocking agents, and multiorgan system failure are important risk factors. 30 Indeed, almost all of our patients had low muscle mass, which was reflected by the muscle strength measurement by handgrip revealing low values, corresponding to 19% of the predicted values for age. During the post‐ICU rehabilitation period, we observed a significant increase of handgrip strength up to 64.7% of the predicted values for age at day 60. Low muscle mass on chest CT was observed in 75% of the patients in the post‐ICU ward and decreased significantly to 27% after rehabilitation ward. All patients pursued physical therapy even after hospital discharge. Low muscle mass can profoundly influence post‐ICU rehabilitation and ensuing functional limitations may persist even after hospital discharge in 65% of patients. 30 , 31 Moreover, the consequences may be long‐term, as illustrated in a study evaluating 109 survivors of acute respiratory distress syndrome. All patients had persistent functional disability at 1 year after discharge, reflected in pulmonary‐function testing, a 6‐minute walk test, and a quality‐of‐life evaluation. 31
At the moment of ICU discharge, 60% of patients had post‐ICU swallowing disorders related to prolonged endotracheal intubation. A recent prospective study including 1304 medical and surgical ICU patients reported an incidence rate of postextubation dysphagia of up to 18.3%. 32 These differences could be attributed to the duration of invasive mechanical ventilation with a median of 33 days in our cohort vs 0.7 days in the aforementioned study. Related complications include increased risk for aspiration‐induced pneumonia, delayed resumption of oral intake, subsequent malnutrition, and prolonged hospital length of stay. 12 Maintaining EN after ICU discharge through the use of a nasogastric tube or a PEG is mandatory to provide optimal nutrition support, similar to our cohort. During the follow‐up, all patients received standardized medical nutrition therapy. Energy and protein intake varied between respectively 26–32 kcal/kg/d and 1–1.5 g/kg/d. At the end of follow‐up, energy and protein intake corresponded to respectively 83.3% and 63.3% of proposed targets, based on the ESPEN guidelines. 10 In our cohort, most patients discharged from the hospital spontaneously stopped ONS consumption and pursued exclusive oral nutrition. Similarly, in a larger post‐ICU cohort study including 32 adult non–COVID‐19 critically ill patients, the median estimated energy and protein requirements were 2000 (1650–2550) kcal and 112 (84–129) g, respectively. 33 Nevertheless, intake was much lower, resulting in 62% of energy and 54% of protein requirements received. A recent randomized control trial of 652 patients discharged from the hospital studied the role of high‐protein ONS (HP‐ONS) vs placebo ONS on malnourished patients. HP‐ONS reduced 90 days’ mortality compared with placebo (4.8% vs 9.7%). Despite the decrease in mortality rate, the number needed to treat in the posthospital discharge setting to prevent one death was relatively high at 20.3 (95% CI: 10.9, 121.4), thus nuancing the effect of ONS. 34 Nevertheless, according to recent recommendations, ONS are recommended for all ICU survivors for at least 3 months following hospital discharge. 14
At the end of the 2‐month follow‐up, all but 2 patients had been discharged from the hospital. All patients had an improved nutrition status. These results show encouraging data concerning the nutrition outcome of critical COVID‐19 patients after discharge from the ICU. However, the present study has some limitations. First of all, the sample size is small, there is no control group, and the study was conducted without a standardized medical nutrition therapy protocol. Moreover, energy requirements were determined based on weight‐based formulas and not indirect calorimetry, given the difficulties to correctly sterilize the equipment and ensure safety in the specific setting of SARS‐CoV‐2 infection. 10 Finally, the 2‐month follow‐up period may be too short to assess long‐term improvement of functional disability.
In conclusion, the present study represents the first report regarding outcome of medical nutrition therapy in critical COVID‐19 patients following ICU discharge. Although malnutrition and low muscle mass with decreased muscle strength is present in the majority of patients after a prolonged ICU stay with mechanical ventilation, standardized medical nutrition therapy and an intense physical rehabilitation allows significant improvement of nutrition parameters. Nutrition support with adequate protein intake (>1.5 g/kg/d) remains crucial even after hospital discharge.
FUNDING INFORMATION
None declared.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Alice Hoyois and Marianna Arvanitakis equally contributed to the conception and design of the research; Marianna Arvanitakis contributed to the design of the research; Alice Hoyois, Asuncion Ballarin, Justine Thomas, Olivier Lheureux, Jean‐Charles Preiser, Emmanuel Coppens, Silvia Perez Bogerd, Olivier Taton, Sylvie Farine, and Pauline Van Ouytsel contributed to the acquisition and analysis of the data; Alice Hoyois, Emmanuel Coppens, and Marianna Arvanitakis contributed to the interpretation of the data; and Alice Hoyois and Marianna Arvanitakis drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Supporting information
Supplementary information
Supplementary information
ACKNOWLEDGMENTS
The authors are indebted to the health workers (nurses and dietitians) of the post‐ICU COVID‐19 rehabilitation unit who helped in collecting data. No funding was required for this study.
Hoyois A, Ballarin A, Thomas J, et al. Nutrition evaluation and management of critically ill patients with COVID‐19 during post–intensive care rehabilitation. Journal of Parenteral and Enteral Nutrition. 2021;45:1153–1163. 10.1002/jpen.2101
REFERENCES
- 1. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, Mcginn T, Davidson KW. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puthucheary ZA, Rawal J, Mcphail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591‐1600. [DOI] [PubMed] [Google Scholar]
- 3. English KL, Paddon‐Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13(1):34‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. AD . Leblanc R, Pientok C, Rowe R. Regional changes in muscle mass following I7 weeks of bed rest. J Appl Physiol. 2018;73(5):2172‐2178. [DOI] [PubMed] [Google Scholar]
- 5. Gomes F, Schuetz P, Bounoure L, et al. ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr. 2018;37(1):336‐353. [DOI] [PubMed] [Google Scholar]
- 6. Singer P, Reintam A, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48‐79. [DOI] [PubMed] [Google Scholar]
- 7. Looijaard WGPM, Dekker IM, Beishuizen A, Girbes ARJ, Straaten HMO, Weijs PJM. Early high protein intake and mortality in critically ill ICU patients with low skeletal muscle area and ‐density. Clin Nutr. 2020. Jul;39(7):2192–2201. [DOI] [PubMed] [Google Scholar]
- 8. Weijs PJM, Mogensen KM, Rawn JD, Christopher KB. Protein intake, nutritional status and outcomes in ICU survivors: a single center cohort study. J Clin Med. 2019;8(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pironi L, Sasdelli AS, Ravaioli F, et al. Malnutrition and nutritional therapy in patients with SARS‐CoV‐2 disease. Clin Nutr. 2020;40(3):1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barazzoni R, Bischoff SC, Breda J, et al. endorsed by the EC . ESPEN expert statements and practical guidance for nutritional management of individuals with SARS‐CoV‐2 infection. Clin Nutr. 2020;39(6):1631‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martindale R, Patel JJ, Taylor B, Arabi YM, Warren M, Mcclave SA. Nutrition therapy in critically ill patients with coronavirus disease 2019. JPEN J Parenter Enteral Nutr. 2020;44(7):1174‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms, and clinical management. Crit Care. 2019;23(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peterson SJ, Tsai AA, Scala CM, Sowa DC. Adequacy of oral intake in critically ill patients. JADA. 2010;110(3):427‐433. [DOI] [PubMed] [Google Scholar]
- 14. Raymond A, Van Zanten H, De Waele E, Wischmeyer PE. Nutrition therapy and critical illness: practical guidance for the ICU, post‐ICU, and long‐term convalescence phases. Crit Care. 2019;23(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Charlson ME, Peter Pompei KLA, Mackenzie CR. A new method of classifying prognostic in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 16. Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL, Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754‐1758. [DOI] [PubMed] [Google Scholar]
- 17. Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3 Investigators. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Derstine BA, Holcombe SA, Ross BE, Wang NC, Su GL, Wang SC. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sullivan JA, Su GL, Wang SC. Quantifying sarcopenia reference values using lumbar and thoracic muscle areas in a healthy population. J Nutr Heal Aging. 2018;21(10):1‐6. [DOI] [PubMed] [Google Scholar]
- 20. Cattermole GN, Graham CA, Rainer TH. Mid‐arm circumference can be used to estimate weight of adult and adolescent patients. Emerg Med J. 2017;34(4):231‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guerra RS, Fonseca I, Pichel F, Restivo MT, Amaral TF. Handgrip strength cutoff values for undernutrition screening at hospital admission. Eur J Clin Nutr. 2014;68(12):1315‐1321. [DOI] [PubMed] [Google Scholar]
- 22. Lau O, Pek K, Chew J, et al. The Simplified Nutritional Appetite Questionnaire (SNAQ) as a screening tool for risk of malnutrition: optimal cut off, factor structure, and validation in healthy community‐dwelling older adults. Nutrients. 2010;12(9):2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hiesmayr M, Schindler K, Pernicka E, et al. Decreased food intake is a risk factor for mortality in hospitalised patients : the NutritionDay survey 2006. Clin Nutr. 2009;28(5):484‐491. [DOI] [PubMed] [Google Scholar]
- 24. Bohannon RW, Peolsson A, Massy‐westropp N, Desrosiers J, Bear‐lehman J. Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive meta‐analysis. Physiotherapy. 2006;92(1):11‐15. [Google Scholar]
- 25. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Causs C, Pattou F, Wallet F, et al. COHC and LCSG . Prevalence of obesity among adult inpatients with COVID‐19 in France. Lancet Diabetes Endocrinol. 2020;8(7):562‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hussain A, Mahawar K, Xia Z, Yang W, El‐Hasani S. Obesity and mortality of COVID‐19. Meta‐analysis. Obes Res Clin Pract. 2020;14(4):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Gunst J, Van den Berghe G. Intensive care nutrition and postintensive care recovery. Crit Care Clin. 2018;34(4):573‐583. [DOI] [PubMed] [Google Scholar]
- 29. Diaz‐granados N, Sc M, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293‐1304. [DOI] [PubMed] [Google Scholar]
- 30. Kress JP, Hall JB. ICU‐acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626‐1635. [DOI] [PubMed] [Google Scholar]
- 31. Matte‐martyn A, Sc B, Diaz‐granados N, et al. One‐year outcomes in survivors of the acute respiratory distress syndrome. NEJM. 2003;348(8):683‐693. [DOI] [PubMed] [Google Scholar]
- 32. Schefold JC, Berger D, Zürcher P, et al. Dysphagia in Mechanically Ventilated ICU Patients (DYnAMICS): a prospective observational trial. 2017;45(12):2061‐2069. [DOI] [PubMed] [Google Scholar]
- 33. Ridley EJ, Parke RL, Davies AR, et al. What happens to nutrition intake in the post‐intensive care unit hospitalization period ? An observational cohort study in critically ill adults. JPEN J Parenter Enter Nutr. 2019;43(1):88‐95. [DOI] [PubMed] [Google Scholar]
- 34. Deutz NE, Matheson EM, Matarese LE, et al. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: a randomized clinical trial. Clin Nutr. 2016;35(1):18‐26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Supplementary information


