Abstract
Weather and the susceptibility of children to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection is still a debated question and currently a hot topic, particularly in view of important decisions regarding opening schools. Therefore, we performed this prospective analysis of anti‐SARS‐CoV‐2 immunoglobulin G (IgG) antibodies in children with known household exposure to SARS‐CoV‐2 and compared their IgG status with the other adults exposed to the index case in the same household. A total of 30 families with a documented COVID‐19 index case were included. A total of 44 out of 80 household contacts (55%) of index patients had anti SARS‐CoV‐2 IgG antibodies. In particular, 16/27 (59,3%) adult partners had IgG antibodies compared with 28/53 (52,3%) of pediatric contacts (p > .05). Among the pediatric population, children ≥5 years of age had a similar probability of having SARS‐CoV‐2 IgG antibodies (21/39, 53.8%) compared to those less than 5 years old (7/14, 50%) (p > .05). Adult partners and children also had a similar probability of having SARS‐CoV‐2 IgG antibodies. Interestingly, 10/28 (35.7%) of children and 5/27 (18.5%) of adults with SARS‐CoV‐2 IgG antibodies were previously diagnosed as COVID‐19 cases. Our study shows evidence of a high rate of IgG antibodies in children exposed to SARS‐CoV‐2. This report has public health implications, highlighting the need to establish appropriate guidelines for school openings and other social activities related to childhood.
Keywords: children, COVID‐19, household, SARS‐CoV‐2, seroprevalence
1.
Eight months after the first outbreak in China, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic is still putting a strain on the health systems around the world. A much‐debated aspect of the disease concerns its impact on children.
A thorough study of the prevalence and contagiousness of the disease among children produced conflicting results: on one hand, low infection rates among children 1 and a low number of severe disease 2 have been described, on the other hand, a serious, albeit rare, inflammatory complication has been reported. 3 Similarly, although the school environment has been associated with low transmission rates, 4 recently published data highlighted an easy spread of the disease among children in a summer camp. 5 This is of particular concern, as the return to school is one of the most critical steps to be addressed in phase two of the pandemic.
To better characterize the possibility of children to be infected with the novel coronavirus, we performed this study aiming to assess the prevalence of anti‐SARS‐CoV‐2 immunoglobulin G (IgG) antibodies in children with known household exposure to SARS‐CoV‐2, and to compare these data with adult partners exposed to the same index case. The full study is still ongoing, aiming to evaluate the presence of neutralizing antibodies in adults and children exposed to SARS‐CoV‐2 and to prospectively follow‐up these families during the next fall/winter, to understand if a specific antibody pattern protects from further SARS‐CoV‐2 infection.
On April 21, 2020, while still in the middle of the national peak of COVID‐19 pandemic, the Fondazione Policlinico Universitario Agostino Gemelli IRCCS in Rome, Italy, established a postacute outpatient service for individuals discharged from the hospital after recovery from COVID‐19. All patients who met World Health Organization criteria for discontinuation of quarantine (absence of fever for 3 consecutive days, improvement of other symptoms, and 2 negative test results for SARS‐CoV‐2 24 h apart) were followed up. At enrollment in the study, real‐time reverse transcriptase‐polymerase chain reaction for SARS‐CoV‐2 was performed and patients with a negative test result were included. 6 Patients were offered a comprehensive medical assessment with detailed history and physical examination. Data on all epidemiological (including household composition) clinical characteristics, including clinical and pharmacological history, lifestyle factors, vaccination status, and body measurements, were collected in a structured electronic data collection system. The COVID‐19 postacute outpatient service is currently active, and further details about the patient evaluation protocol are described. 7 Preliminary results from this study protocol highlighted the important findings in adults experiencing chronic symptoms after an acute COVID‐19 infection. 7
For the purpose of understanding the real burden of SARS‐CoV‐2 infection rates in children, we asked previously hospitalized adults with COVID‐19, enrolled in the postacute outpatient service and living with children younger than 18 years of age, to be enrolled in a substudy aimed to evaluate the IgG antibody seropositivity of children with known exposure to adults with COVID‐19 (index case). We defined an index case as the first identified laboratory‐confirmed case in the household. Household contacts of COVID‐19 patients underwent serology test. We defined as household contact a person who lived in the household of the COVID‐19 patient (index case) at the time of diagnosis, for example, the other partner and/or children living in the same house. Nonhousehold contacts were not included in the study. Similarly, index patients not living with children younger than 18 years of age were excluded.
We grouped pediatric contacts by age 0–5 and 5–18, since most of the currently published studies did not specifically assess the infection rates of the youngest age groups.
The CE certified version of the Vircell COVID‐19 ELISA IgG antibody kit (Vircell Spain S.L.U., Granada, Spain) was used to detect IgG antibodies against SARS‐CoV‐2 according to manufacturer's recommendation (https://en.vircell.com/products/covid-19-elisa/). The kit has a sensitivity of 85% and specificity of 98% for IgG antibody detection.
We conducted statistical analyses using Stata v.16.
The study was approved by the Ethic Committee of our Institution. Written informed consent was obtained from the study participants.
Of 405 adults with COVID‐19 evaluated in the outpatient post‐COVID unit, 33 were living in a household with children under 18 years of age. Of those 33 eligible, 30 (90.9%) agreed to participate and were considered index cases. At the time of COVID‐19 diagnosis of the index case, a total of 80 household contacts were living in the same household and were enrolled in the study, of which 53 were children (median age 10, range: 0–18) and 27 adult partners (median age 45, range: 26–56) (Figure 1). Samples were collected on a mean of 77.1 days (SD 25.4, range: 30–130 days) after the index case's first diagnosis of COVID‐19.
Figure 1.
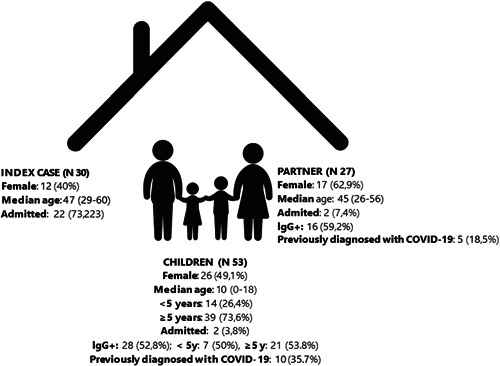
Main epidemiological and microbiological characteristics of the study cohort
Anti‐SARS‐CoV‐2 IgG antibodies were present in 44 out of 80 household contacts (55%), of which 16/27 (59.3%) were adult partners and 28/53 (52.8%) were pediatric contacts (p > .05). Of these, 15/16 (93.7%) IgG‐positive adult contacts and 20/28 (71.4%) IgG‐positive pediatric contacts developed COVID‐19‐related symptoms. Similar relative frequencies of seropositivity were present in children ≥5 years of age (21/39, 53.8%) and in those less than 5 years (7/14, 50%) (p > .05). Adult partners and children also had a similar relative frequency of SARS‐CoV‐2 IgG antibodies. Among contacts, 10/28 children (35.7%) and 5/27 adults (18.5%) with SARS‐CoV‐2 IgG antibodies eventually received a diagnosis, confirmed microbiologically via PCR on nasopharyngeal swab. These diagnoses among household contacts happened after the initial diagnosis of the index case, and they were discovered as part of the epidemiologic assessment activated by the Local Health Authorities once the index case had been reported by the Hospital.
However, it is fundamental to highlight that during the first period of the pandemic not all patients requiring a nasopharyngeal swab were able to access appropriate services, since Italy has been the first severely involved European country, and epidemiologic services were not fully established yet. In fact, only a part of symptomatic household contacts has been tested with nasopharyngeal swabs. In particular, of 53 pediatric contacts, only 31 (58.5%) underwent PCR testing whereas, among 27 adult contacts, only 19 (70.7%) underwent PCR testing, in the context of a contact tracing program.
Our report shows the very similar prevalence of seropositivity among different age groups of household members exposed to an index case. Overall, this report contributes significantly to the currently available data, suggesting that children are susceptible to infection, likely similar to adults. SARS‐CoV‐2 infection rate was higher in our cohort compared with a large contact tracing study performed in South Korea. 8 They reported 3 positive contacts out of 57 (5.3%, 95% confidence interval [CI]: 1.3–13.7) in the age group 0–9 years old and 43 positive contacts out of 231 (18.6%, 95% CI: 14.0–24.0) in the age group 10–19 years old. This difference could be related to the study design involving serology. Importantly, more than 60% of the household contacts we evaluated, including children, had not been diagnosed with SARS‐CoV‐2 infection before this study probably because most of them did not develop symptoms and were not tested with nasopharyngeal swabs. This strongly suggests that the real burden of the SARS‐CoV‐2 pandemic, in particular for pediatric cases, is highly underestimated. 9 , 10
A national seroprevalence study performed by the Italian Ministry of Health assessed the IgG status of 64,660 volunteers living in Italy 10 between May 25th and July 15th, 2020. Overall, the study showed that 2.5% of all tested people developed blood IgG antibodies against SARS‐CoV‐2, leading the Ministry of Health to hypothesize that 1,482,000 Italians acquired the virus during the first wave. The prevalence within the region where our hospital—a regional referral COVID‐19 hospital—is located (Lazio), is 1% of the assessed volunteers. During the first wave of the pandemic, in fact, Central (where also our region is located) and Southern Italy have been relatively spared compared with Northern Italy. This study, however, did not assess pediatric patients, therefore we do not have national data regarding the pediatric population to use as a comparison with our study.
Our study has some limitations to be addressed. First, there is no control group with no index case. Also, we do not have proof that the household contacts have been infected by the index case instead of a further contact, or a common source infected both the index case and the “household contact.” A false positive IgG antibody result is also a possibility, although unlikely. However, the Italian Government established one of the strongest lockdowns in the world, lasting for several months, and only a gradual controlled return to regular activities, with summer camps for children closed until mid‐July, schools opening in mid‐September and remote working still highly supported at the time of the writing of this paper. For these reasons, the probabilities that the household contacts have been infected by the index cases are high.
Our findings, by highlighting that children of all age groups have high probability of being infected with SARS‐CoV‐2 if closely exposed to an index case, have Public Health implications. Considering that recent studies suggested that children can have similar (or even higher) viral loads compared to adults on nasopharyngeal swabs, even if asymptomatic, 11 our study further supports the need for appropriate procedural guidelines of childhood activities, including schools, during the era of COVID‐19. Although access to school is a priority right of children, with several benefits on all aspects of child development, the growing evidence that children can easily be infected with SARS‐CoV‐2 and contribute to viral spread should be used to implement public health recommendations, including hand and respiratory hygiene, physical distancing, masking and active surveillance to reduce and/or prevent SARS‐CoV‐2 transmission within childhood environments.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors are grateful to dr Vittoria Ferrari for the final revision of the language.
Members of Gemelli Against COVID‐19 Post‐Acute Care team: Steering committee: Landi F, Gremese E. Coordination: Bernabei R, Fantoni M, Gasbarrini A. Field investigators: Gastroenterology team: Settanni CR; Geriatric team: Benvenuto F, Bramato G, Carfì A, Ciciarello F, Lo Monaco MR, Martone AM, Marzetti E, Napolitano C, Pagano F, Rocchi S, Rota E, Salerno A, Tosato M, Tritto M, Calvani R, Catalano L, Picca A, Savera G; Infectious disease team: Cauda R, Tamburrini E, Borghetti A, Di Gianbenedetto S, Murri R, Cingolani A, Ventura G, Taddei E, Moschese D, Ciccullo A; Internal Medicine team: Stella L, Addolorato G, Franceschi F, Mingrone G, Zocco MA; Microbiology team: Sanguinetti M, Cattani P, Marchetti S, Posteraro B, Sali M; Neurology team: Bizzarro A, Lauria A; Ophthalmology team: Rizzo S, Savastano MC, Gambini G, Cozzupoli GM, Culiersi C; Otolaryngology team: Passali GC, Paludetti G, Galli J, Crudo F, Di Cintio G, Longobardi Y, Tricarico L, Santantonio M; Pediatric team: Buonsenso D, Valentini P, Pata D, Sinatti D, De Rose C; Pneumology team: Richeldi L, Lombardi F, Calabrese A; Psychiatric team: Sani G, Janiri D, Giuseppin G, Molinaro M, Modica M; Radiology team: Natale L, Larici AR, Marano R; Rheumatology team: Paglionico A, Petricca L, Gigante L, Natalello G, Fedele AL, Lizzio MM, Tolusso B, Alivernini S; Vascular team: Santoliquido A, Santoro L, Nesci A, Popolla V.
Buonsenso D, Valentini P, De Rose C, et al. Seroprevalence of anti‐SARS‐CoV‐2 IgG antibodies in children with household exposure to adults with COVID‐19: preliminary findings. Pediatric Pulmonology. 2021;56:1374–1377. 10.1002/ppul.25280
Contributor Information
Danilo Buonsenso, Email: danilobuonsenso@gmail.com.
Piero Valentini, Email: piero.valentini@policlinicogemelli.it.
DATA AVAILABILITY STATEMENT
All the data and material are available upon request to the corresponding author.
REFERENCES
- 1. Parri N, Lenge M, Buonsenso D. Coronavirus infection in pediatric emergency departments (CONFIDENCE) research group. Children with covid‐19 in Pediatric emergency departments in Italy. N Engl J Med. 2020;383(2):187‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Götzinger F, Santiago‐García B, Noguera‐Julián A, et al. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;S2352‐4642(20):30177‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020:e2010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macartney K, Quinn HE, Pillsbury AJ, et al. and the NSW COVID‐19 Schools study teamtransmission of SARS‐CoV‐2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health. 2020;S2352‐4642(20):30251‐0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Szablewski CM, Chang KT, Brown MM, et al. SARS‐CoV‐2 transmission and infection among attendees of an overnight camp—Georgia, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1023‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gemelli Against COVID‐19 Post‐Acute Care Study Group . Post‐COVID‐19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carfì A, Bernabei R, Landi F. Gemelli against COVID‐19 post‐acute care study group. persistent symptoms in patients after acute COVID‐19. JAMA. 2020:e2012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:26‐2468. 10.3201/eid2610.201315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buonsenso D, Zampino G, Valentini P. Novel coronavirus disease 2019 infection in children: The dark side of a worldwide outbreak. Front Pediatr. 2020;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Indagine di siero‐prevalenza sul SARS‐CoV‐2 condotta dal Ministero della salute e dall'ISTAT. http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=4998;L. Accessed on August 8th 2020.
- 11. Heald‐Sargent T, Muller WJ, Zheng X, Rippe J, Patel AB, Kociolek LK. Age‐related differences in nasopharyngeal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) levels in patients with mild to moderate coronavirus disease 2019 (COVID‐19). JAMA Pediatr. 2020;174:902. 10.1001/jamapediatrics.2020.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data and material are available upon request to the corresponding author.


