Abstract
Aims
Coronavirus disease 2019 (COVID‐19) is a still growing pandemic, causing many deaths and socio‐economic damage. Elevated expression of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) entry receptor angiotensin‐converting enzyme 2 on cardiac cells of patients with heart diseases may be related to cardiovascular burden. We have thus analysed cardiovascular and inflammatory microRNAs (miRs), sensitive markers of cardiovascular damage, in critically ill, ventilated patients with COVID‐19 or influenza‐associated acute respiratory distress syndrome (Influenza‐ARDS) admitted to the intensive care unit and healthy controls.
Methods and results
Circulating miRs (miR‐21, miR‐126, miR‐155, miR‐208a, and miR‐499) were analysed in a discovery cohort consisting of patients with mechanically‐ventilated COVID‐19 (n = 18) and healthy controls (n = 15). A validation study was performed in an independent cohort of mechanically‐ventilated COVID‐19 patients (n = 20), Influenza‐ARDS patients (n = 13) and healthy controls (n = 32). In both cohorts, RNA was isolated from serum and cardiovascular disease/inflammatory‐relevant miR concentrations were measured by miR‐specific TaqMan PCR analyses. In both the discovery and the validation cohort, serum concentration of miR‐21, miR‐155, miR‐208a and miR‐499 were significantly increased in COVID‐19 patients compared to healthy controls. Calculating the area under the curve using receiver operating characteristic analysis miR‐155, miR‐208a and miR‐499 showed a clear distinction between COVID‐19 and Influenza‐ARDS patients.
Conclusion
In this exploratory study, inflammation and cardiac myocyte‐specific miRs were upregulated in critically ill COVID‐19 patients. Importantly, miR profiles were able to differentiate between severely ill, mechanically‐ventilated Influenza‐ARDS and COVID‐19 patients, indicating a rather specific response and cardiac involvement of COVID‐19.
Keywords: COVID‐19, SARS‐CoV‐2, Acute respiratory distress syndrome, Influenza, microRNA, Biomarker
Circulating levels of inflammation and cardiac myocyte‐specific miRs are dysregulated in the blood of critically ill COVID‐19 patients compared to healthy individuals and also severely ill Influenza‐ARDS patients.
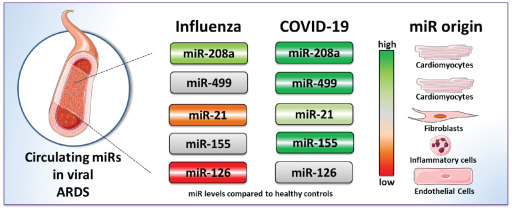
Introduction
Coronavirus disease 2019 (COVID‐19) is still a rapidly growing pandemic disease, which has claimed already more than 1.5 million lives worldwide. Despite massive efforts by the scientific community and enormous accumulation of new insights, the complexity of COVID‐19 caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is still far from being completely understood. Infection with this β‐genus SARS‐CoV‐2 can lead to the potentially lethal COVID‐19, primarily characterized by pneumonia, which can progress into acute respiratory distress syndrome (ARDS). A relatively high incidence of cardiac complications along with respiratory manifestations in COVID‐19 patients has raised concerns, and might be significantly contributing to overall mortality. 1 , 2 , 3 Moreover, multiple organ dysfunctions in infected patients might be triggered and aggravated by a maladaptive immune response that causes systemic inflammatory response associated with endothelial dysfunction. 4 , 5 , 6 , 7
As per the initial understanding, patients with pre‐existing cardiovascular diseases are at higher risk for severe courses of COVID‐19, presumably related to higher susceptibility to endothelial injury due to their pre‐existing vascular burden or higher expression of its entry receptor angiotensin‐converting enzyme (ACE2) in cardiovascular cells including myocytes, fibrocytes and endothelial cells. 8 , 9 The spectrum of cardiovascular manifestations ranges from elevated markers of cardiovascular damage such as troponins to severe arrhythmias and even myocardial infarctions. 3 , 10 Therefore, analysing markers pertaining to cardiovascular properties in COVID‐19 patients might be helpful in predicting unfavourable outcome and complications.
MicroRNAs (miRs) are short non‐coding ribonucleic acids which play essential regulatory roles in various cellular functions and disease processes. Moreover, their ease of detection in our circulatory system has established them as potential sensitive disease biomarkers especially in the cardiovascular context. 11 , 12 , 13 , 14 , 15 Therefore, we sought to identify changes in selected miRs; cardiovascular disease and inflammation‐associated (miR‐155), myocyte‐associated miRs (myomiRs miR‐499 and miR‐208a), fibromiRs (miR‐21) and endothelial miRs (miR‐126) in the blood from mechanically‐ventilated intensive care unit (ICU) patients with COVID‐19‐associated pneumonia in comparison to influenza‐associated ARDS (Influenza‐ARDS) patients and healthy controls.
Methods
Clinical data
Clinical data (demographics, cardiovascular risk factors, cardiac pre‐medication, laboratory, organ function scores at time of sampling, clinical outcomes) and serum were obtained from ICU patients with severe COVID‐19 pneumonia requiring invasive ventilation (one patient received high‐flow nasal oxygen therapy at time of sampling but intubated shortly after) and severe, mechanically‐ventilated Influenza‐ARDS patients. The investigation conforms to the principles outlined in the 1964 Declaration of Helsinki and later amendments. 16 All patients or their health representatives provided written consent. Healthy volunteers were chosen as controls. The study was approved by the local institutional review board (#8164_BO_K_2018 and #8980_BO_K_2020).
RNA isolation
RNA was isolated from 100 µL of serum using the miRNeasy Serum/Plasma Advanced Kit (Qiagen), as described by the manufacturer. Synthetic cel‐miR‐39‐3p (1.6x108 copies/µL) (Qiagen) was spiked‐in before RNA isolation for normalization in subsequent quantitative real‐time polymerase chain reaction (qRT‐PCR). RNA was eluted from the columns with 30 µL of water. 12 , 17 The RNA was treated with heparinase to remove traces of heparin known to interfere with PCR reactions.
Polymerase chain reaction‐based analysis of microRNAs
The isolated RNA was reverse transcribed to complementary DNA (cDNA) utilising TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems) following manufactures guidelines. RT‐qPCR was performed with specific TaqMan miR assays (Applied Biosystems) for Cel‐miR‐39, hsa‐miR‐21‐5p, hsa‐miR‐126‐3p, hsa‐miR‐155‐5p, hsa‐miR‐208a‐3p and hsa‐miR‐499‐5p on a Viia7 system (Thermo Fisher Scientific). miRs with Cq≥36 were deemed to be undetected and set to Cq = 36. Relative miR concentrations were calculated using the 2‐dCq method (dCq = Cq[miR] − Cq[Cel‐miR‐39]). Relative miR levels were log2 transformed. 12
Statistical analysis
Statistical analysis of miR concentrations between the two groups was performed via Mann–Whitney test in the discovery cohort. In the validation cohort, Dunn's multiple comparisons test was used to compare healthy controls, Influenza‐ARDS and COVID‐19 patients. For group discrimination by levels of selected miRs, receiver operating characteristic (ROC) curves were used and the area under the curve (AUC) was calculated. Clinical characteristics depending on miR levels were compared by Chi2 or Wilcoxon U test, as appropriate after subgrouping by median concentration of individual miRs. All analyses and graph production were performed using STATA v16.1 (USA) and GraphPad Prism 9 (USA).
Results
In this exploratory study we initially compared serum miR concentrations in critically ill COVID‐19 patients with that of healthy individuals as a discovery cohort. The control group consisted of 15 subjects (median age 31 years, 93% male) while the COVID‐19 group comprised 18 subjects (median age 59 years, 94% male). Demographic data of the discovery cohort are described in online supplementary Table S1 . In the discovery cohort miR‐21, miR‐155, miR‐208a and miR‐499 were significantly up‐regulated in COVID‐19 patients compared to healthy controls whereas miR‐126 levels were significantly decreased (Figure 1 ).
Figure 1.
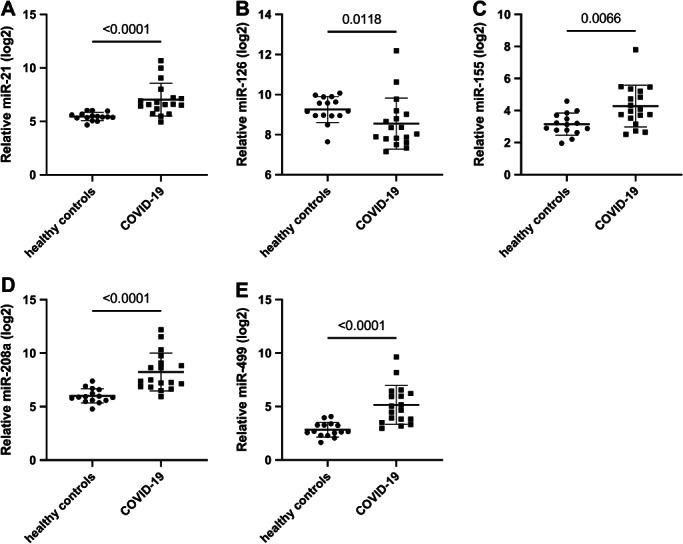
Levels of the indicated microRNAs (miRs) in healthy controls and COVID‐19 patients. Data are presented as dot plots and mean ± standard deviation. Mann–Whitney test was used for statistical comparison.
For validation, we recruited an independent cohort of mechanically‐ventilated COVID‐19 patients (n = 20, median age 59.5 years, 70% male), as well as invasively‐ventilated Influenza‐ARDS patients (n = 13, median age 56 years, 85% male) with similar respiratory compromise (Table 1 ) and reasonably age‐matched healthy controls (n = 32; median age 50 years; 62.5% male). When comparing cardiovascular risk factors between the COVID‐19 and Influenza‐ARDS groups, with the exception of smoking history no significant differences in the number of patients with diabetes, arterial hypertension, dyslipidaemia and obesity were observed (Table 1 ). No significant differences in cardiac pre‐medication (use of angiotensin‐converting enzyme inhibitors, AT1 inhibitors, beta‐blockers, diuretics, calcium channel blockers and aldosterone antagonists) were found (Table 1 ). Procalcitonin, lactate dehydrogenase, troponin T, creatine kinase and Sequential Organ Failure Assessment score were significantly higher in the Influenza‐ARDS group compared to COVID‐19 patients. The median noradrenaline dose was higher in the Influenza‐ARDS group (Table 1 ).
Table 1.
Demographics of the validation cohort
| Characteristics | COVID‐19 (n = 20) | Influenza‐ARDS (n = 13) | P‐value |
|---|---|---|---|
| Age (years), median (IQR) | 59.5 (46–68) | 56 (49–58) | 0.554 |
| Male sex, n (%) | 14 (70) | 11 (85) | 0.338 |
| Body mass index (kg/m2), median (IQR) | 29.4 (25.7–33.4) | 25 (24.5–29.2) | 0.107 |
| Cardiovascular risk factors, n (%) | |||
| Diabetes | 6 (30) | 0 (0) | 0.060 |
| Arterial hypertension | 11 (55) | 5 (38) | 0.481 |
| Dyslipidaemia | 3 (15) | 4 (31) | 0.393 |
| Obesity | 5 (25) | 4 (31) | >0.999 |
| Ever smoker | 0 (0) | 6 (46) | 0.002 |
| Cardiac pre‐medication, n (%) | |||
| ACE inhibitor | 3 (15) | 1 (8) | 0.638 |
| AT1 inhibitor | 2 (10) | 3 (23) | 0.360 |
| Beta‐blocker | 5 (25) | 3 (23) | >0.999 |
| Diuretic | 4 (20) | 1 (8) | 0.625 |
| Calcium channel blocker | 2 (10) | 2 (15) | >0.999 |
| Aldosterone antagonist | 0 (0) | 1 (8) | 0.394 |
| Basal echocardiographic data, n (%) | |||
| No. of existing data | 7 (35) | 8 (62) | |
| Preserved LVEF | 7 (100) | 8 (100) | >0.999 |
| LV hypertrophy | 3 (43) | 3 (38) | >0.999 |
| Laboratory at time of sampling | |||
| CRP (mg/L), median (IQR) | 123 (92–197) | 197 (141–296) | 0.069 |
| Procalcitonin (ng/L), median (IQR) | 0.45 (0.2–1.0) | 3.55 (1.55–9.55) | <0.001 |
| IL‐6 (ng/L), median (IQR) | 98 (32–304) | – | |
| Ferritin (µg/L), median (IQR) | 1513 (1065–2267) | 1613 (620–5017) | 0.910 |
| Leucocytes (gpt/L), median (IQR) | 8.8 (7.1–16.4) | 8.6 (6.5–12.6) | 0.407 |
| Lactate (mmol/L), median (IQR) | 1.7 (1.3–2.0) | 1.2 (1.0–1.8) | 0.154 |
| D‐dimer (mg/L), median (IQR) | 1.24 (0.8–3.7) | 1.8 (1.1–4.4) | 0.325 |
| LDH (U/L), median (IQR) | 433 (358–572) | 550 (515–908) | 0.004 |
| NT‐proBNP (ng/L) | 482 (278–803) | 1211 (380–2611) | 0.068 |
| Troponin T (ng/L), median (IQR) | 12 (6–25) | 42 (14–110) | 0.011 |
| Creatine kinase (U/L), median (IQR) | 301 (97–589) | 918 (721–1846) | 0.003 |
| paO2/FiO2 (mmHg), median (IQR) | 97 (64–153) | 83 (60–100) | 0.137 |
| SOFA score at day of sampling (IQR) | 8 (6–9) | 11 (10–12) | 0.001 |
| Treatment modalities at time of sampling | |||
| Invasive ventilation, n (%) | 18 (90) | 13 (100) | 0.239 |
| PEEP (mbar), median (IQR) | 14 (10–16) | 15 (14–16) | 0.483 |
| Pmax (mbar), median (IQR) | 28 (25–30) | 26 (25–28) | 0.640 |
| ECMO, n (%) | 3 (15) | 5 (38) | 0.124 |
| Vasopressor use, n (%) | 14 (70) | 12 (92) | 0.423 |
| Noradrenaline dose (µg/kg/min), median (IQR) | 0.175 (0.057–0.267) | 0.306 (0.212–0.563) | 0.030 |
| Treatment modalities and outcomes during ICU stay | |||
| Renal replacement therapy, n (%) | 6 (30) | 8 (62) | 0.073 |
| ECMO, n (%) | 8 (40) | 7 (54) | 0.435 |
| ECMO, days, median (IQR) | 9 (7–15) | 12 (3–20) | 0.799 |
| ICU, days, median (IQR) | 18 (12–26) | 18 (15–21) | 0.920 |
| Vasopressor, days, median (IQR) | 8 (3–12) | 8 (4–13) | 0.792 |
| Ventilation, days, median (IQR) | 13 (8–20) | 15 (9–17) | 0.736 |
| 28‐day ICU mortality, n (%) | 2 (10) | 5 (38) | 0.051 |
Healthy control subjects were part of the validation cohort (n = 32, median age 50 years, 62.5% male).
ACE, angiotensin‐converting enzyme; AT1, angiotensin II receptor type 1; CRP, C‐reactive protein; ECMO, extracorporeal membrane oxygenation; FiO2, fraction of inspired oxygen; ICU, intensive care unit; IL‐6, interleukin‐6; IQR, interquartile range; LDH, lactate dehydrogenase; LV, left ventricular; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; paO2, arterial partial pressure of oxygen; PEEP, positive end‐expiratory pressure; Pmax, pressure maximum; SOFA, Sequential Organ Failure Assessment.
In line with the validation cohort, miR‐21 was increased in the COVID‐19 group compared to healthy controls and Influenza‐ARDS patients (Figures 1 and 2 A ). Changes in miR‐126 concentration could not be confirmed in the validation cohort (Figures 1 and 2 B ). Up‐regulation of miR‐155 and miR‐499 in COVID‐19 patients was confirmed in the second cohort both compared to healthy controls and Influenza‐ARDS patients (Figures 1 and 2 C,E ), whereas miR‐208a was upregulated in both severe COVID‐19 and Influenza‐ARDS groups compared to healthy controls (Figures 1 and 2 D ).
Figure 2.
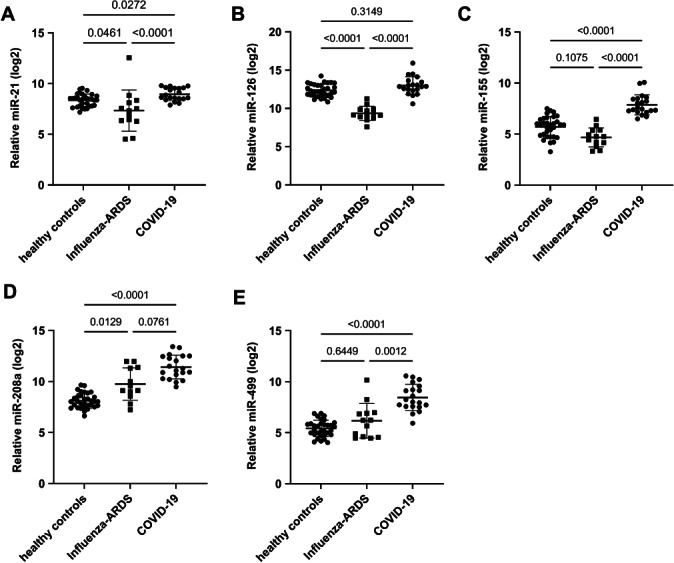
Levels of the indicated microRNAs (miRs) in healthy controls, influenza‐associated acute respiratory distress syndrome (Influenza‐ARDS) and COVID‐19 patients. Data are presented as dot plots and mean ± standard deviation. Dunn's multiple comparisons test was used for statistical comparison.
ROC analyses of inflammation and cardiac‐associated miRs miR‐155, miR‐208a and miR‐499 showed a strong discrimination between COVID‐19 and Influenza‐ARDS by these markers of myocardial damage [AUC: 1.000 (miR155); 0.796 (miR‐208a); 0.865 (miR‐499)] (Figure 3 ).
Figure 3.
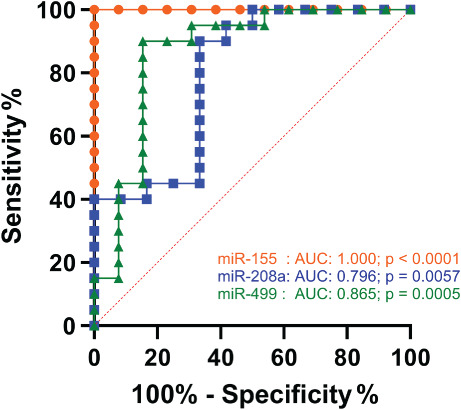
Receiver operating characteristic curve analysis of multiple microRNAs (miR‐155, miR‐208a, miR‐499) was used to distinguish between the COVID‐19 and the influenza‐associated acute respiratory distress syndrome (Influenza‐ARDS) group. AUC, area under the curve.
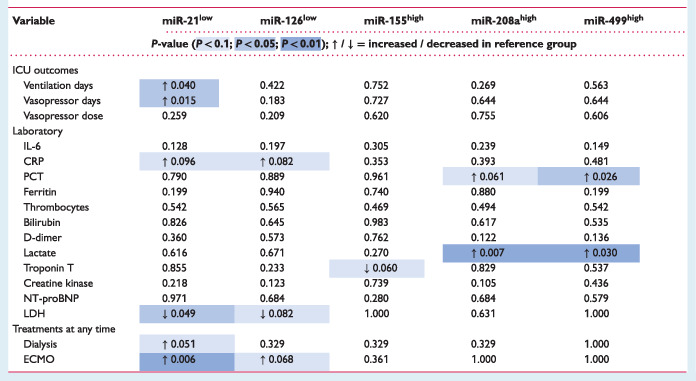
Stratification into low and high miR concentrations divided by the median concentration revealed an association of lower miR‐21 levels with more ventilation and vasopressor days as well as a higher rate of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy (Table 2 ). Higher miR‐208a and miR‐499 levels were associated with elevated procalcitonin and lactate (Table 2 ).
Table 2.
Differences in clinical and laboratory data at time of sampling between COVID‐19 patients of the validation cohort with high/low* levels of the investigated microRNAs
|
CRP, C‐reactive protein; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IL‐6, interleukin‐6; LDH, lactate dehydrogenase; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; PCT, procalcitonin.
Discussion
In this exploratory study, we aimed to investigate altered levels of circulating cardiovascular and inflammatory miRs in severe COVID‐19 patients requiring invasive ventilation to identify biomarkers for myocardial damage and disease severity. The rationale for the design of this study was the well‐described association of miR‐155 with inflammation, miR‐208a and miR‐499 with myocardial/cardiomyocyte damage, and miR‐21 and miR‐126 with cardiac fibroblast and endothelial cell dysfunction, respectively. 18 To our knowledge, this is the first study reporting the interlink of miR levels in blood of COVID‐19 patients and clinical parameters. Our study identified significantly altered levels of cardiac‐associated miRs in COVID‐19 patients indicating a strong association of COVID‐19 with cardiovascular ailments and respective biomarkers.
In order to strengthen the quality and significance of the exploratory study, we performed our analysis across two waves of the COVID‐19 pandemic to include a broader pool of patient samples. We initially analysed a discovery cohort during the first wave of COVID‐19 and then, after recruiting new patients with the emergence of a second wave of COVID‐19, a validation study was performed. Except for miR‐126, all miRs were consistently up‐regulated in the COVID‐19 group compared to healthy controls in both the discovery and the validation cohort. In line with the clinical picture of the disease, inflammatory miRs, such as miR‐155 19 and heart‐muscle specific miRs, also called myomiRs, such as miR‐208a 20 and miR‐499, 20 , 21 were significantly up‐regulated in COVID‐19 patients. Interestingly, the altered levels of miR‐155 and miR‐499 could further distinguish between COVID‐19 and Influenza‐ARDS groups, even though both the diseases are phenotypically very similar. With the levels of inflammation associated miR‐155 being highest at the site, the markedly elevated levels in COVID‐19 might be reflective of the since widely‐appreciated endothelialitis, which is COVID‐19‐specific. 22 In line with this, miR‐126, which is known to be protective from endothelial damage and deprived in chronic cardiovascular disease, 23 was decreased in our discovery cohort, albeit this finding was not reproducible in the validation cohort. Within the COVID‐19 group, the patients with higher miR‐208a and miR‐499 also had higher procalcitonin and lactate levels. However, this clinical correlation should not be overestimated as procalcitonin and lactate were relatively low on average. A correlation between deregulated miR levels and ECMO support has also been observed previously; however, whether ECMO support caused the miR deregulation or miR deregulation called for ECMO support is not very clear. 24 In this regard, it has been demonstrated that the use of ECMO is not associated with significant changes in circulating miRs within 24 h before and after ECMO implantation. 25
Interestingly, fibrosis‐associated miR‐21 11 was found to be increased in acute COVID‐19 compared to healthy controls and Influenza‐ARDS patients. The up‐regulation of miR‐21, miR‐155, miR‐208a and miR‐499 in COVID‐19 survivors might be a predictor of chronic myocardial damage and inflammation. Of note, despite troponin levels being higher in the Influenza‐ARDS group, myocardial specific miR‐208a and miR‐499 were more up‐regulated in COVID‐19. In this context, a recent cardiovascular magnetic resonance (CMR) imaging study in patients recently recovered from SARS‐CoV‐2 infection provided alarming results. In 78 out of 100 patients CMR abnormalities were found and 60 patients presented with ongoing myocardial inflammation while high‐sensitive troponin levels were only elevated in 5% of the cohort, 26 indicating troponin is not a useful marker of myocardial remodelling processes in COVID‐19. Importantly, these findings were independent of pre‐existing conditions and severity and overall course of COVID‐19. 26 This highlights the need for easily accessible biomarkers, such as circulating miRs, which may provide predictive information on potential long‐term cardiovascular consequences. Overall, we believe that the significance of this study is strengthened by the fact that patients in both the COVID‐19 and Influenza‐ARDS groups had a similar cardiac pre‐existing disease spectrum prior to hospitalization, but the miR concentration is significantly different in severe ARDS. This confirms that unlike influenza, which mainly triggers a pulmonary response, COVID‐19 infection triggers multiorgan involvement with inflammation, endothelial cell damage and cardiac involvement.
Although this study encompasses a rather small patient population, our exploratory approach divided into two independent cohorts provides important insights into the pathophysiological aspects of miRs in COVID‐19. These should serve as a foundation for larger studies and also serve to analyse the long‐term course of COVID‐19 patients.
Supporting information
Table S1. Demographics of the discovery cohort.
Acknowledgements
We thank all patients and healthy volunteers, who participated in this study. Open access funding enabled and organized by Projekt DEAL.
Funding
This work was supported by PRACTIS – Clinician Scientist Program of Hannover Medical School, funded by the German Research Foundation (DFG, ME 3696/3–1) to A.D. and B.S. and by a grant from the DFG to S.D. (DA 1209/4–3). This work was further supported by COVID‐19 research grant funded by Deutsche Herzstiftung e.V., Frankfurt a.M. to A.D. and T.T. and by the German Research Foundation (DFG; KFO 311) to J.B., M.H., T.W. and T.T.
Conflict of interest: T.T. and J.B. filed and licensed patents in the field of non‐coding RNAs (outside the submitted work). T.T. is founder and holds shares of Cardior Pharmaceuticals GmbH (outside the submitted work). J.B. reports personal fees from Abbott, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo, Medtronic, MSD, Novartis, Pfizer, Servier, grants and personal fees from Abiomed, CvRX, Vifor, grants from Zoll, outside the submitted work. C.B. filed and licensed patents in the fields of telomerase and non‐coding RNA therapy (outside the submitted work). M.M.H. reports personal fees from Actelion, Bayer, Acceleron, GSK, Janssen, MSD, Pfizer, outside the submitted work. I.P. reports non‐financial support from TEVA, Chiesi, outside the submitted work. All other authors have nothing to disclose.
References
- 1. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pericàs JM, Hernandez‐Meneses M, Sheahan TP, Quintana E, Ambrosioni J, Sandoval E, Falces C, Marcos MA, Tuset M, Vilella A, Moreno A, Miro JM; Hospital Clínic Cardiovascular Infections Study Group. COVID‐19: from epidemiology to treatment. Eur Heart J 2020;41:2092–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID‐19. Am J Emerg Med 2020;38:1504–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev 2020;19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Groß S, Jahn C, Cushman S, Bär C, Thum T. SARS‐CoV‐2 receptor ACE2‐dependent implications on the cardiovascular system: from basic science to clinical implications. J Mol Cell Cardiol 2020;144:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stahl K, Gronski PA, Kiyan Y, Seeliger B, Bertram A, Pape T, Welte T, Hoeper MM, Haller H, David S. Injury to the endothelial glycocalyx in critically ill patients with COVID‐19. Am J Respir Crit Care Med 2020;202:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicin L, Abplanalp WT, Mellentin H, Kattih B, Tombor L, John D, Schmitto JD, Heineke J, Emrich F, Arsalan M, Holubec T, Walther T, Zeiher AM, Dimmeler S. Cell type‐specific expression of the putative SARS‐CoV‐2 receptor ACE2 in human hearts. Eur Heart J 2020;41:1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA‐21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008;456:980–984. [DOI] [PubMed] [Google Scholar]
- 12. Derda AA, Thum S, Lorenzen JM, Bavendiek U, Heineke J, Keyser B, Stuhrmann M, Givens RC, Kennel PJ, Schulze PC, Widder JD, Bauersachs J, Thum T. Blood‐based microRNA signatures differentiate various forms of cardiac hypertrophy. Int J Cardiol 2015;196:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Gonzalo‐Calvo D, Vea A, Bar C, Fiedler J, Couch LS, Brotons C, Llorente‐Cortes V, Thum T. Circulating non‐coding RNAs in biomarker‐guided cardiovascular therapy: a novel tool for personalized medicine? Eur Heart J 2019;40:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sieweke JT, Pfeffer TJ, Biber S, Chatterjee S, Weissenborn K, Grosse GM, Hagemus J, Derda AA, Berliner D, Lichtinghagen R, Hilfiker‐Kleiner D, Bauersachs J, Bar C, Thum T, Bavendiek U. miR‐21 and NT‐proBNP correlate with echocardiographic parameters of atrial dysfunction and predict atrial fibrillation. J Clin Med 2020;9:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu D, Chatterjee S, Xiao K, Riedel I, Wang Y, Foo R, Bar C, Thum T. MicroRNAs targeting the SARS‐CoV‐2 entry receptor ACE2 in cardiomyocytes. J Mol Cell Cardiol 2020;148:46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rickham PP. Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. Br Med J 1964;2:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaguszewski M, Osipova J, Ghadri JR, Napp LC, Widera C, Franke J, Fijalkowski M, Nowak R, Fijalkowska M, Volkmann I, Katus HA, Wollert KC, Bauersachs J, Erne P, Luscher TF, Thum T, Templin C. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J 2014;35:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartmann D, Fiedler J, Sonnenschein K, Just A, Pfanne A, Zimmer K, Remke J, Foinquinos A, Butzlaff M, Schimmel K, Maegdefessel L, Hilfiker‐Kleiner D, Lachmann N, Schober A, Froese N, Heineke J, Bauersachs J, Batkai S, Thum T. MicroRNA‐based therapy of GATA2‐deficient vascular disease. Circulation 2016;134:1973–1990. [DOI] [PubMed] [Google Scholar]
- 19. Mahesh G, Biswas R. MicroRNA‐155: a master regulator of inflammation. J Interferon Cytokine Res 2019;39:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress‐dependent cardiac growth and gene expression by a microRNA. Science 2007;316:575–579. [DOI] [PubMed] [Google Scholar]
- 21. van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 2009;17:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zietzer A, Steffen E, Niepmann S, Dusing P, Hosen MR, Liu W, Jamme P, Al‐Kassou B, Goody PR, Zimmer S, Reiners KS, Pfeifer A, Bohm M, Werner N, Nickenig G, Jansen F. MicroRNA‐mediated vascular intercellular communication is altered in chronic kidney disease. Cardiovasc Res 2020. Nov 2. 10.1093/cvr/cvaa322 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24. Herrera‐Rivero M, Zhang R, Heilmann‐Heimbach S, Mueller A, Bagci S, Dresbach T, Schroder L, Holdenrieder S, Reutter HM, Kipfmueller F. Circulating microRNAs are associated with pulmonary hypertension and development of chronic lung disease in congenital diaphragmatic hernia. Sci Rep 2018;8:10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scettri M, Seeba H, Staudacher DL, Robinson S, Stallmann D, Heger LA, Grundmann S, Duerschmied D, Bode C, Wengenmayer T, Ahrens I, Hortmann M. Influence of extracorporeal membrane oxygenation on serum microRNA expression. J Int Med Res 2019;47:6109–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa‐Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020;5:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics of the discovery cohort.


