Abstract
Background
As the pandemic continues to unfold, effective, technology‐based solutions are needed to help patients with atrial fibrillation (AF) maintain their health and well‐being during the outbreak of COVID‐19.
Methods
This single‐center, pilot study investigated the effects of a 4‐week (eight sessions) virtual AF self‐management program. Questionnaires were completed at baseline and 1 week after the intervention, and assessed AF knowledge, adherence to self‐management behaviors, mental health, physical function, and disease‐specific quality of life in patients with AF. Secondary outcomes included knowledge of COVID‐19, intervention, acceptability, and satisfaction.
Results
Of 68 patients who completed baseline questionnaires, 57 participated in the intervention and were included in the analysis (mean age of 73.4 ± 10.0 years, 60% male). Adherence to AF self‐monitoring behaviors, including monitoring their heart rate (p < .001), heart rhythm (p = .003), and blood pressure (p = .013) were significantly improved at the end of the intervention compared with baseline. Symptom identification (p = .007) and management (p < .001) also improved. Reductions in sleep disturbance (p < .001), anxiety (p = .014), and depression (p = .046) were also observed. Misinformation and inaccurate beliefs about COVID‐19 were significantly reduced at the end of the intervention compared with baseline.
Conclusions
This pilot study suggests that a virtual patient education program could have beneficial effects on adherence to guideline‐recommend self‐care of AF, emotional wellbeing, physical function, and knowledge of COVID‐19 in patients with AF. Future randomized studies in larger samples are needed to determine the clinical benefits of the intervention.
Keywords: atrial fibrillation, digital health, intervention; patient education; telehealth
1. INTRODUCTION
Atrial fibrillation (AF) is a leading cause of prolonged disability, repeat hospitalizations and premature death in the United States. 1 , 2 Although guidelines promote the crucial role of patient education and self‐management behaviors (e.g., symptom identification, medication adherence, lifestyle modification, and management of non‐cardiovascular comorbidities) in preventing complications of AF (e.g., thromboembolic events) 2 , 3 and improving health‐related quality of life, 4 , 5 many patients struggle to understand their condition and achieve optimal management of AF.
The outbreak of novel coronavirus disease 2019 (COVID‐19) and the extraordinary measures taken to reduce the spread of the virus introduced an entirely new set of challenges for patients with AF. Persons with underlying cardiovascular disease have a higher incidence of severe illness, complications and death from COVID‐19. 6 , 7 , 8 They are also more susceptible to the secondary health impacts of a national emergency (e.g., disruptions to routine care and health care services) which can exacerbate pre‐existing stressors such as poverty, unemployment, and food, and housing insecurity. 9 Anxiety, isolation, and depression, which are more common in patients with AF than in the general population, 10 may also worsen during a pandemic and have been associated with nonadherence, 11 AF recurrence, 12 and increased morbidity 13 and mortality in prior studies. 14 For these reasons, early detection and proactive management of these issues is essential to mitigate patients’ risk of clinical decompensation, adverse health events, and unplanned hospital admissions during the COVID‐19 pandemic.
Initiatives to improve education and self‐management for patients with AF are well established, 4 , 15 , 16 but these clinic‐based interventions have been less accessible during COVID‐19, especially during the acute phases of the pandemic and periods of mandatory quarantine. To address this critical gap in care, we conducted a single‐center, pilot study of a virtual AF patient education program designed to provide continuous education and support to persons with AF during COVID‐19. The AF‐At‐Home Program was conceptually based on prior self‐management interventions for patients with AF 3 , 4 , 15 , 16 and was adapted for rapid delivery during the pandemic. Additional components of the intervention focused on enhancing coping strategies, activating personal resources, and equipping patients with the knowledge, tools, and skills they need to maintain their physical and emotional health during the COVID‐19 pandemic. The purpose of this study was to examine the feasibility, acceptability, and preliminary efficacy of the AF‐At‐Home Program.
2. METHODS
2.1. Study design, population, and procedures
A pre‐post design was employed for this pilot study, which was conducted with patients treated at an outpatient electrophysiology clinic at an academic medical center in North Carolina during an acute phase of the pandemic (April 28, 2020 to June 2, 2020) when mandatory shelter‐in‐place orders were issued for all residents in the state of North Carolina. Patients were prospectively screened for eligibility using automated EHR algorithms. 17 , 18 Persons 18 years or older with a documented diagnosis of AF and who were enrolled in the EHR‐based patient portal (i.e., EPIC MyChart) were eligible for the study. Individuals were excluded if they could not give informed consent or lacked access to an internet‐enabled device (i.e., smartphone, tablet, or computer) which was required for participation.
Individuals meeting inclusion criteria were sent an invitation to participate in the intervention through the EHR‐based patient portal. Patients who provided consent to participate were emailed a packet of information about the program and instructions to help troubleshoot technical issues. Patients did not receive compensation for completing study questionnaires or for participating in the intervention. The protocol and procedures were approved by the institutional review board at the University of North Carolina; all participants provided electronic informed consent.
2.2. AF‐At‐Home program
The AF‐At‐Home Program was developed to improve AF management by focusing on self‐monitoring, skill development, and behavioral risk factor modification. The program included eight 1‐h group sessions, occurring 2 days per week over 4 consecutive weeks and was delivered with a secure video‐conferencing platform. Each session included 40 min of didactic instruction followed by 20 min of interactive group discussion and questions answered by the session leader. Sessions were led by a diverse group of health care professionals from cardiovascular electrophysiology, cardiac psychology, endocrinology, clinical pharmacy, and social work. Content was based on guideline‐recommended topics for AF patient education 2 and included information on AF symptom recognition and management, therapeutic strategies, stroke prevention, behavioral strategies to mitigate lifestyle risk factors (healthy diet, physical activity, minimizing alcohol and other substances), comorbidity management (e.g., blood pressure monitoring and control, heart failure therapy), developing an action plan for acute AF exacerbations, and skills for reducing stress associated with AF (details of each session are provided in the Supplementary Appendix). In addition to standard AF patient education, sessions focused on enhancing coping strategies, activating personal resources, and equipping patients with the knowledge, tools, and skills they need to maintain their physical and emotional health during the pandemic (e.g., accessing routine care via telehealth, the importance of seeking medical care for acute cardiovascular symptoms, healthy eating during food shortages, and the psychological, social, and financial impact of the pandemic). To reinforce learning, patients were provided digital educational materials and website links to video recordings of the didactic portion of the session.
2.3. Data collection and outcomes
Baseline demographic and clinical data were collected by medical chart abstraction for all study participants. Standard definitions 1 , 2 and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD‐10) codes were used to obtain data on demographic characteristics, AF history, prior procedures, current medical therapies, general medical history, and lifestyle factors. Personnel who collected medical record data were blinded to study outcomes.
Patients completed questionnaires at baseline (pre‐intervention) and again 5 weeks later (1‐week post‐intervention). The primary outcomes for this study were AF‐related health knowledge, adherence to guideline‐recommended self‐management behaviors, mental and physical health outcomes, and general and AF‐specific quality of life. Secondary outcomes included self‐reported knowledge of COVID‐19 and assessment of intervention acceptability and satisfaction with the program.
2.3.1. AF knowledge and adherence to self‐management behaviors
A series of questions evaluated patients' knowledge and understanding of AF, adherence to self‐management behaviors, and confidence in implementing self‐management skills (details provided in Table S1 of the Supplementary Appendix). Items were similar to those used in prior studies of AF self‐management behaviors. 19 Patients were asked to indicate the frequency of completing specific AF self‐management behaviors and to rate their overall level of confidence in performing these behaviors using a Likert scale, with higher scores indicating greater adherence or confidence in completing the prescribed skill.
2.3.2. Mental health and physical function
The National Institutes of Health Patient‐Reported Outcomes Measurement Information System (PROMIS)–29 profile, version 2.0, was used to assess global health status and quality of life. The PROMIS‐29 is a self‐administered, extensively validated, quality of life questionnaire with eight domains that assess the following symptoms during the previous 7 days: pain intensity and interference, fatigue, sleep disturbance, physical functioning, depression, anxiety, and ability to participate in social roles and activities. 20 There is also a single item scale for pain intensity. PROMIS raw scores are transformed into t scores, with a mean of 50 and standard deviation (SD) of 10 representing the U.S. general population. Scores range from 0 to 100 on all PROMIS measures, with higher scores representing more of a given domain (e.g., higher score denotes more function). 20 For example, a higher value may represent worsening pain, pain interference, fatigue, sleep disturbance, depression and anxiety, and an improvement in physical functioning, and ability to participate in social roles and activities.
2.3.3. AF quality of life
AF‐related quality of life was assessed with the Atrial Fibrillation Effects on Quality of Life questionnaire (AFEQT), a widely used 20‐item measure of patients’ AF symptoms, daily activities, treatment concerns, and satisfaction with treatment during the past month. 21 Respondents rated items on a 7‐point Likert‐based scale. AFEQT scores range from 0 to 100, with higher scores representing the best possible AF‐related quality of life (no impairment) and 0 representing the worst. A 5‐point change in the AFEQT is indicative of clinically meaningful change in an individual patient. 22
2.3.4. Knowledge, beliefs, and behaviors related to COVID‐19
Given the rapid spread of inaccurate or misleading medical information about COVID‐19 during the pandemic, 23 separate questions assessed patients’ knowledge of COVID‐19 and beliefs about its potential effect on their health and health care utilization. Specifically, patients were asked to indicate if the following statements were true, false, or if they were unsure: (1) taking nonsteroidal anti‐inflammatory drugs (NSAIDs), such as ibuprofen or naproxen, increases my risk of becoming sick or having worse symptoms of COVID‐19; (2) taking medications, such as hydroxychloroquine, can prevent or treat symptoms of COVID‐19; (3) certain medications people take to manage their heart condition (angiotensin converting enzyme inhibitors [ACE‐I] or angiotensin receptor blocker [ARB]) increase the risk of COVID‐19 infection and may make symptoms of COVID‐19 worse; and (4) if I become infected with COVID‐19, I should stop taking these medications (ACE‐I, ARB) immediately. In addition, patients were asked to indicate if they agree or disagree with the following statements: (5) “I would delay seeking care for acute symptoms of a heart attack or stroke due to fear of COVID‐19" and (6)"If I had to go to the hospital for worsening cardiac symptoms, I worry that I would not get the medical care I need because of COVID‐19.” To further understand health information seeking behaviors among patients with AF during the pandemic, a separate question asked about their primary source of information on COVID‐19 (television news, newspaper, social media [e.g., Twitter, Facebook], radio, smartphone app, magazines, family/friends, doctors/medical team).
2.3.5. Acceptability and satisfaction with the program
Patients’ provided feedback about their experience and satisfaction with the program at follow‐up. Items were rated on a 5‐point scale (1—Strongly disagree; 2—Disagree; 3—Agree; 4—Strongly agree; 5—N/A, I did not take part in the educational program). Questions included: (1) This educational program helped me understand how to manage my AF during COVID‐19; (2) when I completed the program, I felt more confident about managing my AF during a public health emergency; (3) I am satisfied with this COVID‐19–AF educational program.
2.4. Sample size calculation
We determined that recruitment of at least 49 patients would provide a power of more than 80% to detect an effect of the intervention on PROMIS domain scores from pre to post intervention, with a two‐sided alpha level of 0.05, and a planned drop‐out rate of 25% which is the median dropout rate for studies of internet‐based education and lifestyle interventions. 24 However, the decision was made to expand enrollment to accommodate a potentially higher dropout rate (≥50%) due to the pandemic.
2.5. Statistical analysis
Study outcomes were analyzed separately among those who participated in at least one session of the intervention (program participants, n = 57) and those who attended zero sessions of the intervention but completed the questionnaire at both study baseline and follow‐up (non‐participants, n = 11) unless otherwise indicated. Categorical data were summarized using frequencies and percentages and continuous data are reported as mean ± SD. The Kolmogorov‐Smirnov test was used to test for data normality, and non‐parametric methods were used where indicated. Baseline characteristics were calculated for the entire sample and compared among program participants and non‐completers using t‐tests and chi‐squared tests, as appropriate. A risk score for stroke (e.g., CHA2DS2‐VASc: congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke/transient ischemic attack (doubled)–vascular disease, age 65–74 years, and sex category [female]) was calculated and used for univariate comparisons.
The Wilcoxon signed rank test was used to compare the primary outcome measures at baseline and study follow‐up. Effect size estimates were calculated for AF‐related quality of life (AFEQT) and mental health and physical function outcomes (PROMIS) using the nonparametric equivalent of the Cohen's d estimate, the r statistic. The r statistic produces estimates of the strength of a relationship between variables (small effect = 0.10 to 0.30: medium effect = 0.31 to 0.49: large effect; ≥0.50). 25 Separate analyses were performed with Wilcoxon signed rank test to examine secondary outcomes among program participants. The effect of intervention compliance was assessed using the Mann‐Whitney U test. Program acceptability and satisfaction were also examined. Less than 1% of patients had missing data; these data were excluded from the analyses. A two‐sided p value of less than .05 was considered significant. Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA). Figure 1 was generated using R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria).
FIGURE 1.
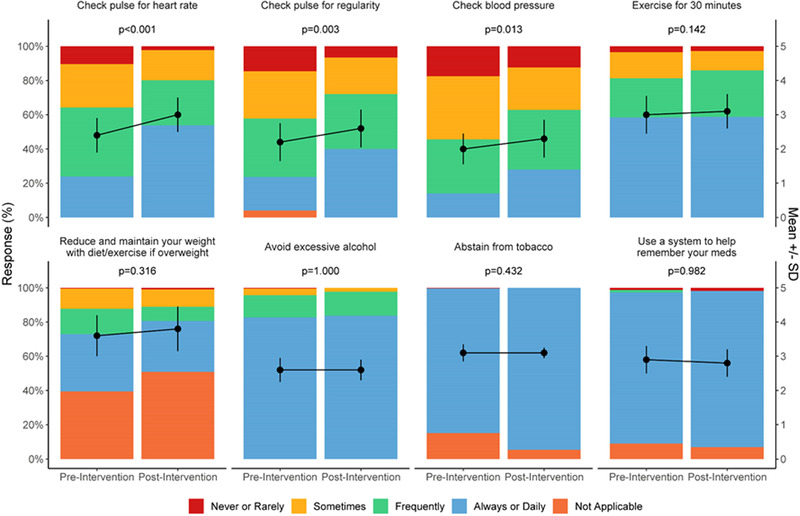
Effects of the atrial fibrillation (AF)‐at‐home program on self‐management skills in patients with AF [Color figure can be viewed at wileyonlinelibrary.com]
3. RESULTS
Sixty‐eight patients consented to participate in the study and completed both baseline and follow up questionnaires. The majority of patients who agreed to participate in the educational program attended at least one session of the intervention (84%) with a mean attendance of 5.4 out of eight sessions (SD = 2.8). Eleven patients completed both the baseline and follow‐up questionnaires but attended zero sessions of the intervention (referred to as non‐participants).
3.1. Baseline characteristics of the sample
Baseline characteristics of the study population are shown in Table 1. Most patients were older (mean age of 73.4 ± 10.0 years), white, males with paroxysmal AF, hypertension, hyperlipidemia, and had prescriptions for beta‐blockers and direct oral anticoagulant (DOAC) therapies. Persons who participated in the AF‐At‐Home Program were more likely to have persistent or permanent AF, have a history of coronary artery disease and had a lower body mass index (BMI) than those who did not participate in the intervention. There were no positive cases of COVID‐19 reported among study participants at follow‐up.
TABLE 1.
Baseline characteristics of atrial fibrillation (AF) patients who did and did not participate in the AF at home program
| Overall sample (N = 68) | Program participation (n = 57) | Did not participate (n = 11) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years)a | 73.4 ± 10.0 | 74.1 ± 9.2 | 69.5 ± 13.5 | .166 |
| Sex | .383 | |||
| Male | 39 (57.4%) | 34 (59.6%) | 5 (45.5%) | |
| Female | 29 (42.6%) | 23 (40.4%) | 6 (54.5%) | |
| Race/ethnicity | .820 | |||
| White | 66 (97.1%) | 55 (96.5%) | 11 (100%) | |
| African American | 1 (1.5%) | 1 (1.8%) | 0 (0%) | |
| Other | 1 (1.5%) | 1 (1.8%) | 0 (0%) | |
| Marital status | .828 | |||
| Married | 54 (79.4%) | 45 (78.9%) | 9 (81.8%) | |
| Divorced | 5 (79.4%) | 4 (7.0%) | 1 (9.1%) | |
| Single | 5 (7.4%) | 4 (7.0%) | 1 (9.1%) | |
| Widowed | 4 (5.9%) | 4 (7.0%) | 0 (0%) | |
| Employment status | .121 | |||
| Employed | 12 (17.6%) | 9 (15.8%) | 3 (27.3%) | |
| Not employed | 43 (63.2%) | 39 (68.4%) | 4 (36.4%) | |
| Unknown | 13 (19.1%) | 9 (15.8%) | 4 (36.4%) | |
| AF history | ||||
| AF Type | .044 | |||
| Paroxysmal | 52 (76.5%) | 41 (71.9%) | 11 (100.0%) | |
| Persistent or permanent | 16 (23.5%) | 16 (28.1%) | 0 (0.0%) | |
| Time since AF diagnosis (months) ‡ | 60.7 ± 52.8 | 57.5 ± 47.2 | 78.0 ± 78.3 | .289 |
| Prior procedures | ||||
| Ablation | 29 (42.6%) | 24 (42.1%) | 5 (45.5%) | .837 |
| LAA occlusion | 2 (2.9%) | 2 (3.5%) | 0 (0.0%) | .528 |
| Prior cardioversion | 30 (44.1%) | 26 (45.6%) | 4 (36.4%) | .572 |
| PM/ICD implant | 19 (27.9%) | 17 (29.8%) | 2 (18.2%) | .431 |
| Cardiovascular comorbidities | ||||
| Hypertension | 38 (55.9%) | 32 (56.1%) | 6 (54.5%) | .922 |
| Previous MI | 12 (17.6%) | 12 (21.1%) | 0 (0.0%) | .094 |
| Coronary heart disease | 20 (29.4%) | 20 (35.1%) | 0 (0.0%) | .019 |
| Hyperlipidemia | 39 (57.4%) | 34 (59.6%) | 5 (45.5%) | .383 |
| Heart failure | 17 (25.0%) | 15 (26.3%) | 2 (18.2%) | .568 |
| TIA/CVA | 8 (11.8%) | 8 (14.0%) | 0 (0.0%) | .186 |
| Diabetes mellitus | 5 (7.4%) | 4 (7.0%) | 1 (9.1%) | .809 |
| Obstructive sleep apnea | 23 (33.8%) | 18 (31.6%) | 5 (45.5%) | .373 |
| Thyroid disease | 14 (20.9%) | 11 (19.6%) | 3 (27.3%) | .569 |
| Chronic lung disease | 15 (22.1%) | 11 (19.3%) | 4 (36.4%) | .211 |
| Chronic kidney disease | 7 (10.3%) | 6 (10.5%) | 1 (9.1%) | .886 |
| Anxiety | 12 (17.9%) | 10 (17.9%) | 2 (18.2%) | .980 |
| Depression | 6 (8.8%) | 6 (10.5%) | 0 (0.0%) | .260 |
| CHA2DS2‐VASc | ||||
| 0 | 2 (2.9%) | 1 (1.8%) | 1 (9.1%) | .187 |
| 1 | 12 (17.6%) | 9 (15.8%) | 3 (27.3%) | .360 |
| ≥2 | 54 (79.4%) | 47 (82.5%) | 7 (63.6%) | .158 |
| Medications | ||||
| Aspirin | 14 (20.6%) | 13 (22.8%) | 1 (9.1%) | .303 |
| P2Y12 | 3 (4.4%) | 3 (5.3%) | 0 (0%) | .436 |
| Anticoagulation therapy | 52 (76.5%) | 46 (80.7%) | 6 (54.5%) | .061 |
| Warfarin | 5 (7.4%) | 4 (7.0%) | 1 (9.1%) | .809 |
| DOAC | 47 (69.1%) | 42 (73.7%) | 5 (45.5%) | .064 |
| Beta blocker | 47 (69.1%) | 41 (71.9%) | 6 (54.5%) | .447 |
| Calcium channel blocker | 4 (6.0%) | 3 (5.3%) | 1 (9.1%) | .633 |
| Antiarrhythmics | 22 (32.8%) | 18 (31.6%) | 4 (36.4%) | .601 |
| Lifestyle factors | ||||
| BMI‡ | 28.1 ± 6.8 | 27.1 ± 5.7 | 33.1 ± 10.1 | .007 |
| Alcohol consumption | 46 (67.6%) | 37 (64.9%) | 9 (81.8%) | .272 |
| Smoking status | .523 | |||
| Current | 1 (1.5%) | 1 (1.8%) | 0 (0%) | |
| Never | 33 (48.5%) | 26 (45.6%) | 7 (63.6%) | |
| Former | 34 (50.0%) | 30 (52.6%) | 4 (36.4%) |
Abbreviations: AF, Atrial fibrillation; MI, myocardial infarction; CAD, coronary artery disease, LAA, left atrial appendage; PM, pacemaker; DOAC, direct oral anticoagulant; ICD, implantable cardioverter‐defibrillator; TIA; transient ischemic attack; CVA; cerebrovascular accident; BMI, body mass index.
aData are presented as means ± SD.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Effect of the intervention on primary outcomes
Adherence to guideline‐recommended self‐monitoring behaviors, including monitoring their heart rate (2.4 ± 1.0 vs. 3.0 ± 1.0; p < .001), heart rhythm (2.2 ± 1.1 vs. 2.6 ± 1.1; p = .003), and blood pressure (2.0 ± 0.9 vs. 2.3 ± 1.1; p = .013) increased significantly over time in the intervention group (Figure 1). Self‐confidence in following medical recommendations (p < .001), symptom identification (p = .007), management (p < .001), and awareness of when to seek emergency medical care for acute exacerbations (p = .001) also improved in the intervention group whereas no improvements in knowledge, self‐care or confidence was observed among non‐participants. Adherence to dietary recommendations also declined (or weight gain occurred) during the study period among non‐participants.
Mental health and physical function were improved at the end of the intervention compared with baseline among program participants (Table 2), as evidenced by significant reductions in anxiety (p = .014; r = 0.33), depression (p = .046; r = 0.26), and sleep disturbance (p < .001; r = 0.80), and improvements in physical function (p = 0006; r = 0.36). There was no change in fatigue, social activities and pain. Furthermore, among persons who did not participate in the intervention, there was no change in mental health, physical function and AF‐related quality of life based on AFEQT total and subscale scores. However, there was a modest reduction in sleep disturbance (p = .029) among non‐participants at the end of the follow‐up period.
TABLE 2.
Differences in primary outcomes at baseline and study follow‐up
| Program participation | N | Baseline | Follow‐up | p | Effect size | |
|---|---|---|---|---|---|---|
| AFEQT total score | ||||||
| Yes | 57 | 76.7 ± 17.9 | 79.2 ± 16.1 | .252 | 0.15 | |
| No | 11 | 80.9 ± 11.2 | 81.7 ± 15.0 | .824 | ||
| AFEQT symptom subscale | ||||||
| Yes | 57 | 81.9 ± 19.4 | 84.1 ± 15.6 | .476 | 0.10 | |
| No | 11 | 79.2 ± 16.9 | 79.2 ± 19.6 | .819 | ||
| AFEQT daily activity | ||||||
| Yes | 57 | 73.4 ± 24.6 | 75.5 ± 23.9 | .260 | 0.15 | |
| No | 11 | 82.8 ± 15.5 | 85.2 ± 16.4 | .266 | ||
| AFEQT treatment concern | ||||||
| Yes | 57 | 77.6 ± 17.0 | 81.0 ± 16.1 | .139 | 0.20 | |
| No | 11 | 79.3 ± 11.5 | 77.6 ± 25.3 | .964 | ||
| AFEQT current control | ||||||
| Yes | 54 | 79.0 ± 19.2 | 80.9 ± 21.1 | .251 | 0.015 | |
| No | 10 | 70.0 ± 30.2 | 71.7 ± 30.5 | .659 | ||
| AFEQT treatment relieved | ||||||
| Yes | 51 | 78.8 ± 21.1 | 79.7 ± 22.7 | .624 | 0.06 | |
| No | 10 | 70.0 ± 32.2 | 70.0 ± 24.6 | .826 | ||
| PROMIS‐physical function | ||||||
| Yes | 57 | 47.7 ± 8.8 | 49.3 ± 7.9 | .006 | 0.36 | |
| No | 11 | 51.8 ± 6.3 | 51.9 ± 6.1 | .645 | ||
| PROMIS‐anxiety | ||||||
| Yes | 56 | 51.8 ± 9.4 | 49.5 ± 8.4 | .014 | 0.33 | |
| No | 11 | 53.0 ± 10.8 | 54.3 ± 10.8 | .646 | ||
| PROMIS‐depression | ||||||
| Yes | 55 | 48.5 ± 7.4 | 46.6 ± 7.3 | .046 | 0.26 | |
| No | 11 | 46.7 ± 9.2 | 48.4 ± 10.7 | .346 | ||
| PROMIS‐fatigue | ||||||
| Yes | 56 | 47.4 ± 10.7 | 46.9 ± 10.0 | .919 | 0 | |
| No | 11 | 45.8 ± 6.9 | 46.0 ± 10.2 | .718 | ||
| PROMIS‐sleep disturbance | ||||||
| Yes | 57 | 56.0 ± 2.6 | 45.4 ± 8.2 | <.001 | 0.80 | |
| No | 11 | 57.8 ± 2.7 | 48.8 ± 10.7 | .029 | ||
| PROMIS‐social activities | ||||||
| Yes | 56 | 51.4 ± 10.6 | 52.1 ± 10.5 | .626 | 0.06 | |
| No | 10 | 55.5 ± 10.0 | 55.8 ± 9.1 | 1.000 | ||
| PROMIS‐pain | ||||||
| Yes | 57 | 48.0 ± 8.0 | 48.1 ± 7.5 | .852 | 0.03 | |
| No | 11 | 50.0 ± 7.5 | 49.8 ± 6.9 | .789 |
Notes
a:Data are presented as means ± SD. For PROMIS domains, a positive value represents worsening pain, pain interference, fatigue, sleep disturbance, depression, and anxiety and an improvement in physical functioning and ability to participate in social roles and activities.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.3. Effect of the intervention on knowledge, beliefs, and behaviors related to COVID‐19
As shown in Table 3, uncertainty and inaccurate information about COVID‐19 was high at baseline among intervention participants. Specifically, 16.1% believed taking NSAIDs increases their risk of infection or experiencing worse symptoms of COVID‐19 while another 35.7% were unsure. Eight percent believed that taking hydroxychloroquine could prevent or treat COVID‐19 and 29.8% were unsure. Nearly two‐thirds of AF patients were unsure if taking commonly prescribed medications for cardiovascular disease (ACE‐I and ARBs) increased their risk of COVID‐19 infection or worse outcomes and just over half of AF patients (52.6%) were unsure if they should immediately discontinue taking ACE‐I and ARBs if infected by the virus. Following the intervention, the number of patients who reported feelings of uncertainty and incorrect information about COVID‐19 decreased substantially. Moreover, inaccurate beliefs about the risks of NSAIDs (p = .021), ACE‐I and ARBs (p = .024) and COVID‐19 was reduced at the end of the intervention compared with baseline. Similarly, the proportion of patients who correctly stated that hydroxychloroquine does not prevent/treat COVID‐19, and that they should not abruptly discontinue their cardiovascular medications if infected by the virus increased from baseline to follow‐up.
TABLE 3.
Knowledge, beliefs, and behaviors related to COVID‐19
| AF‐At‐Home Program Participants | |||
|---|---|---|---|
| Baseline | Follow‐up | p | |
| NSAIDs increase the risk of COVID‐19 infection and worse outcomes | .021 | ||
| True | 9 (16.1%) | 13 (23.2%) | |
| False | 27 (48.2%) | 33 (58.9%) | |
| Unsure | 20 (35.7%) | 10 (17.9%) | |
| Hydroxychloroquine can prevent or treat COVID‐19 | .054 | ||
| True | 5 (8.8%) | 4 (7.1%) | |
| False | 35 (61.4%) | 46 (82.1%) | |
| Unsure | 17 (29.8%) | 6 (10.5%) | |
| ACE‐I and ARBs increase the risk of COVID‐19 infection and worse outcomes | .024 | ||
| True | 7 (12.3%) | 11 (19.6%) | |
| False | 13 (22.8%) | 22 (39.3%) | |
| Unsure | 37 (64.9%) | 23 (41.1%) | |
| Discontinue taking ACE‐I and ARBs immediately if infected by COVID‐19 | <.001 | ||
| True | 2 (3.5%) | 2 (3.5%) | |
| False | 25 (43.9%) | 43 (75.4%) | |
| Unsure | 30 (52.6%) | 12 (21.1%) | |
| Delay or avoid seeking medical attention for symptoms of a heart attack or stroke due to fears of contracting COVID‐19a | ‐ | ||
| Agree | ‐ | 4 (7%) | ‐ |
| Disagree | ‐ | 53 (93%) | ‐ |
| If I had to go to the hospital for worsening cardiac symptoms, I would not get the medical care I need because of COVID‐19a | |||
| Agree | ‐ | 13 (22.8%) | ‐ |
| Disagree | ‐ | 44 (77.2%) | ‐ |
a
Items were not assessed in the baseline questionnaire–data presented are for the follow‐up questionnaire.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Very few patients reported that they would delay seeking medical care for acute cardiovascular symptoms due to fears of COVID‐19 infection (7.0%). However, nearly one in five reported concerns about receiving sub‐optimal care if they were hospitalized for AF during the pandemic. In addition, Figure S1 shows that patients with AF obtain information on COVID‐19 from a variety of electronic and print media sources (Supplementary Appendix). Television news programs were the most the common source of information on COVID‐19 (86%) followed by the patient's medical team (77.2%) and the newspaper (68.4%).
3.4. Intervention adherence, acceptability, and satisfaction
There was no correlation between the number of intervention sessions attended and the primary or secondary outcomes (data not shown). A majority of program participants (75%) reported improvements in AF knowledge and self‐management skills after completing the AF‐At Home‐Program. Participants also reported feeling more confident in their ability to recognize symptoms and manage AF exacerbations (58.8% strongly agreed, 39.2% agreed) and more than two‐thirds reported they were highly satisfied with the program.
4. DISCUSSION
COVID‐19 has revealed the clear and pressing need for technology‐based approaches to delivering continuous education and support to patients with AF during a public health emergency. In this pilot study, we demonstrated the feasibility of developing and rapidly deploying a tailored AF‐self management intervention delivered by a broad range of health care professionals during a period of mandatory quarantine. The results of this study show that the AF‐At‐Home program was effective at increasing self‐confidence in disease management and adherence to guideline‐recommended AF self‐management behaviors, including self‐ monitoring (heart rate, heart rhythm, and blood pressure), symptom identification and management and may have broader applications in routine care outside beyond this pandemic. The program was also effective in reducing sleep disturbance, anxiety and depression. Additionally, we observed a high prevalence of misinformation and inaccurate beliefs about COVID‐19 in this sample of AF patients at baseline. Preliminary findings from this investigation suggest that the AF‐At‐Home program and other technology‐based, direct‐to‐consumer, communication strategies may be effective at reducing uncertainty and inaccurate beliefs about COVID‐19 in vulnerable persons.
Previous studies have suggested that clinic‐based interventions are effective in increasing disease‐specific knowledge, long‐term adherence to anticoagulation therapy, reducing symptom burden and improving quality of life. 4 , 9 , 15 , 16 Advancements in technology have provided new opportunities to expand access to these self‐care programs which have been shown to be safe, cost‐effective, and associated with high patient and provider satisfaction. 26 However, data on the effectiveness of virtual self‐care programs for AF are limited and few studies have examined patients’ engagement with, and acceptance of, technology‐enabled interventions. In one study, an online education program for persons with AF undergoing direct cardioversion or pulmonary vein isolation procedures was associated with modest improvements in AF‐ and procedure‐related knowledge. 27 Another study of a smartphone app for AF showed improvements in disease knowledge, medication adherence, and quality of life. 28 However, those studies were limited to specific subgroups of patients with AF and few apps have demonstrated adoption, reach, and sustainability after their initial evaluation, particularly among persons who are less‐educated, lower income or from a minority background. 29
We extend this work by demonstrating the acceptability and preliminary efficacy of a structured AF self‐management program that was adapted for rapid delivery to address the secondary health impacts of an ongoing public health emergency. We further demonstrate that such an intervention can be delivered 100% remotely, thereby minimizing the risk of COVID‐19 exposure among health care providers and patients. The high percentage of women (40%) and older adults who participated in this study also suggests that the technology‐based programs may facilitate access to underserved populations by overcoming traditional barriers to nonattendance (e.g., inadequate transportation, lack of insurance, and work obligations, and caregiver responsibilities). 30 In addition, although family involvement was not explicitly assessed in this study, we observed a high rate of participation among spouses and caregivers during the intervention. Family members often play a critical role in facilitating lifestyle modifications 31 and couples‐based interventions have been shown to be more effective at improving cardiovascular risk factor management than programs that target only the patient. 32 , 33 Thus, virtual models of AF care may have additional benefits that warrant further study.
The intervention did not have an effect on AF‐related quality of life. The absence of an effect on AF‐related quality of life could have been due to the relatively brief duration of the study which was selected for its practicability and implementation in a wide variety of health care settings and is consistent with the American Heart Association's goals for integrating telehealth solutions into existing care delivery systems. 34 Since the AFEQT is designed to assess symptoms over a 30‐day period, changes in disease‐specific quality of life may not have had sufficient time to manifest. Alternatively, physical stress, emotional isolation, and alterations in daily activities due to COVID‐19 may have attenuated effects of the intervention. Similarly, disruptions to routine care and the transition to telehealth visits may have adverse effects on treatment satisfaction and other factors measured by the AFEQT.
Perhaps one of the most concerning findings from this study was the high rate of inaccurate knowledge, beliefs and behavioral responses to COVID‐19 among persons with AF. Fear and uncertainty have fueled the rapid spread of false information about the virus which is then repeated and “re‐tweeted” on television programs, the internet and social media platforms. 23 This can have dire consequences on population health during a disease outbreak, as it may intensify public fear, mistrust, and paranoia and hamper efforts to deploy effective containment strategies. Patients with AF may be especially vulnerable to these messages, as they may be more likely to discontinue effective therapies because of what they heard on tv and read online. 35 While robust data are lacking, preliminary findings from this study suggest that patients with AF patients are more likely to get information about COVID‐19 from television news shows rather than their medical providers which may have, in part, contributed to the high rates of misinformation about COVID‐19 reported at baseline. There has also been speculation that patients may delay or forgo care for acute cardiovascular symptoms during the pandemic due to fear of infection or receiving inadequate care at the hosptial. 36 Data from our study suggests that while the majority of patients in this sample would seek emergency care for acute cardiovascular symptoms, a significant proportion of patients were afraid that they would receive suboptimal care. Our intervention appeared to attenuate uncertainty and inaccurate beliefs about COVID‐19 among participants, suggesting that timely, direct communication from trusted medical providers may be beneficial at reducing misinformation and fear which are both emerging threats to patient safety.
This intervention was specifically developed to address disruptions in medical services during the most acute phase of the COVID‐19 pandemic, however, the need for safe, socially‐distant interventions will remain during more “chronic” phases of the pandemic, and possibly for months even after a vaccine becomes available. It also remains to be seen whether these services can be adopted and implemented in routine care, and whether the AF‐At‐Home program is effective in populations with less access to technology, low health literacy, and persons living in rural communities, as these populations may be disproportionately affected by digital inequalities and disruption to routine care during a public health emergency. Further randomized clinical studies are also needed to quantify the impact of the AF‐At‐Home program on clinically meaningful outcomes such as reductions in thromboembolic events, and utilization of inpatient, outpatient, or emergency medical services, and to identify patients who would benefit from this type of technology‐based intervention.
4.1. Limitations
This study is not without limitations. First, this study was conducted at an academic medical center with predominantly older, white patients with varying levels of AF knowledge, adherence and health literacy at baseline. Thus, the generalizability of these results to other patients with AF and to other types of organizations may be limited. The lack of randomization is another limitation of this study. Second, while we were able to examine within‐subject changes in the primary outcomes among persons who did and did not participate in the intervention, the study did not include a pre‐specified comparison condition (e.g., usual care) and was not powered to examine between‐group differences in outcomes. Third, in response to the disruptions to routine care that occurred while stay‐at‐home orders were in place, the recruitment period was intentionally short (8 days). We recognize that this limits generalizability, but we felt that rapid, direct communication with patients was the priority. Nevertheless, this may have introduced a selection bias, as persons who regularly use technology may have responded to the study invitation in that timeframe. The reliance on self‐report measures that are prone to social desirability bias may also have affected our results. Similarly, while specific changes in AF knowledge were evaluated, additional dimensions of disease knowledge and health behavior change should be examined in future studies (e.g., knowledge of anticoagulation, procedures, and preventative health behaviors). Fourth, as with any observational study, it is possible that residual confounding may have affected our results. Finally, this pilot study was too small and too short in duration to assess the effect of the intervention on meaningful clinical outcomes or adjust for important clinical covariates in our analyses. Well‐designed trials are needed to clarify these issues and to determine the optimal frequency and timing of intervention sessions to maximize program value in routine clinical care.
5. CONCLUSIONS
As the pandemic continues to unfold, effective, technology‐based solutions are needed to help patients with AF maintain the health and wellbeing. Findings from this proof‐of‐concept study indicate that a virtual self‐management program for persons with AF may improve disease self‐management, mental health, and physical function during the pandemic and may have broader applications in routine care outside beyond the pandemic. The program was also effective in reducing misinformation and inaccurate beliefs about COVID‐19. Larger studies with longer follow‐up are needed to determine the efficacy of this intervention in reducing complications of AF and improving important quality of life outcomes.
CONFLICTS OF INTEREST
Anil K. Gehi, MD: Research Grant: Bristol‐Myers Squib Foundation, Honoraria/Consulting Fees: Biosense‐Webster, Abbott, Biotronik, Zoll Medical. Jennifer Walker, MSN, ANP has received salary support from the Bristol‐Myers Squib Foundation. Sriram Machineni, MD: Research funding: Novo Nordisk, Boeringher Ingelheim, Consulting Fees: Novo Nordisk, Rhythm Pharmaceuticals.
DATA SHARING STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We want to thank Tanya Lulla, Lindsay Mosteller and Brittany Becker for their contribution to this study. This project was funded by a COVID‐19 grant from the Bristol Myers Squib Foundation awarded to Dr. Gehi. Dr. Rosman's effort was sponsored by a grant from the National Heart, Lung, and Blood Institute (K23HL141644).
Rosman L, Armbruster T, Kyazimzade S, et al. Effect of a virtual self‐management intervention for atrial fibrillation during the outbreak of COVID‐19. Pacing Clin Electrophysiol. 2021;44:451–461. 10.1111/pace.14188
REFERENCES
- 1. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42:373‐498. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104‐132. [DOI] [PubMed] [Google Scholar]
- 3. Barnason S, White‐Williams C, Rossi LP, et al. Evidence for therapeutic patient education interventions to promote cardiovascular patient self‐management: a scientific statement for healthcare professionals from the American Heart Association. Circ Cardiovasc Qual Outcomes. 2017;10:e000025. [DOI] [PubMed] [Google Scholar]
- 4. Clarkesmith DE, Pattison HM, Khaing PH, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2017;4:CD008600‐CD008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaufman BG, Kim S, Pieper K, et al. Disease understanding in patients newly diagnosed with atrial fibrillation. Heart. 2018;104:494‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81:e16‐e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng Y‐Y, Ma Y‐T, Zhang J‐Y, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reza N, DeFilippis EM, Jessup M. Secondary impact of the COVID‐19 pandemic on patients with heart failure. Circ Heart Fail. 2020;13:e007219. [DOI] [PubMed] [Google Scholar]
- 10. Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:e441‐419. [DOI] [PubMed] [Google Scholar]
- 11. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta‐analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101‐2107. [DOI] [PubMed] [Google Scholar]
- 12. Lampert R, Jamner L, Burg M, et al. Triggering of symptomatic atrial fibrillation by negative emotion. J Am Coll Cardiol. 2014;64:1533‐1534. [DOI] [PubMed] [Google Scholar]
- 13. Valtorta NK, Kanaan M, Gilbody S, Ronzi S, Hanratty B. Loneliness and social isolation as risk factors for coronary heart disease and stroke: systematic review and meta‐analysis of longitudinal observational studies. Heart. 2016;102:1009‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frasure‐Smith N, Lespérance F, Habra M, et al. Elevated depression symptoms predict long‐term cardiovascular mortality in patients with atrial fibrillation and heart failure. Circulation. 2009;120:140. [DOI] [PubMed] [Google Scholar]
- 15. Beyth RJ, Quinn L, Landefeld CS. A multicomponent intervention to prevent major bleeding complications in older patients receiving warfarin. A randomized, controlled trial. Ann Intern Med. 2000;133:687‐695. [DOI] [PubMed] [Google Scholar]
- 16. Hendriks JML, de Wit R, Crijns HJGM, et al. Nurse‐led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33:2692‐2699. [DOI] [PubMed] [Google Scholar]
- 17. Kannan V, Wilkinson KE, Varghese M, et al. Count me in: using a patient portal to minimize implicit bias in clinical research recruitment. J Am Med Inform Assoc. 2019;26:703‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Obeid JS, Beskow LM, Rape M, et al. A survey of practices for the use of electronic health records to support research recruitment. J Clin Transl Sci. 2017;1:246‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lane DA, Ponsford J, Shelley A, Sirpal A, Lip GY. Patient knowledge and perceptions of atrial fibrillation and anticoagulant therapy: effects of an educational intervention programme. The West Birmingham Atrial Fibrillation Project. Int J Cardiol. 2006;110:354‐358. [DOI] [PubMed] [Google Scholar]
- 20. Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS(®)‐29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018;27:1885‐1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spertus J, Dorian P, Bubien R, et al. Development and validation of the Atrial Fibrillation Effect on QualiTy‐of‐Life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:15‐25. [DOI] [PubMed] [Google Scholar]
- 22. Holmes DN, Piccini JP, Allen LA, et al. Defining clinically important difference in the atrial fibrillation effect on quality‐of‐life score. Circ Cardiovasc Qual Outcomes. 2019;12:e005358. [DOI] [PubMed] [Google Scholar]
- 23. Wessler BS, Kent DM, Konstam MA. Fear of coronavirus disease 2019—An emerging cardiac risk. JAMA Cardiol. 2020;5(9):981‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mutsaerts MAQ, Kuchenbecker WKH, Mol BW, Land JA, Hoek A. Dropout is a problem in lifestyle intervention programs for overweight and obese infertile women: a systematic review. Human Reproduction. 2013;28:979‐986. [DOI] [PubMed] [Google Scholar]
- 25. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2‐18. [DOI] [PubMed] [Google Scholar]
- 26. Anderson L, Sharp GA, Norton RJ, et al. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;6:07130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desteghe L, Germeys J, Vijgen J, et al. Effectiveness and usability of an online tailored education platform for atrial fibrillation patients undergoing a direct current cardioversion or pulmonary vein isolation. Int J Cardiol. 2018;272:123‐129. [DOI] [PubMed] [Google Scholar]
- 28. Turchioe MR, Jimenez V, Isaac S, Alshalabi M, Slotwiner D, Creber RM. Review of mobile applications for the detection and management of atrial fibrillation. Heart Rhythm O2. 2020;1:35‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banerjee A. Bridging the global digital health divide for cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2017;10:e004297. [DOI] [PubMed] [Google Scholar]
- 30. Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the million hearts cardiac rehabilitation collaborative. Mayo Clin Proc. 2017;92:234‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shiffman D, Louie JZ, Devlin JJ, Rowland CM, Mora S. Concordance of cardiovascular risk factors and behaviors in a multiethnic US Nationwide cohort of married couples and domestic partners. JAMA Network Open. 2020;3:e2022119‐e2022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richards EA, Franks MM, McDonough MH, Porter K. ‘Let's move:’ a systematic review of spouse‐involved interventions to promote physical activity. Int J Health Promot Educ. 2018;56:51‐67. [Google Scholar]
- 33. Dougherty CM, Thompson EA, Kudenchuk PJ. Patient plus partner trial: a randomized controlled trial of 2 interventions to improve outcomes after an initial implantable cardioverter‐defibrillator. Heart Rhythm. 2019;16:453‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eapen ZJ, Turakhia MP, McConnell MV, et al. Defining a mobile health roadmap for cardiovascular health and disease. J Am Heart Assoc. 2016;5:e003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hill JA, Agewall S, Baranchuk A, et al. Medical misinformation: vet the message!. Heart Rhythm. 2019;16:332‐333. [DOI] [PubMed] [Google Scholar]
- 36. Kiss P, Carcel C, Hockham C, Peters SAE. The impact of the COVID‐19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes. 2020;7(1):18‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


